Leptin Modulates the Expression of miRNAs-Targeting POMC mRNA by the JAK2-STAT3 and PI3K-Akt Pathways
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Culture and Reagents
2.2. Quantitative RT-PCR (qRT-PCR) Analysis
2.3. Luciferase Reporter Assays
2.4. Gene Ontology and KEGG Pathways Analysis
2.5. Statistical Analysis
3. Results
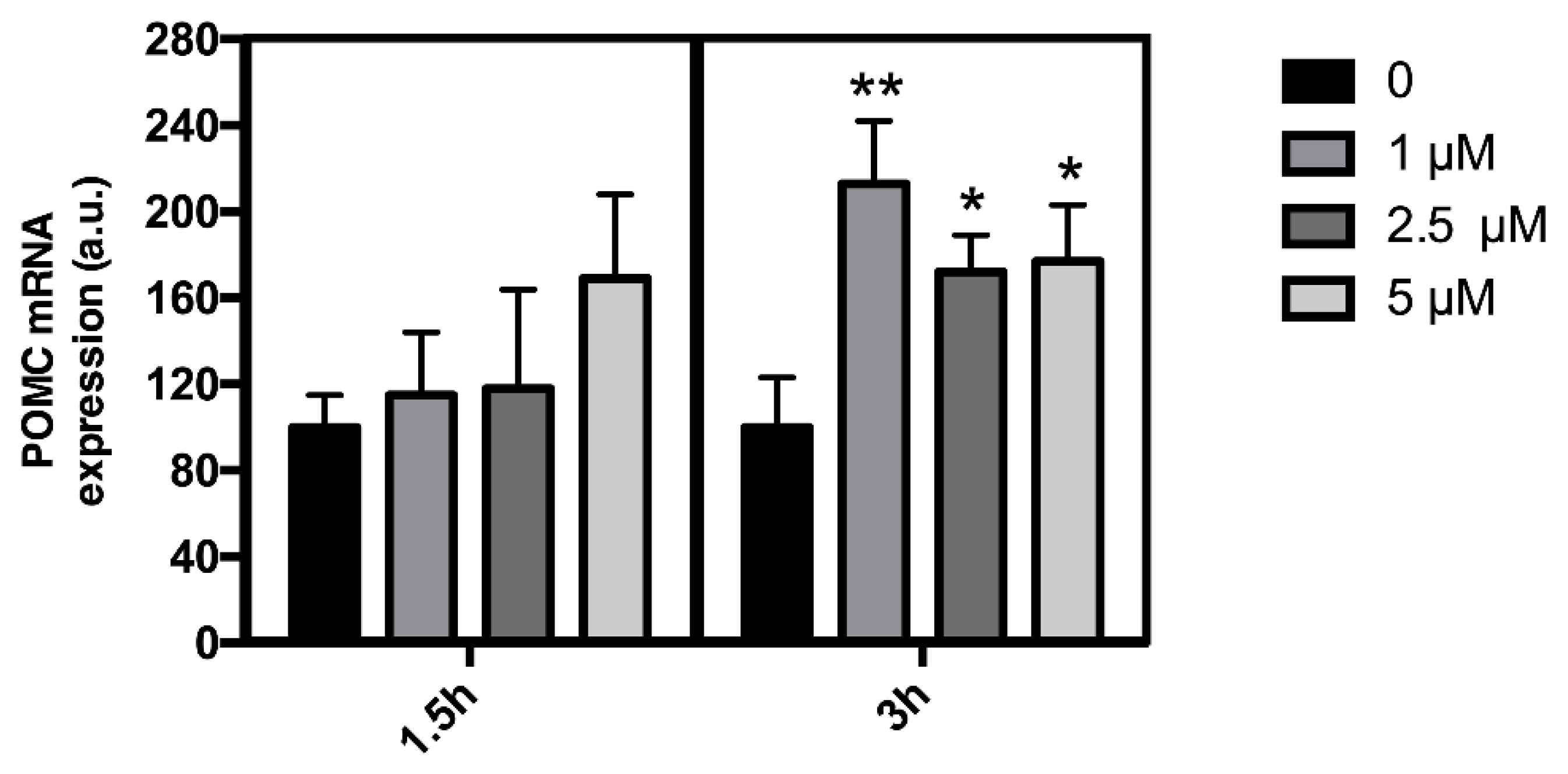
3.1. Effect of Leptin on POMC Expression in mHypoA-POMC/GFP Cell Line
3.2. miR-383, miR-384-3p, and miR-488 Target POMC 3′UTR
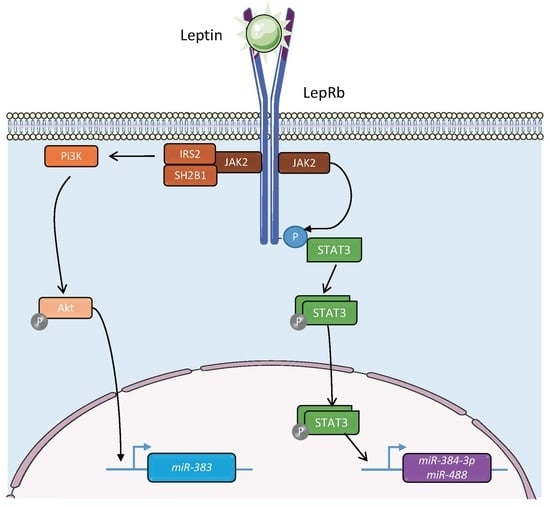
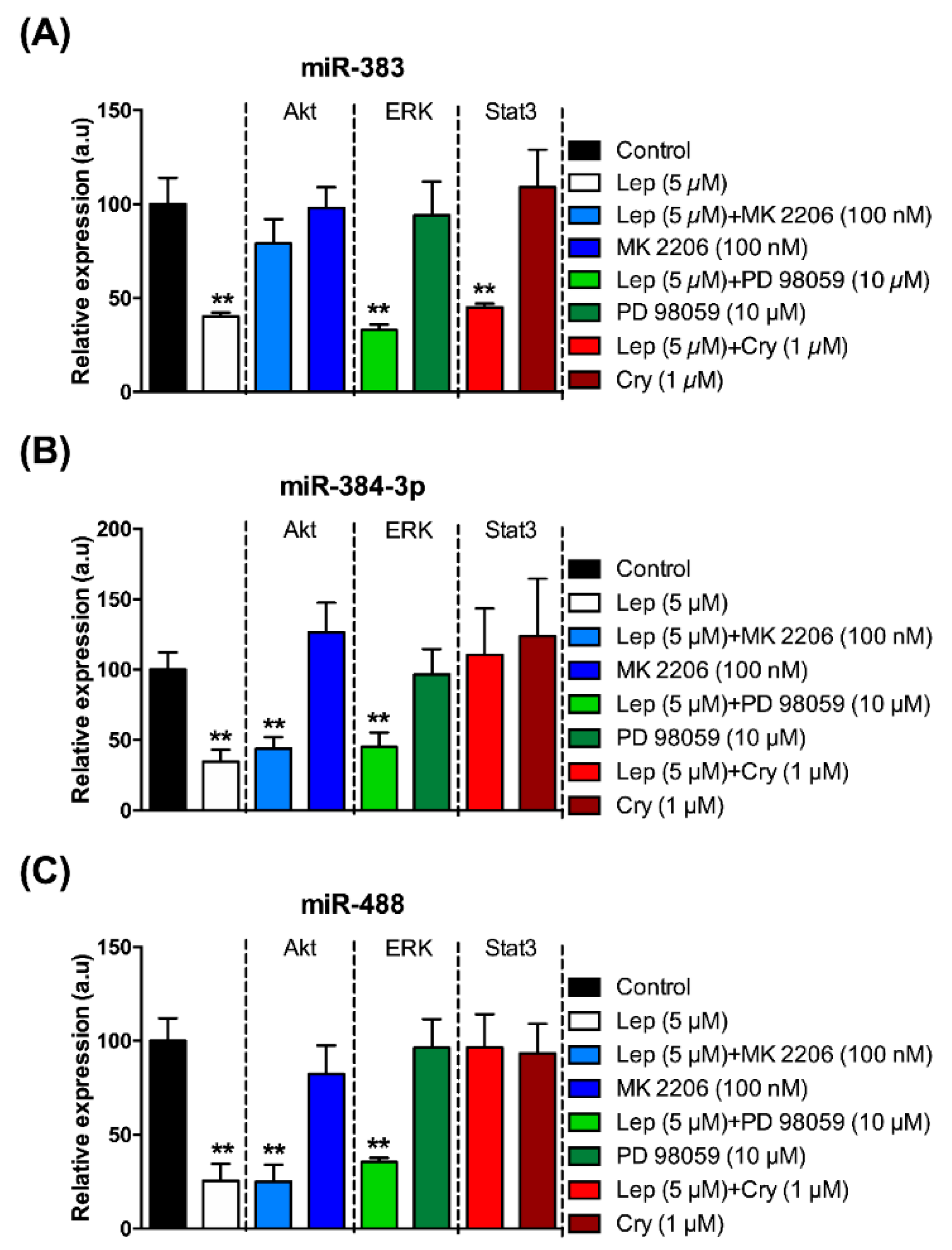
3.3. Regulation of the Expression of the miRNAs of Interest by Leptin via the STAT3, AKT, and ERK Pathways
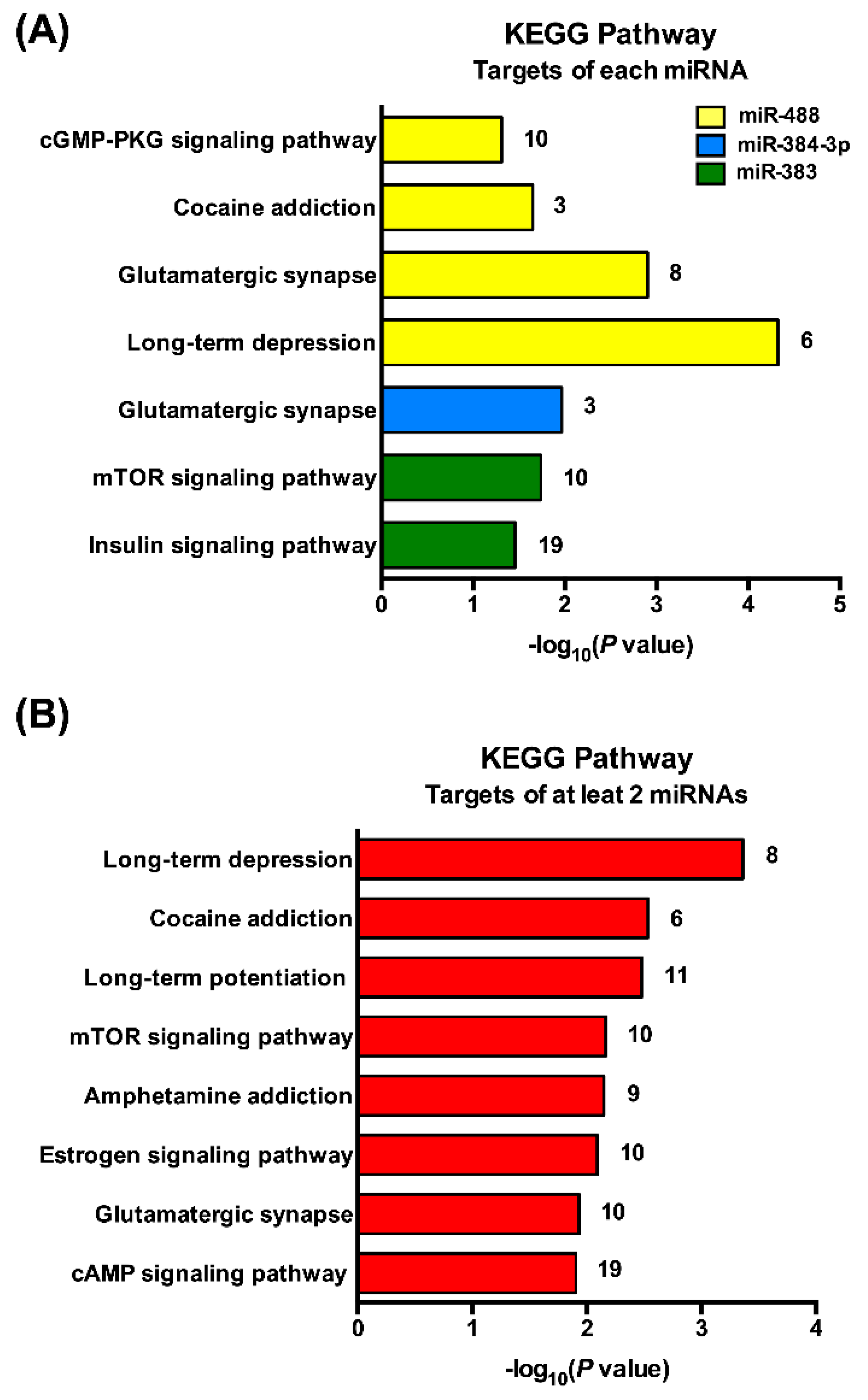
3.4. Pathway Analysis of miRNAs Targeting POMC
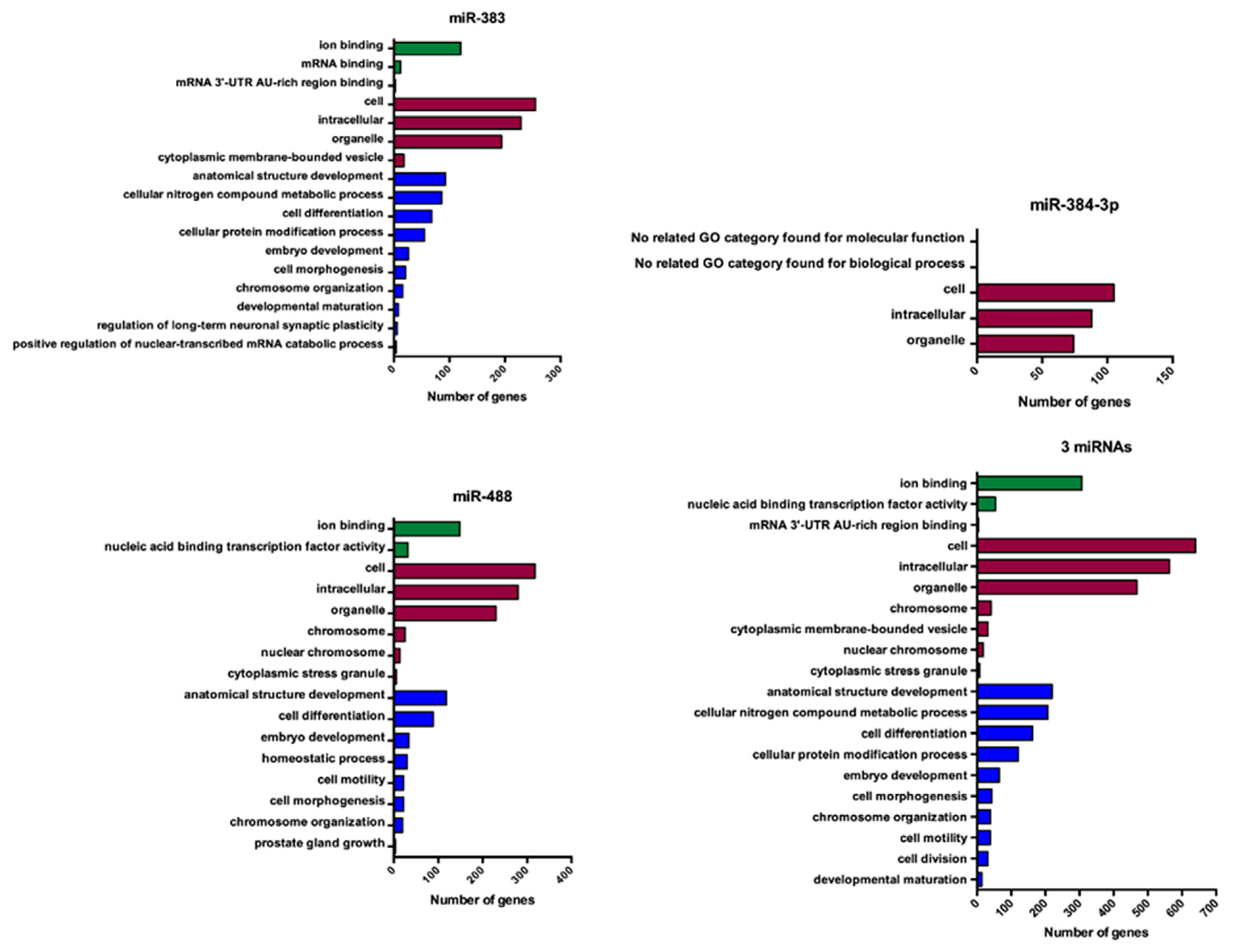
3.5. Gene Ontology Analysis of miRNAs Targeting POMC
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. The role of microRNA in the modulation of the melanocortinergic system. Front. Neurosci. 2017, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005, 8, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Gomis, R.; Claret, M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014, 220, T25–T46. [Google Scholar] [CrossRef]
- Zhang, Y. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Balthasar, N.; Coppari, R.; McMinn, J.; Liu, S.M.; Lee, C.E.; Tang, V.; Kenny, C.D.; McGovern, R.A.; Chua, S.C., Jr.; Elmquist, J.K.; et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004, 42, 983–991. [Google Scholar] [CrossRef]
- Tartaglia, L.A. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Xu, A.W.; Ste-Marie, L.; Kaelin, C.B.; Barsh, G.S. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (POMC) neurons causes decreased POMC expression, mild obesity, and defects in compensatory refeeding. Endocrinology 2007, 148, 72–80. [Google Scholar] [CrossRef]
- Vaisse, C.; Halaas, J.L.; Horvath, C.M.; Darnell, J.E.; Stoffel, M.; Friedman, J.M. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat. Genet. 1996, 14, 95–97. [Google Scholar] [CrossRef]
- Myers, M.G.; Cowley, M.A.; Münzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef]
- Rahmouni, K.; Sigmund, C.D.; Haynes, W.G.; Mark, A.L. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 2009, 58, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Cao, Y.; Hayashi, Y.; Nakata, M.; Takano, E.; Yada, T.; Zhang, C.; Ogawa, W.; Oki, M.; Chua, S.; et al. PDK-1/FoxO1 pathway in POMC neurons regulates POMC expression and food intake. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E787–E798. [Google Scholar] [CrossRef] [PubMed]
- Derghal, A.; Djelloul, M.; Airault, C.; Pierre, C.; Dallaporta, M.; Troadec, J.-D.; Tillement, V.; Tardivel, C.; Bariohay, B.; Trouslard, J.; et al. Leptin is required for hypothalamic regulation of miRNAs targeting POMC 3′UTR. Front. Cell Neurosci. 2015, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Derghal, A.; Djelloul, M.; Azzarelli, M.; Degonon, S.; Tourniaire, F.; Landrier, J.-F.; Mounien, L. MicroRNAs are involved in the hypothalamic leptin sensitivity. Epigenetics 2018, 13, 1127–1140. [Google Scholar] [CrossRef]
- Schneeberger, M.; Altirriba, J.; García, A.; Esteban, Y.; Castaño, C.; García-Lavandeira, M.; Alvarez, C.V.; Gomis, R.; Claret, M. Deletion of miRNA processing enzyme Dicer in POMC-expressing cells leads to pituitary dysfunction, neurodegeneration and development of obesity. Mol. Metab. 2012, 2, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Greenman, Y.; Kuperman, Y.; Drori, Y.; Asa, S.L.; Navon, I.; Forkosh, O.; Gil, S.; Stern, N.; Chen, A. Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Mol. Endocrinol. 2013, 27, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Croizier, S.; Park, S.; Maillard, J.; Bouret, S.G. Central Dicer-miR-103/107 controls developmental switch of POMC progenitors into NPY neurons and impacts glucose homeostasis. eLIFE 2018, 7, e40429. [Google Scholar] [CrossRef]
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. An Emerging role of micro-RNA in the effect of the endocrine disruptors. Front. Neurosci. 2016, 10, 318. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Landrier, J.-F.; Derghal, A.; Mounien, L. MicroRNAs in obesity and related metabolic disorders. Cells 2019, 8, 859. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Xu, Y.; Xie, X.; Wang, T.; Ko, J.-H.; Zhou, T. The role of RNA structure at 5′ untranslated region in microRNA-mediated gene regulation. RNA 2014, 20, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ajay, S.S.; Yook, J.I.; Kim, H.S.; Hong, S.H.; Kim, N.H.; Dhanasekaran, S.M.; Chinnaiyan, A.M.; Athey, B.D. New class of microRNA targets containing simultaneous 5′-UTR and 3′-UTR interaction sites. Genome Res. 2009, 19, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.J.; Coller, H.A. The code within the code: microRNAs target coding regions. Cell Cycle 2010, 9, 1533–1541. [Google Scholar] [CrossRef]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef]
- Wang, J.; Lu, M.; Qiu, C.; Cui, Q. TransmiR: A transcription factor-microRNA regulation database. Nucleic Acids Res. 2010, 38, D119–D122. [Google Scholar] [CrossRef]
- Li, Z.; Ji, L.; Su, S.; Zhu, X.; Cheng, F.; Jia, X.; Zhou, Q.; Zhou, Y. Leptin up-regulates microRNA-27a/b-3p level in hepatic stellate cells. Exp. Cell Res. 2018, 366, 63–70. [Google Scholar] [CrossRef]
- Nazarians-Armavil, A.; Chalmers, J.A.; Lee, C.B.; Ye, W.; Belsham, D.D. Cellular insulin resistance disrupts hypothalamic mHypoA-POMC/GFP neuronal signaling pathways. J. Endocrinol. 2014, 220, 13–24. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Muiños-Gimeno, M.; Espinosa-Parrilla, Y.; Guidi, M.; Kagerbauer, B.; Sipilä, T.; Maron, E.; Pettai, K.; Kananen, L.; Navinés, R.; Martín-Santos, R.; et al. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol. Psychiatry 2011, 69, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, S.; Xue, L.; Li, Y.; Wang, J.; He, X.; Zhu, Z.; Dong, C. MiR-488 determines coat pigmentation by down-regulating the pigment-producing gene pro-opiomelanocortin. Cell. Mol. Biol. 2016, 62, 37–43. [Google Scholar] [PubMed]
- Mounien, L.; Marty, N.; Tarussio, D.; Metref, S.; Genoux, D.; Preitner, F.; Foretz, M.; Thorens, B. Glut2-dependent glucose-sensing controls thermoregulation by enhancing the leptin sensitivity of NPY and POMC neurons. FASEB J. 2010, 24, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Mounien, L.; Do Rego, J.-C.; Bizet, P.; Boutelet, I.; Gourcerol, G.; Fournier, A.; Brabet, P.; Costentin, J.; Vaudry, H.; Jégou, S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology 2009, 34, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Toda, C.; Santoro, A.; Kim, J.D.; Diano, S. POMC neurons: From birth to death. Annu. Rev. Physiol. 2017, 79, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Sternson, S.M.; Atasoy, D.; Betley, J.N.; Henry, F.E.; Xu, S. An emerging technology framework for the neurobiology of appetite. Cell Metab. 2016, 23, 234–253. [Google Scholar] [CrossRef]
- Koch, M.; Varela, L.; Kim, J.G.; Kim, J.D.; Hernández-Nuño, F.; Simonds, S.E.; Castorena, C.M.; Vianna, C.R.; Elmquist, J.K.; Morozov, Y.M.; et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 2015, 519, 45–50. [Google Scholar] [CrossRef]
- McIlwraith, E.K.; Loganathan, N.; Belsham, D.D. Regulation of Gpr173 expression, a putative phoenixin receptor, by saturated fatty acid palmitate and endocrine-disrupting chemical bisphenol A through a p38-mediated mechanism in immortalized hypothalamic neurons. Mol. Cell. Endocrinol. 2019, 485, 54–60. [Google Scholar] [CrossRef]
- Salehi, A.; Loganathan, N.; Belsham, D.D. Bisphenol A induces POMC gene expression through neuroinflammatory and PPARγ nuclear receptor-mediated mechanisms in POMC-expressing hypothalamic neuronal models. Mol. Cell. Endocrinol. 2019, 479, 12–19. [Google Scholar] [CrossRef]
- Tse, E.K.; Belsham, D.D. Palmitate induces neuroinflammation, ER stress, and POMC mRNA expression in hypothalamic mHypoA-POMC/GFP neurons through novel mechanisms that are prevented by oleate. Mol. Cell. Endocrinol. 2018, 472, 40–49. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, L.-G.; Yao, H.-B.; Zhang, J.; Ding, K.-F. Upregulation of microRNA-383 inhibits the proliferation, migration and invasion of colon cancer cells. Oncol. Lett. 2018, 15, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Wang, J.; Song, Y.; Wang, Q.; Wu, Q. MicroRNA-383 regulates cell viability and apoptosis by mediating Wnt/β-catenin signaling pathway in non-small cell lung cancer. J. Cell. Biochem. 2018, 120, 7918–7926. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Tian, H.; Liu, L.; Zhang, X.-S.; Li, W.-Q.; Deng, Y.-M.; Yao, G.-D.; Yin, M.-M.; Sun, F. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell Death Dis. 2010, 1, e94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, G.; Lai, W.; Liu, S.; Shuai, J. MicroRNA-383 upregulation protects against propofol-induced hippocampal neuron apoptosis and cognitive impairment. Exp. Med. 2018, 15, 3181–3188. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.; Jiao, Y.; Hao, M.; Tang, X. MicroRNA-383 suppresses the PI3K-AKT-MTOR signaling pathway to inhibit development of cervical cancer via down-regulating PARP2. J. Cell. Biochem. 2018, 119, 5243–5252. [Google Scholar] [CrossRef]
- Cota, D.; Proulx, K.; Smith, K.A.B.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef]
- Yang, S.-B.; Tien, A.-C.; Boddupalli, G.; Xu, A.W.; Jan, Y.N.; Jan, L.Y. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 2012, 75, 425–436. [Google Scholar] [CrossRef]
- Xia, F.; Sun, J.-J.; Jiang, Y.-Q.; Li, C.-F. MicroRNA-384-3p inhibits retinal neovascularization through targeting hexokinase 2 in mice with diabetic retinopathy. J. Cell. Physiol. 2018, 234, 721–730. [Google Scholar] [CrossRef]
- Khlaifia, A.; Matias, I.; Cota, D.; Tell, F. Nutritional status-dependent endocannabinoid signalling regulates the integration of rat visceral information. J. Physiol. 2017, 595, 3267–3285. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Y. Hunger states control the directions of synaptic plasticity via switching cell type-specific subunits of NMDA receptors. J. Neurosci. 2015, 35, 13171–13182. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-H.; Zheng, L.; He, H.-P.; Zheng, D.-L.; Wei, Z.-Q.; Wang, N.; Dong, J.; Ma, W.-J.; Zhang, T.-C. STAT3 regulated ATR via microRNA-383 to control DNA damage to affect apoptosis in A431 cells. Cell. Signal. 2015, 27, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Ozsolak, F.; Poling, L.L.; Wang, Z.; Liu, H.; Liu, X.S.; Roeder, R.G.; Zhang, X.; Song, J.S.; Fisher, D.E. Chromatin structure analyses identify miRNA promoters. Genes Dev. 2008, 22, 3172–3183. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derghal, A.; Astier, J.; Sicard, F.; Couturier, C.; Landrier, J.-F.; Mounien, L. Leptin Modulates the Expression of miRNAs-Targeting POMC mRNA by the JAK2-STAT3 and PI3K-Akt Pathways. J. Clin. Med. 2019, 8, 2213. https://doi.org/10.3390/jcm8122213
Derghal A, Astier J, Sicard F, Couturier C, Landrier J-F, Mounien L. Leptin Modulates the Expression of miRNAs-Targeting POMC mRNA by the JAK2-STAT3 and PI3K-Akt Pathways. Journal of Clinical Medicine. 2019; 8(12):2213. https://doi.org/10.3390/jcm8122213
Chicago/Turabian StyleDerghal, Adel, Julien Astier, Flavie Sicard, Charlène Couturier, Jean-François Landrier, and Lourdes Mounien. 2019. "Leptin Modulates the Expression of miRNAs-Targeting POMC mRNA by the JAK2-STAT3 and PI3K-Akt Pathways" Journal of Clinical Medicine 8, no. 12: 2213. https://doi.org/10.3390/jcm8122213
APA StyleDerghal, A., Astier, J., Sicard, F., Couturier, C., Landrier, J.-F., & Mounien, L. (2019). Leptin Modulates the Expression of miRNAs-Targeting POMC mRNA by the JAK2-STAT3 and PI3K-Akt Pathways. Journal of Clinical Medicine, 8(12), 2213. https://doi.org/10.3390/jcm8122213