Spontaneous SSCD Auto-Plugging: Clinical, Electrophysiological and Radiological Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
- −
- Clinical atypia, defined as presentation not fully consistent with the Bárány Society’s recommended diagnostic criteria [8], characterized by one or more of the following features:
- −
- No or lesser than expected conductive hearing loss (CHL) in pure tone audiometry: no or slight air bone gap (ABG)
- −
- Normal cVEMP threshold, normal oVEMP amplitude
- −
- No bone conduction hyperacusis (including autophony)
- −
- No pulsatile tinnitus (PT)
- −
- No nystagmus or dizziness induced by pressure or sound
- −
- Arguments for another possible vestibular disease or disorder.
- −
- And a large dehiscence in HRCT, defined here as >4 mm in the Pöschl plane (Suspicion of auto-plugging)
2.2. Audio-Vestibular Assessment
2.3. Radiological Assessment
3. Results
3.1. Clinical and Radiological Findings
3.2. Audio-Vestibular Findings
3.3. Imaging Presentation
4. Discussion
4.1. Radiological Elements
4.2. Further Pathophysiological Elements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABG | Air bone gap |
| BPPV | Benign paroxysmal positional vertigo |
| CFD | Cochleo-facial dehiscence |
| CHL | Conductive hearing loss |
| HRCT | High resolution computed tomodensitometry |
| MRI | Magnetic resonance imagery |
| PT | Pulsatile tinnitus |
| SSC | Superior semicircular canal |
| SSCD | Superior semi-circular canal dehiscence |
| SNHL | Sensorineural hearing loss |
| TMW | Third mobile window |
| VEMP | Vestibular evoked myogenic potentials |
| VHIT | Video Head Impulse test |
| VOR | Vestibulo-ocular reflex |
References
- Cawthorne, T. Otosclerosis. J. Laryngol. Otol. 1955, 69, 437–456. [Google Scholar] [CrossRef]
- Tullio, P. Das Ohr und die Entstehung der Sprache und Schrift; Urban and Schwarzenberg: Berlin, Germany, 1929. [Google Scholar]
- Minor, L.B.; Solomon, D.; Zinreich, J.S.; Zee, D.S. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 249–258. [Google Scholar] [CrossRef]
- Minor, L.B. Superior canal dehiscence syndrome. Am. J. Otol. 2000, 21, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wackym, P.A.; Balaban, C.D.; Zhang, P.; Siker, D.A.; Hundal, J.S. Third window syndrome: Surgical management of cochlea-facial nerve dehiscence. Front. Neurol. 2019, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N.; Rosowski, J.J. Conductive hearing loss caused by third-window lesions of the inner ear. Otol. Neurotol. 2008, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Iversen, M.M.; Rabbitt, R.D. Biomechanisms of third window syndrome. Front. Neurol. 2020, 11, 891. [Google Scholar] [CrossRef]
- Ward, B.K.; van de Berg, R.; van Rompaey, V.; Bisdorff, A.; Hullar, T.E.; Welgampola, M.S.; Carey, J.P. Consensus document of the committee for the classification of vestibular disorders of the Bárány Society. Superior semicircular canal dehiscence syndrome: Diagnostic criteria. J. Vestib. Res. 2021, 31, 131–141. [Google Scholar] [CrossRef]
- Ward, B.K.; Agrawal, Y.; Nguyen, E.; Della Santina, C.C.; Limb, C.J.; Francis, H.W.; Minor, L.B.; Carey, J.P. Hearing outcomes after surgical plugging of the superior semicircular canal by a middle cranial fossa approach. Otol. Neurotol. 2012, 33, 1386–1391. [Google Scholar] [CrossRef]
- Vlastarakos, P.V.; Proikas, K.; Tavoulari, E.; Kikidis, D.; Maragoudakis, P.; Nikolopoulos, T.P. Efficacy assessment and complications of surgical management for superior semicircular canal dehiscence: A meta-analysis of published interventional studies. Eur. Arch. Otorhinolaryngol. 2009, 266, 177–186. [Google Scholar] [CrossRef]
- Brandolini, C.; Modugno, G.C. Do signs of natural plugging of superior semicircular canal dehiscence exist? Am. J. Otolaryngol. 2012, 33, 268–271. [Google Scholar] [CrossRef]
- Carey, J.P.; Migliaccio, A.A.; Minor, L.B. Semicircular canal function before and after surgery for superior canal dehiscence. Otol. Neurotol. 2007, 28, 356–364. [Google Scholar] [CrossRef]
- Mukherjee, P.; Chiarovano, E.; Cheng, K.; Manzari, L.; McGarvie, L.A.; MacDougall, H.G. Video-head impulse test in superior canal dehiscence. Acta Otolaryngol. 2021, 141, 471–475. [Google Scholar] [CrossRef]
- Cremer, P.D.; Minor, L.B.; Carey, J.P.; Della Santina, C.C. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology 2000, 55, 1833–1841. [Google Scholar] [CrossRef]
- Castellucci, A.; Brandolini, C.; Del Vecchio, V.; Giordano, D.; Pernice, C.; Bianchin, G.; Maiolo, V.; Ferri, G.G. Temporal bone meningocele associated with superior canal dehiscence. Otol. Neurotol. 2018, 39, e506–e508. [Google Scholar] [CrossRef]
- Ionescu, E.C.; Idriss, S.; Reynard, P.; Ltaief-Boudrigua, A.; Thai-Van, H. Persistent positional vertigo in a patient with partial “auto-plugged” superior semicircular canal dehiscence: A case study. J. Int. Adv. Otol. 2022, 18, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, E.C.; Mustea, E.; Reynard, P.; Thai-Van, H. Comment on Castellucci et al. Impaired vestibulo-ocular reflex on video head impulse test in superior canal dehiscence: “Spontaneous plugging” or endolymphatic flow dissipation? Audiol. Res. 2023, 13, 802–820. Audiol. Res. 2024, 14, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, A.; Martellucci, S.; Malara, P.; Botti, C.; Del Vecchio, V.; Brandolini, C.; Ferri, G.G.; Ghidini, A.; Armato, E. Possible pathomechanisms accounting for both sound/pressure-induced eye movements and video head impulse test data in superior canal dehiscence. Acta Otolaryngol. 2021, 141, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, A.; Malara, P.; Martellucci, S.; Alfarghal, M.; Brandolini, C.; Piras, G.; Armato, E.; Ruberto, R.R.; Brizzi, P.; Presutti, L.; et al. Impaired vestibulo-ocular reflex on video head impulse test in superior canal dehiscence: “Spontaneous plugging” or endolymphatic flow dissipation? Audiol. Res. 2023, 13, 802–820. [Google Scholar] [CrossRef]
- Welgampola, M.S.; Myrie, O.A.; Minor, L.B.; Carey, J.P. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology 2008, 70, 464–472. [Google Scholar] [CrossRef]
- Malara, P.; Martellucci, S.; Castellucci, A. Defining potential pathomechanisms behind an impaired canal function at the video-head impulse test in canal dehiscence. Reply to Ionescu et al. comment on “Castellucci et al. Impaired vestibulo-ocular reflex on video head impulse test in superior canal dehiscence: “Spontaneous plugging” or endolymphatic flow dissipation?”. Audiol. Res. 2025, 15, 32. [Google Scholar] [CrossRef]
- Castellucci, A.; Dumas, G.; Abuzaid, S.M.; Armato, E.; Martellucci, S.; Malara, P.; Alfarghal, M.; Ruberto, R.R.; Brizzi, P.; Ghidini, A.; et al. Posterior semicircular canal dehiscence with vestibulo-ocular reflex reduction for the affected canal at the video-head impulse test: Considerations to pathomechanisms. Audiol. Res. 2024, 14, 317–332. [Google Scholar] [CrossRef]
- Niesten, M.E.; Hamberg, L.M.; Silverman, J.B.; Lou, K.V.; McCall, A.A.; Windsor, A.; Curtin, H.D.; Herrmann, B.S.; Grolman, W.; Nakajima, H.H.; et al. Superior canal dehiscence length and location influences clinical presentation and audiometric and cervical vestibular-evoked myogenic potential testing. Audiol. Neurootol. 2014, 19, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, A.; Piras, G.; Del Vecchio, V.; Crocetta, F.M.; Maiolo, V.; Ferri, G.G.; Ghidini, A.; Brandolini, C. The effect of superior canal dehiscence size and location on audiometric measurements, vestibular-evoked myogenic potentials and video-head impulse testing. Eur. Arch. Otorhinolaryngol. 2021, 278, 997–1015. [Google Scholar] [CrossRef]
- Lee, S.Y.; Bae, Y.J.; Kim, M.; Song, J.J.; Choi, B.Y.; Koo, J.W. Changes in vestibulo-ocular reflex gain after surgical plugging of superior semicircular canal dehiscence. Front. Neurol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Renteria, A.E.; Elblidi, A.; Altamami, N.; Alhabib, S.; Saliba, I. Video head impulse test demonstrates a residual function after plugging of dehiscent superior semicircular canal. Otol. Neurotol. 2023, 44, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Çeliker, F.B.; Özgür, A.; Çeliker, M.; Beyazal, M.; Turan, A.; Terzi, S.; Inecikili, M.F. The efficacy of magnetic resonance imaging for the diagnosis of superior semicircular canal dehiscence. J. Int. Adv. Otol. 2017, 13, 68–71. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.; Choi, J.Y.; Bae, Y.J.; Kim, M.; Song, J.J.; Choi, B.Y.; Jeong, W.K.; Koo, J.W. Quantitative three-dimensional image analysis of the superior canal after surgical plugging to treat superior semicircular canal dehiscence. Sci. Rep. 2021, 11, 16112. [Google Scholar] [CrossRef]
- Eberhard, K.E.; Siker, D.; Yawn, R.; Klokker, M.; Cayé-Thomasen, P.; Lee, D.J. Current trends, controversies, and future directions in the evaluation and management of superior canal dehiscence syndrome. Front. Neurol. 2021, 12, 638574. [Google Scholar] [CrossRef]
- Ionescu, E.C.; Reynard, P.; Damien, M.; Ltaïef-Boudrigua, A.; Hermann, R.; Gianoli, G.J.; Thai-Van, H. Why should multiple dehiscences of the otic capsule be considered before surgically treating patients with superior semicircular canal dehiscence? A radiological monocentric review and a case series. Front. Neurol. 2023, 14, 1209567. [Google Scholar] [CrossRef]
- Nadaraja, G.S.; Monfared, A.; Jackler, R.K. Spontaneous cerebrospinal fluid leak through the posterior aspect of the petrous bone. J. Neurol. Surg. B Skull Base 2012, 73, 71–75. [Google Scholar] [CrossRef]
- Pelosi, S.; Bederson, J.B.; Smouha, E.E. Cerebrospinal fluid leaks of temporal bone origin: Selection of surgical approach. J. Neurol. Surg. Part B Skull Base 2010, 20, 253–259. [Google Scholar] [CrossRef]
- Kuo, P.; Bagwell, K.A.; Mongelluzzo, G.; Schutt, C.A.; Malhotra, A.; Khokhar, B.; Kveton, J.F. Semicircular canal dehiscence among idiopathic intracranial hypertension patients. Laryngoscope 2018, 128, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Berkiten, G.; Gürbüz, D.; Akan, O.; Tutar, B.; Tunç, M.K.; Karaketir, S.; Bircan, H.S.; Berkiten, E.; Sarı, H.; Atar, Y.; et al. Dehiscence or thinning of bone overlying the superior semicircular canal in idiopathic intracranial hypertension. Eur. Arch. otorhinolaryngol. 2022, 279, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Johanis, M.; De Jong, R.; Miao, T.; Hwang, L.; Lum, M.; Kaur, T.; Willis, S.; Arsenault, J.J.; Duong, C.; Yang, I.; et al. Concurrent superior semicircular canal dehiscence and endolymphatic hydrops: A novel case series. Int. J. Surg. Case Rep. 2021, 78, 382–386. [Google Scholar] [CrossRef] [PubMed]


| Patient | Age (Years) Sex | Audiometric Findings Air Bone Gap | Symptoms | VHIT (Gain) | VEMPs Threshold (dB) (Amplitude) |
|---|---|---|---|---|---|
| SSC/HSC/PSC | |||||
| 1. | 68 F | LE: CHL 30 dB (0.25 kHz) 50 dB (0.5 kHz) | Isolated vertigo (sometimes while bending over or blowing nose) | Normal RE: 1/1/1 LE: 0.9/1/1 | cVEMPs RE: 95 dB (38.07 µV) LE: 70 dB (550.30 µV) oVEMPs RE:Absent; LE: 95 dB |
| 2. | 53 F | LE: mild SNHL (No CHL) | PT, autophony (LE) Dizziness | Normal RE: 1/1/1 LE: 1/1/0.9 | cVEMPs RE: 95 dB (428.56 µV) LE: 60 dB (901.89 µV) oVEMPs RE:Absent; LE: 95 dB |
| 3. | 77 F | LE: mixed hearing loss; CHL: 40 dB (0.25 kHz) | -Recurrent BPPV despite vestibular rehabilitation | Normal RE: 1/0.8/0.8 LE: 0.9/1/0.9 | cVEMPs RE: 95 dB (71.05 µV) LE: 60 dB (948.89 µV) oVEMPs: Not available |
| 4. | 85 F | LE: CHL 45 dB (0.25 kHz) 20 dB (0.5 kHz) | -Dizziness. -No auditory symptoms | LE SSC impairment RE: 0.9/0.9/0.7 LE: 0.6/0.9/0.7 | cVEMPs RE: 95 dB (35.98 µV) LE: 50 dB (790.74 µV) oVEMPs RE: absent; LE: 95 dB |
| 5. | 41 F | LE: CHL 45 dB (0.25 Khz) 20 dB (0.5 kHz) | -Autophony (LE) -Vertigos when sneezing | Normal RE: 0.8/1/1 LE: 0.8/1/1 | cVEMPs RE: 50 dB (596.95 µV) LE: 50 dB (585.12 µV) oVEMPs RE: 95 dB; LE: 95 dB |
| 6. | 42 M | Bilateral CHL RE: 20 dB (0.25 and 0.5 kHz) LE: 30 dB (0.25 kHz) 15 dB (0.5 kHz) | -Dizziness -Non-PT (bilateral) | Bilateral SSC impairment RE: 0.6/0.9/0.7 LE: 0.6/0.9/0.7 | cVEMPs RE: 50 dB (817 µV) LE: 50 dB (728 µV) oVEMPs RE: 70 dB; LE: 60 dB |
| 7. | 57 M | SNHL(bilateral) RE: moderate LE: mild | -Autophony (RE) -Tullio -Vertigo at Valsalva (closed glottis) -Dizziness. | Normal RE: 0.9/1/0.8 LE: 1/1/0.8 | cVEMPs RE: 60 dB (356 µV) LE: 50 dB (530 µV) oVEMPs RE: 95 dB; LE: 95 dB |
| 8. | 41 M | LE: Normal | -Non-PT (LE) (THI 68) | Normal RE: 1/1/1 LE: 1/1/1 | cVEMPs RE: 95 dB (150 µV) LE: 95 dB (400 µV) oVEMPs Not available |
| 9. | 41 M | LE CHL 30 dB (0.25 and 0.5 kHz) | -Autophony (LE) -PT (LE) | Normal RE:0.8/0.9/0.8 LE:0.9/1/0.9 | cVEMPs RE: 70 dB (637.91 µV) LE: 50 dB (1539.67 µV) oVEMPs RE: abs; LE: 90 dB |
| 10. | 37 M | LE CHL 40 dB (0.25 kHz) 20 dB (0.5 kHz) | -Ear fullness, PT (LE) -Autophony (LE) | Normal RE: 0.9/0.9/0.9 LE: 0.8/1/1 | cVEMPs RE: 95 dB (204.16 µV) LE: 60 dB (755.39 µV) oVEMPs RE: abs; LE: 95 dB |
| 11. | 50 F | RE: moderate SNHL LE: CHL 20 dB (0.25 and 0.5 kHz) | -PT (LE) | Normal RE: 1/0.9/1 LE: 0.9/1/1 | cVEMPs RE: 95 dB (322.15 µV) LE: 40 dB (1709.61 µV) oVEMPs RE:abs; LE: 60 dB |
| Patient | Arguments for HRCT Prescription | Atypia (Suspecting Auto-Plugging) | HRCT | MRI |
|---|---|---|---|---|
| 1. | CHL assessment (on the LE) | Nearly asymptomatic | Unilateral SSCD LE: 4 mm | LE: Partial auto-plugging |
| 2. | Autophony as very invalidating symptom | Mild SNHL, without CHL | Unilateral SSCD LE: 5.2 mm | LE: Partial auto-plugging |
| 3. | Persistent (typical) BPPV after several repositioning maneuvers | Persistent BPPV after repositioning maneuvers | Bilateral SSCD RE: 4 mm LE: 5 mm | LE: Complete auto-plugging |
| 4. | MRI: suspicion of left SSCD CHL assessment (on the LE) | Asymptomatic (including Valsalva) Tumarkin | Bilateral SSCD LE: 4 mm RE: 3 mm (near dehiscence?) | LE: Partial auto-plugging |
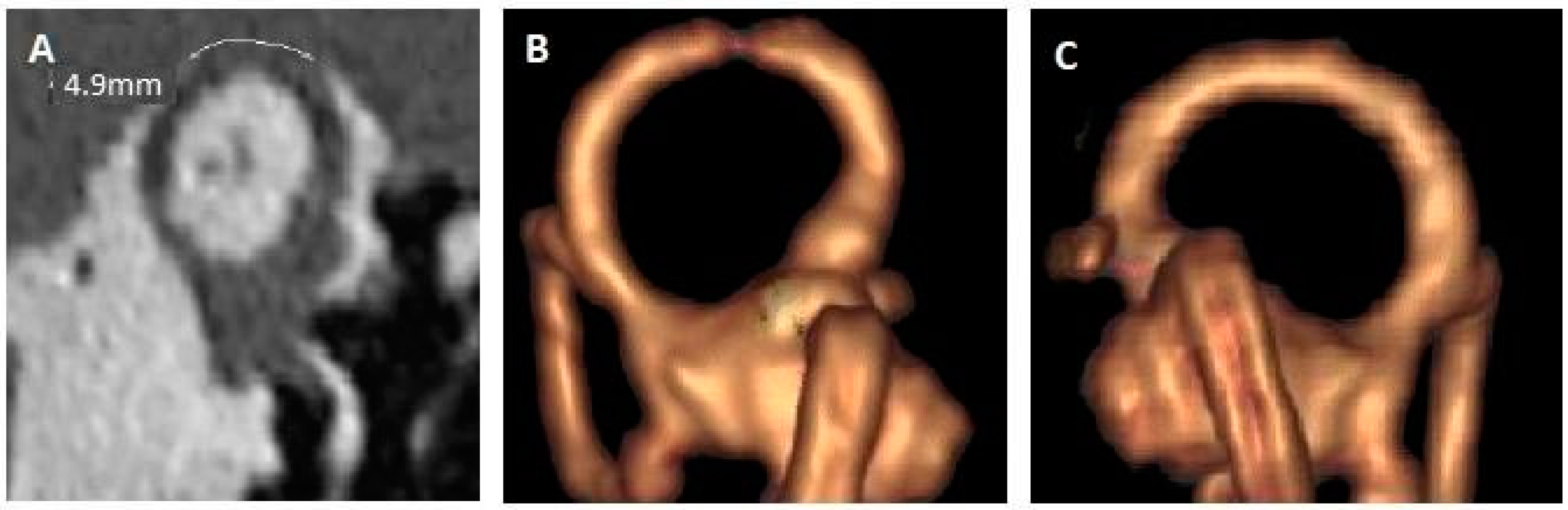
| 5. (Figure 2) | LE: CHL and autophony Tinnitus bilateral Vertigo when sneezing | No dizziness, no noise induced vertigo, no PT | Bilateral SSCD RE: 2 mm LE: 5 mm | LE: Partial auto-plugging |
| 6. (Figure 3) | Dizziness Bilateral continuous tinnitus | No vertigo No PT | Bilateral SSCD RE: 7 mm LE: 7 mm | RE: Complete auto-plugging LE: partial auto-plugging |
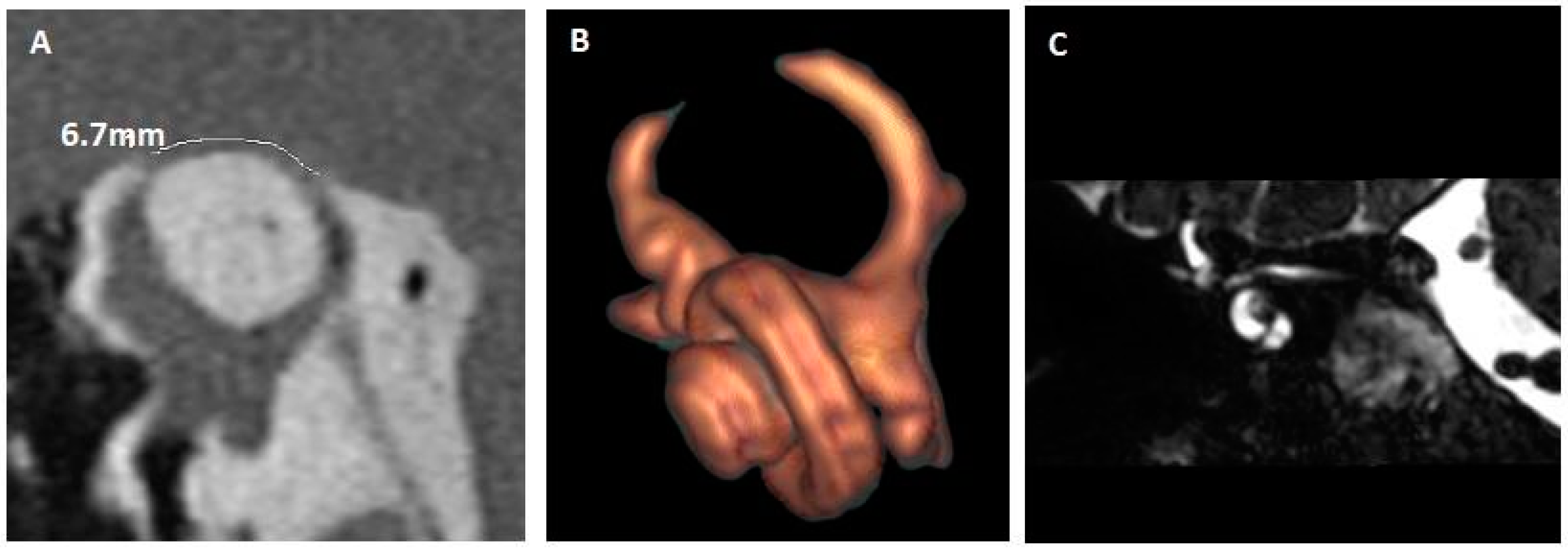
| 7. (Figure 4) | RE: Autophony Tullio | No CHL No PT | Bilateral SSCD RE: 6.7 mm LE: 1.4 mm | RE: Complete auto-plugging |
| 8. | SSCD suspected on MRI (asked for unilateral no PT) | No CHL No vertigo Tinnitus (No PT) | Bilateral SSCD RE: 6.4 mm LE: 1.9 mm | RE: Complete auto-plugging |
| 9. | LE: CHL | No vestibular symptom | Bilateral SSCD LE: 5.7 mm RE: 6.6 mm | LE: complete auto-plugging RE: partial auto-plugging |
| 10. | LE: CHL Autophony | No pressure or noise induced vertigo | Unilateral SSCD LE: 4.7 mm | LE: complete auto-plugging |
| 11. | SSCD suspected on MRI (unilateral SNHL) | No pressure or noise induced vertigo; No autophony | Bilateral SSCD LE: 4.2 mm RE: 1.5 mm | LE: complete auto-plugging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynard, P.; Mustea, E.; Ltaief-Boudrigua, A.; Castellucci, A.; Thai-Van, H.; Ionescu, E.C. Spontaneous SSCD Auto-Plugging: Clinical, Electrophysiological and Radiological Evidence. J. Clin. Med. 2025, 14, 8054. https://doi.org/10.3390/jcm14228054
Reynard P, Mustea E, Ltaief-Boudrigua A, Castellucci A, Thai-Van H, Ionescu EC. Spontaneous SSCD Auto-Plugging: Clinical, Electrophysiological and Radiological Evidence. Journal of Clinical Medicine. 2025; 14(22):8054. https://doi.org/10.3390/jcm14228054
Chicago/Turabian StyleReynard, Pierre, Eugenia Mustea, Aïcha Ltaief-Boudrigua, Andrea Castellucci, Hung Thai-Van, and Eugen C. Ionescu. 2025. "Spontaneous SSCD Auto-Plugging: Clinical, Electrophysiological and Radiological Evidence" Journal of Clinical Medicine 14, no. 22: 8054. https://doi.org/10.3390/jcm14228054
APA StyleReynard, P., Mustea, E., Ltaief-Boudrigua, A., Castellucci, A., Thai-Van, H., & Ionescu, E. C. (2025). Spontaneous SSCD Auto-Plugging: Clinical, Electrophysiological and Radiological Evidence. Journal of Clinical Medicine, 14(22), 8054. https://doi.org/10.3390/jcm14228054









