Abstract
Background: Megaprosthetic replacement is widely used following tumour resection but remains challenged by periprosthetic joint infection (PJI) and variable functional outcomes. This narrative review aims to summarise current evidence on infection rates, prevention strategies, and functional outcomes following proximal humerus megaprosthetic reconstruction. We hypothesise that antibacterial coatings and improved soft-tissue techniques reduce infection rates and enhance functional recovery. Methods: A comprehensive narrative review of PubMed, Web of Science, and the Cochrane Library was performed using the terms proximal humerus, shoulder, bone tumor, sarcoma, neoplasm, infection, megaprosthesis, and endoprosthetic replacement. Reference lists were screened manually. Case reports and series with fewer than five patients were excluded. Twenty-seven clinical studies (more than 1100 patients; mainly osteosarcoma, chondrosarcoma, and metastatic lesions) were included and qualitatively analyzed. Results: The reported infection rates ranged from 4% to 20%, with higher risk in patients receiving adjuvant therapy. Silver-coated implants reduced PJI compared with uncoated designs (e.g., 11.2% → 9.2% in primary implants; 29.2% → 13.7% in revisions) without systemic toxicity. Alternative antibacterial coatings (e.g., silver- or copper-enriched hydroxyapatite) showed promising early results but remain supported by limited clinical data. Soft-tissue stabilization with Trevira tube or synthetic mesh improved joint stability and did not increase infection risk. Functional outcomes, usually assessed by MSTS or TESS, were moderate to good (≈60–80%) overall, with better scores when the deltoid and axillary nerve were preserved or when reverse total shoulder arthroplasty was possible. Conclusions: Proximal humerus megaprosthetic reconstruction benefits from meticulous soft-tissue handling, selective use of antibacterial technologies, and multidisciplinary management. The current literature is mainly retrospective, heterogeneous, and non-comparative. Prospective multicenter studies are needed to clarify the long-term effectiveness of silver or alternative coatings, soft-tissue reconstruction techniques, and emerging custom-made 3D-printed prostheses.
1. Introduction
The proximal humerus is a common site for primary and metastatic bone tumors, accounting for 7–10% of bone sarcomas. It is the second most frequent site for osseous sarcomas and the third for osteosarcomas. After the femur, the humerus is the second most affected long bone for pathological fractures, with an incidence of 16–27%, significantly impacting load-bearing and daily activities [1,2,3]. Shoulder reconstruction aims to restore stability, preserve deltoid and rotator cuff function when possible, and achieve pain-free mobility to maximize quality of life after major bone loss [4]. Although this review focuses on tumor-related megaprosthetic reconstruction, these broader reconstructive principles provide essential context for understanding the challenges of achieving a stable and functional shoulder.
Surgical treatment for bone tumors largely depends on tumor histology and typically involves wide resection, often resulting in significant bone defects. However, recent advancements in adjuvant therapies, including multi-target chemotherapy and localized radiation, have enabled an increasing number of patients to undergo limb-sparing tumor resections [5,6,7,8].
Several reconstructive options are available, including allografts, alloprosthetic composites (APC), megaprosthetic (megaendoprostetic) replacement (MPR), and reverse shoulder arthroplasty (RSA) [9]. The primary objective of reconstruction is to achieve a stable and functional restoration of the arm and shoulder while minimizing postoperative complications [1,2,3]. Although these principles are shared across different anatomical sites—such as the acetabulum, where surgery aims to relieve pain, restore function, and maintain mechanical stability—the proximal humerus remains uniquely challenging due to its demanding soft-tissue envelope and the critical role of the deltoid and axillary nerve in postoperative function [10].
The recent literature has demonstrated that MPR is the most commonly performed and preferred surgical technique. Given its favorable outcomes, MPR is now considered the gold standard for the treatment of primary bone tumors, pathological fractures, and impending fractures in patients with a good prognosis [1,2,11,12,13,14].
Complications of this surgical approach include soft tissue failure (painful instability, subluxations, dislocations), aseptic loosening, and infection. In the proximal humerus, while subluxations and dislocations are the most common failure causes, infections and mechanical complications are also significant, though less frequent [1,13,15,16,17].
This narrative review aims to summarise current evidence on infection rates, prevention strategies, and functional outcomes following proximal humerus megaprosthetic reconstruction. We hypothesize that antibacterial coatings and improved soft-tissue techniques reduce infection rates and enhance functional recovery.
2. Materials and Methods
A comprehensive literature search was performed in PubMed, Web of Science, and the Cochrane Library to identify studies addressing causes of failure in proximal humeral megaprostheses. The following keywords and their combinations were used in an exploratory way: proximal humerus, shoulder, bone tumor, sarcoma, neoplasm, infection, megaprosthesis, endoprosthetic replacement. Additional relevant articles were identified by manually screening the reference lists of included studies. No restrictions were applied regarding publication year or study design.
Studies were considered if they were published in English and reported proximal humerus reconstruction with megaprostheses, including data on infection or failure modes and, when available, functional outcomes. Case reports with fewer than five patients, studies lacking specific data on the proximal humerus, and articles without original clinical data were excluded.
Two authors (Messina F., Meschini C.) independently screened titles, abstracts, and full texts; disagreements were resolved by consensus or, when necessary, with a senior author (Ziranu A.). Journal titles, author names, and institutional affiliations were not concealed at any stage, and no direct contact was made with study authors for patient-specific data.
Because this work is a narrative review and not a formal systematic review, the search and selection process was designed to be broad and inclusive but not protocol-driven. A simplified flow diagram (Figure 1) is provided for transparency to illustrate how records were screened and excluded; however, this does not represent a PRISMA-guided systematic review, and no quantitative synthesis or formal risk-of-bias assessment were performed (Figure 1).
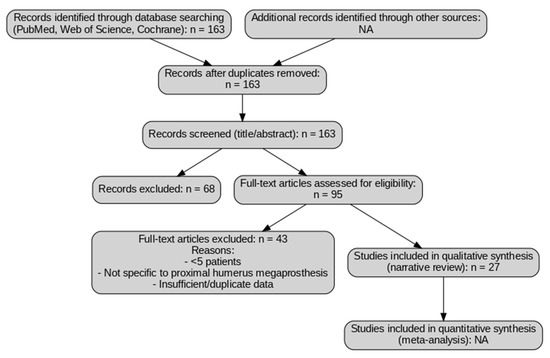
Figure 1.
Study selection flow diagram for narrative review. The diagram illustrates the selection process of the studies included in this review. A total of 163 records were identified through database searching (PubMed, Web of Science, and Cochrane Library). After screening titles and abstracts, 95 full-text articles were selected, of which 43 were excluded for the following reasons: small sample size (<5 patients), lack of specific focus on proximal humerus megaprosthesis, or insufficient/duplicate data. Finally, 27 studies were included in the qualitative synthesis. No quantitative synthesis (meta-analysis) was performed, as this work is a narrative review. NA: Not applicable.
Use of Generative AI
Generative artificial intelligence tools (ChatGPT, OpenAI) were used to improve the English language and clarity of the text. All content was reviewed and approved by the authors, who take full responsibility for the final manuscript.
3. Results
A total of 163 articles were initially identified through the literature search strategy. After removing duplicates and screening titles and abstracts, 95 studies were selected for full-text review. Of these, about 25–30 studies were included in this narrative synthesis. Several studies were excluded at this stage due to small sample sizes (<5 patients), lack of data specific to proximal humerus reconstruction, absence of a focus on megaprosthetic techniques, or insufficient reporting of infection and functional outcomes. The selected literature was then analyzed to provide a cross-sectional overview of the current evidence, emphasizing infection rates, prosthetic innovations, and postoperative functional results in patients undergoing proximal humeral reconstruction. Infection rates ranged from 4% to 20%, with the highest incidence in primary sarcoma patients receiving adjuvant therapy [8,11,16,18]. Functional outcomes were generally moderate to good (MSTS/TESS 60–80%), reaching up to 96% when deltoid and axillary nerve preservation was achieved [19,20,21,22].
Infection rates in proximal humerus megaprostheses varied widely, with higher risk observed in patients with primary bone sarcomas receiving chemotherapy and/or radiotherapy [8,11,16,18,19]. Silver-coated implants consistently demonstrated lower infection rates, particularly in revision settings, without reports of systemic toxicity [20,23,24,25]. Alternative antibacterial coatings such as silver- or copper-enriched hydroxyapatite have shown promising early results but remain based on limited clinical evidence [23,26,27,28].
Functional outcomes, typically assessed using MSTS or TESS scores, were moderate to good (≈60–80%) overall but showed high variability depending on soft tissue preservation and reconstruction type [19,20,21,22]. Emerging approaches such as 3D-printed custom-made prostheses show encouraging mid-term outcomes with improved anatomical fit [23]. These findings underscore the multifactorial nature of achieving durable, infection-free and functionally satisfactory outcomes after proximal humeral tumor resection and megaprosthetic reconstruction, and highlight the need for continued innovation and high-quality clinical studies.
A detailed summary of infection rates, prosthetic innovations, and functional results is provided in Table 1.

Table 1.
Summary of Key Findings on Infection Rates, Functional Outcomes, and Mechanical Complications in Proximal Humerus Megaprosthetic Reconstruction.
4. Discussion
Periprosthetic joint infection (PJI) is a well-documented complication in primary prosthetic implants, occurring in approximately 1–2% of cases. However, when modular endoprostheses (MPR) are used following extensive bone resections in oncologic or revision surgery, the infection rate can rise significantly, reaching up to 50% [25,30,31].
In the context of proximal humerus replacement, PJI appears to be a rare but serious complication, possibly owing to the rich vascular supply and soft-tissue coverage around the shoulder. Henderson et al. reported an overall infection rate of 6.3% in patients with shoulder replacements [32]. Kumar et al. documented five infections (four superficial and one deep) in a cohort of 100 patients, all caused by Staphylococcus aureus [33]. Similarly, Arhens et al. identified one wound dehiscence and three deep infections requiring surgical intervention among 102 patients who underwent shoulder MPR [16]. Cannon et al., in their study on the functional outcomes of 83 patients undergoing total proximal humerus replacement, reported two infections, including one leading to implant removal [34]. A systematic review and meta-analysis by Fiore et al., which included 382 patients, identified nine infections following proximal humeral MPR [13], whereas Ross et al. found no deep infections in their 25-patient series [35].
It is important to note that the available evidence on proximal humerus megaprosthetic reconstruction is highly heterogeneous. Most included studies are retrospective case series with small sample sizes and variable follow-up durations [13,16,20,24]. Moreover, standardized definitions of complications and consistent reporting of functional outcomes (e.g., MSTS, TESS) are often lacking [21,34]. Such variability limits direct comparability and may lead to over- or underestimation of infection rates and functional results. The predominance of non-comparative studies and the absence of robust prospective data further reduce the strength and generalizability of current conclusions [11,13]. Functional outcomes following proximal humeral megaprosthesis are influenced by multiple patient- and procedure-related factors. Extensive resections that sacrifice the deltoid muscle or axillary nerve markedly reduce shoulder stability and motion, whereas preservation of these structures is associated with better function [20,21,36,37]. Recent registry analyses and large multicenter series have provided more robust outcome data for shoulder megaprostheses, particularly reverse configurations, reporting satisfactory mid-term function and manageable complication rates [36,37]. These data support the current trend toward reverse total shoulder arthroplasty when feasible and confirm its ability to achieve good functional outcomes in oncologic reconstruction.
Patient comorbidities and general health status can impair recovery, while timely and structured postoperative rehabilitation plays a critical role in restoring mobility [34]. Furthermore, procedures performed in high-volume oncologic centers by experienced surgical teams have been linked to improved functional scores, highlighting the impact of surgical expertise and multidisciplinary care on postoperative outcomes [13,16].
To mitigate PJI incidence and improve treatment outcomes, several strategies have been developed. One of the most explored approaches involves modifying implant surfaces with antibiotics, antiseptics, and metal coatings, aiming to reduce bacterial colonization and enhance infection resistance [25,38].
4.1. Overall Summary and New Insights
This Narrative Review provides a comprehensive synthesis of current evidence regarding infection rates, preventive strategies, and functional outcomes after proximal humerus megaprosthetic reconstruction. Reported infection rates ranged from 4% to 20%, with the highest risk in patients receiving adjuvant therapies for primary sarcomas [8,11,16,18,19]. Silver-coated implants and other antibacterial surface modifications consistently demonstrated reduced periprosthetic infection rates, particularly in high-risk or revision cases, while maintaining safety [20,21,22,24,30,39]. Soft-tissue reconstruction techniques—especially the use of the Trevira tube and synthetic meshes—proved effective in enhancing joint stability without increasing infection risk [22,40,41,42,43]. Functional outcomes were generally moderate to good, reaching up to 96% in reverse configurations with preserved deltoid and axillary nerve function [19,21,28,29,36,37].
Compared with previous research, this review adds a focused, anatomy-specific perspective that integrates both infection control and functional recovery strategies in a single framework. It highlights the importance of multidisciplinary surgical management and the emerging role of antibacterial coatings and 3D-printed custom designs in improving oncological limb-salvage outcomes [23,25,26,27,44,45,46].
4.2. Silver Coat
Several in vitro and animal studies have demonstrated the efficacy and safety of silver in preventing periprosthetic infections [26]. In orthopedic prostheses, three commercially available silver-coated modular endoprostheses (MPRs) are designed to reduce infection risk through different coating technologies [26]:
- MUTARS® MPR (Implancast, Buxtehude, Germany) contains the highest silver content among available prostheses. It consists of a titanium-vanadium (TiAl6V4) base, coated with two metallic layers: an inner 0.2 mm gold layer, which facilitates the controlled release of silver ions into periprosthetic tissues, and an outer 15 μm pure silver layer applied via galvanic deposition [24,26].
- METS® prosthesis (Stanmore Implants, Elstree, UK) is originally made of titanium and features a 5 μm silver layer, known as Agluna® (Accentus Medical, Oxfordshire, UK), created through anodization and silver absorption via ion exchange [5].
- Megasystem C® MPR (Waldemar Link, Hamburg, Germany) incorporates PorAg® (Porous Argentum, New York, NY, USA) technology, consisting of a 1 μm deep layer containing silver and an outer 0.1 μm TiAg20N layer. These layers generate an electrochemical reaction, forming an electron cloud around the prosthetic surface. The resulting cathodic reaction disrupts ATP synthesis in bacteria, leading to cell death [30,47].
Clinical studies have evaluated the effectiveness of silver-coated implants in preventing infections following proximal humerus replacement, with promising results.
No infections were reported in Trovarelli et al.’s case series of 22 patients or Schmolders et al.’s study of 30 patients using silver-coated implants [20,25]. Similarly, Perry et al., in a single-center study of 50 patients, observed only one infection, which occurred in the non-coated prosthesis group [20,25].
Beyond reducing early periprosthetic infections, silver-coated implants have been associated with less invasive infection management, a lower likelihood of implant removal, and a reduced risk of amputation. Given these benefits, their most promising use appears to be in revision surgery for infection-related implant failures, where effective infection control is crucial [39] (Table 2).

Table 2.
Clinical evidence on silver-coated megaprostheses.
Other Antibacterial Coating Strategies
In addition to silver coatings, alternative antibacterial strategies for megaprosthetic implants have gained attention. Among these, hydroxyapatite coatings enriched with copper (Cu-HA) have demonstrated promising antimicrobial effects while preserving osteoconductivity, offering a potential alternative in patients where silver-related side effects are a concern [28]. Similarly, silver-containing hydroxyapatite composites (Ag-HA) continue to be explored, particularly for their dual action in infection prevention and bone integration, showing encouraging outcomes in various orthopaedic settings [27]. Furthermore, emerging nanostructured coatings—including antibiotic-loaded hydrogels and self-assembling surfaces—are being investigated to provide a controlled local release of antimicrobial agents and to disrupt early biofilm formation [29,44,45,46]. Despite their promise, these technologies remain supported by low-level and mid-term evidence, and their widespread adoption is currently limited by high production costs, regulatory hurdles, and the lack of long-term clinical data. Future prospective multicenter studies are needed before these coatings can be routinely recommended in oncologic megaprosthese.
4.3. Trevira Tube
Reconstructing the upper limb with modular tumor endoprostheses is a well-established approach for managing primary malignant bone tumors or metastatic disease. However, functional outcomes following upper limb surgery with megaendoprostheses are often less than ideal. This is largely due to the frequent need for axillary nerve resection, resulting in the loss of deltoid muscle function, as well as the common involvement of the rotator cuff [48].
To address joint instability in such cases, the Trevira tube, a knitted polyester structure, has been developed to facilitate the reattachment of remaining muscles and tendons. This allows for fibroblastic ingrowth, contributing to the development of a stable joint [40].
Despite its functional advantages, concerns have been raised regarding the risk of deep tissue infections associated with polyester fibers. However, the current literature provides limited data on soft tissue reconstruction using a Trevira tube following proximal humerus replacement. Schmolder et al. investigated the role of the Trevira tube in 30 patients undergoing endoprosthetic replacement of the proximal humerus after wide resection [25]. Among them, 15 patients received an endoprosthesis combined with a Trevira tube, while in the remaining 15, the surgeon opted against using the tube due to an already stable joint. They reported only one case of periprosthetic joint infection (PJI), occurring after implant revision for luxation in the subgroup with a Trevira tube. However, the Trevira tube was not associated with a statistically significant increase in periprosthetic infection rates [25].
Similarly, Gosheger et al. analyzed 69 cases of megaprosthesis implantation with use of a Trevira tube for soft tissue reattachment around the megaprosthesis, including 16 proximal humerus replacements. Their findings indicated no increased infection rate compared to replacements without the Trevira tube. Additionally, the Trevira tube effectively prevented dislocation [40]. A recent review has also emphasized the critical role of soft-tissue reattachment and biointegration strategies—such as synthetic meshes and polyester sleeves—in enhancing joint stability and potentially reducing infection risk after megaprosthetic reconstruction [41].
In another study, three patients underwent proximal and/or total humerus replacement using the MUTARS system with Trevira tube capsular reconstruction. The Trevira tube successfully prevented dislocation without increasing the infection rate [42].
Henderson et al. reported a 6.3% infection rate in patients with proximal humerus replacements, which was lower than that observed in some series [32]. A higher complication rate was noted in more physically active patients. Tang et al. found that synthetic mesh-based soft tissue reconstruction resulted in lower complication rates and better functional outcomes. Similar observations were made in the current study, where initial soft tissue reconstruction with synthetic mesh was associated with fewer soft tissue complications and infections [43]. Henderson et al. also highlighted that instability-related complications (Type I) are more frequent in patients undergoing shoulder or proximal femur replacement, emphasizing the potential benefits of synthetic mesh in such cases [21] (Table 3).

Table 3.
Clinical results of Trevira tube use in proximal humerus megaprosthesis reconstruction.
4.4. Tumor Type and Adjuvant Treatment
The risk of periprosthetic joint infection (PJI) in proximal humerus and shoulder megaprostheses is significantly influenced by both the underlying tumor type and adjuvant therapies [17]. Primary bone tumors, such as osteosarcoma and chondrosarcoma, are associated with a higher incidence of PJI compared with secondary metastatic lesions. This increased risk is likely multifactorial: chemotherapy and radiotherapy can impair local vascularity, delay wound healing, and suppress the immune response, while patients with aggressive primary tumors often experience greater soft-tissue compromise and systemic immunosuppression [5,6,11,16,20,24,41,49,50]. These factors together may create a more favorable environment for bacterial colonization and infection development. This increased susceptibility is likely due to the need for extensive resection and soft tissue loss, which compromises the local immune response and delays wound healing [6,50]. In a study analyzing mega-arthroplasty in different joints, patients with primary bone tumors exhibited a statistically significant higher risk of PJI than those with metastatic bone disease, suggesting that the aggressive biological behavior of these tumors contributes to infection susceptibility [5,31].
Adjuvant therapies, including chemotherapy and radiotherapy, further exacerbate the risk of infection. Chemotherapy-induced immunosuppression, leukopenia, and impaired wound healing are well-documented risk factors for surgical site infections (SSI) [6,7]. Moreover, postoperative chemotherapy within one month of surgery has been associated with an increased risk of PJI. Similarly, radiation therapy, while effective in local tumor control, can cause significant soft tissue damage, reduce vascularization, and impair host defenses, thereby increasing infection susceptibility [8].
In a study evaluating the functional and oncological outcomes of inverse proximal humerus prostheses, infections were predominantly observed in patients who had received radiotherapy, reinforcing the notion that radiation-induced tissue damage is a key predisposing factor [5,6,22].
However, the inherent complexity of this patient cohort, coupled with the need for aggressive oncologic therapies and the resultant immunosuppression, continues to pose substantial challenges to effective infection prevention. Ongoing research is essential to refine strategies for infection control, especially in high-risk patients undergoing megaprosthetic reconstruction after tumor excision.
4.5. Other Relevant Factors
The recent literature highlights additional factors that may influence outcomes in megaprosthetic reconstructions beyond infection-specific strategies. Theil et al. showed that while longer primary surgeries were linked to mechanical failures, no clear association emerged between surgical duration and infection risk; notably, more prolonged revision surgeries might reduce subsequent failures, possibly due to more extensive debridement efforts [31,51]. Berger et al. reported a 30% infection rate after mega-prosthesis implantation, with persistent infections or amputations occurring in nearly half of infected patients, emphasizing the high morbidity linked to periprosthetic infections in these complex reconstructions [18]. Meanwhile, advanced approaches such as three-dimension-printed custom-made prostheses have demonstrated encouraging mid-term outcomes, potentially improving anatomical fit and reducing mechanical complications [23]. Although blood loss management protocols like tranexamic acid administration have shown benefits in joint arthroplasty [19], their role in tumor-related megaprostheses remains to be fully elucidated. These insights underline the multifactorial nature of complications and the need for tailored, multidisciplinary strategies.
5. Conclusions
The current literature on megaprosthetic reconstruction of the proximal humerus is predominantly composed of small, retrospective series with heterogeneous patient populations and variable follow-up durations. As a result, the true incidence of periprosthetic joint infection (PJI) and the long-term functional outcomes remain incompletely defined.
Silver-coated endoprostheses and alternative antibacterial surface treatments show encouraging early results in lowering infection risk, particularly in revision or high-risk settings, but the supporting evidence is limited and largely non-comparative. Similarly, soft-tissue reconstruction techniques such as the Trevira tube appear to improve joint stability without clearly increasing infection rates, although data are still scarce.
Given these limitations, conclusions regarding the superiority of any specific implant design, coating technology, or reconstruction strategy should be drawn cautiously. Well-designed, prospective, multicenter studies with standardized outcome reporting are urgently needed to clarify the long-term effectiveness of antibacterial coatings, soft-tissue reconstruction methods, and modern implant innovations such as 3D-printed custom-made prostheses.
Until such data become available, the management of proximal humeral reconstructions after tumor resection should rely on a multidisciplinary approach, careful patient selection, meticulous soft tissue handling, and judicious integration of emerging technologies to mitigate infection risk and optimize functional outcomes.
Author Contributions
Conceptualization, F.M., C.M., M.S.O., M.C., A.B. and G.R.; methodology, F.M. and C.M.; validation, F.M., C.M. and A.Z.; formal analysis, F.M.; investigation, F.M.; resources, F.M.; data curation, F.M.; writing—original draft preparation, F.M.; writing—review and editing, F.M., C.M. and A.Z.; visualization, F.M.; supervision, F.M.; project administration, F.M.; funding acquisition, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed.
Acknowledgments
The authors declare that a generative AI tool (GPT-5, OpenAI, San Francisco, CA, USA) was used only to support language editing and formatting according to journal guidelines. All scientific content, analysis, and conclusions are the authors’ own work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klingebiel, S.; Schneider, K.N.; Gosheger, G.; Ackmann, T.; Timme, M.; Rickert, C.; Deventer, N.; Theil, C. Periprosthetic Stress Shielding of the Humerus after Reconstruction with Modular Shoulder Megaprostheses in Patients with Sarcoma. J. Clin. Med. 2021, 10, 3424. [Google Scholar] [CrossRef]
- Rovere, G.; Meschini, C.; Piazza, P.; Messina, F.; Caredda, M.; De Marco, D.; Noia, G.; Maccagnano, G.; Ziranu, A. Proximal humerus fractures treatment in adult patients with bone metastasis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 100–105. [Google Scholar] [PubMed]
- Teunis, T.; Nota, S.P.F.T.; Hornicek, F.J.; Schwab, J.H.; Lozano-Calderón, S.A. Outcome After Reconstruction of the Proximal Humerus for Tumor Resection: A Systematic Review. Clin. Orthop. 2014, 472, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Colò, G.; Fusini, F.; Faoro, L.; Popolizio, G.; Ferraro, S.; Ippolito, G.; Leigheb, M.; Surace, M.F. Current Evidence and Surgical Strategies in the Management of Greater Tuberosity Fracture–Dislocations: A Narrative Review. J. Clin. Med. 2025, 14, 5159. [Google Scholar] [CrossRef] [PubMed]
- Wafa, H.; Grimer, R.J.; Reddy, K.; Jeys, L.; Abudu, A.; Carter, S.R.; Tillman, R.M. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: Case-control study. Bone Jt. J. 2015, 97-B, 252–257. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Altsitzioglou, P.; Papadopoulos, D.V.; Lorenzo, D.; Romanò, C.L.; Benzakour, T.; Tsukamoto, S.; Errani, C.; Angelini, A.; Mavrogenis, A.F. Infections of Tumor Prostheses: An Updated Review on Risk Factors, Microbiology, Diagnosis, and Treatment Strategies. Biology 2023, 12, 314. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ebihara, T.; Kitade, K.; Setsu, N.; Endo, M.; Iida, K.; Matsumoto, Y.; Matsunobu, T.; Oda, Y.; Iwamoto, Y.; et al. Risk Factors of Periprosthetic Infection in Patients with Tumor Prostheses Following Resection for Musculoskeletal Tumor of the Lower Limb. J. Clin. Med. 2020, 9, 3133. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Surgical Site Infection after Bone Tumor Surgery: Risk Factors and New Preventive Techniques. Cancers 2022, 14, 4527. [Google Scholar] [CrossRef]
- Daher, M.; Fares, M.Y.; Gill, S.S.; Boufadel, P.; Jensen, A.R.; Eward, W.C.; Khan, A.Z.; Horneff, J.G.; Abboud, J.A. Allograft-prosthetic composite versus megaprosthesis for proximal humerus reconstruction after tumor resection: A meta-analysis of clinical outcomes. Clin. Shoulder Elb. 2025, 28, 298–305. [Google Scholar] [CrossRef]
- Spinelli, M.S.; Ziranu, A.; Piccioli, A.; Maccauro, G. Surgical treatment of acetabular metastasis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3005–3010. [Google Scholar]
- Dubina, A.; Shiu, B.; Gilotra, M.; Hasan, S.A.; Lerman, D.; Ng, V.Y. What is the Optimal Reconstruction Option after the Resection of Proximal Humeral Tumors? A Systematic Review. Open Orthop. J. 2017, 11, 203–211. [Google Scholar] [CrossRef]
- Houdek, M.T.; Bukowski, B.R.; Athey, A.G.; Elhassan, B.T.; Barlow, J.D.; Morrey, M.E.; Rose, P.S.; Wagner, E.R.; Sanchez-Sotelo, J. Comparison of reconstructive techniques following oncologic intraarticular resection of proximal humerus. J. Surg. Oncol. 2021, 123, 133–140. [Google Scholar] [CrossRef]
- Fiore, M.; Sambri, A.; Giannini, C.; Zucchini, R.; De Cristofaro, R.; De Paolis, M. Anatomical and reverse megaprosthesis in proximal humerus reconstructions after oncologic resections: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2021, 142, 2459–2469. [Google Scholar] [CrossRef]
- Denissen, J.J.P.M.; Koenders, N.; Van Hinte, G.; Groen, F.; Van Der Wees, P.J.; Van Der Geest, I.C.M.; Dierselhuis, E.F. Functional outcomes after reverse shoulder megaprosthesis following resection of malignant bone tumor in the proximal humerus: A systematic review and meta-analysis. JSES Int. 2023, 7, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Pinnamaneni, S.; Damron, T.A. Proximal humerus reconstruction in orthopedic oncology. J. Cancer Metastasis Treat. 2021, 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, H.; Theil, C.; Gosheger, G.; Rödl, R.; Deventer, N.; Rickert, C.; Ackmann, T.; Schwarze, J.; Klingebiel, S.; Schneider, K.N. The Bateman-Type Soft Tissue Reconstruction around Proximal or Total Humeral Megaprostheses in Patients with Primary Malignant Bone Tumors—Functional Outcome and Endoprosthetic Complications. Cancers 2021, 13, 3971. [Google Scholar] [CrossRef]
- Karampikas, V.; Gavriil, P.; Goumenos, S.; Trikoupis, I.G.; Roustemis, A.G.; Altsitzioglou, P.; Kontogeorgakos, V.; Mavrogenis, A.F.; Papagelopoulos, P.J. Risk factors for peri-megaprosthetic joint infections in tumor surgery: A systematic review. SICOT-J 2024, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Parai, C.; Tillander, J.; Bergh, P.; Wennergren, D.; Brisby, H. High Risk for Persistent Peri-Prosthetic Infection and Amputation in Mega-Prosthesis Reconstruction. J. Clin. Med. 2023, 12, 3575. [Google Scholar] [CrossRef]
- Maccagnano, G.; Pesce, V.; Noia, G.; Coviello, M.; Vicenti, G.; Vitiello, R.; Ziranu, A.; Spinarelli, A.; Moretti, B. The effects of a new protocol on blood loss in total knee arthroplasty. Orthop. Rev. 2022, 14, 37625. [Google Scholar] [CrossRef]
- Trovarelli, G.; Cappellari, A.; Angelini, A.; Pala, E.; Ruggieri, P. What Is the Survival and Function of Modular Reverse Total Shoulder Prostheses in Patients Undergoing Tumor Resections in Whom an Innervated Deltoid Muscle Can Be Preserved? Clin. Orthop. 2019, 477, 2495–2507. [Google Scholar] [CrossRef]
- Lang, N.W.; Kasparek, M.F.; Synak, L.; Waldstein, W.; Funovics, P.T.; Windhager, R.; Hobusch, G.M. What sports activity levels are achieved in long-term survivors with modular endoprosthetic humerus reconstruction following primary bone sarcoma resection? Wien. Klin. Wochenschr. 2021, 133, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Rachbauer, A.M.; Schneider, K.N.; Gosheger, G.; Deventer, N. Endoprosthetic Reconstruction of the Proximal Humerus with an Inverse Tumor Prosthesis. Cancers 2023, 15, 5330. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, R.; Matrangolo, M.R.; El Motassime, A.; Perna, A.; Cianni, L.; Maccauro, G.; Ziranu, A. Three-Dimension-Printed Custom-Made Prosthetic Reconstructions in Bone Tumors: A Single Center Experience. Curr. Oncol. 2022, 29, 4566–4577. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Von Eiff, C.; Streitbuerger, A.; Balke, M.; Budny, T.; Henrichs, M.P.; Hauschild, G.; Ahrens, H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J. Surg. Oncol. 2010, 101, 389–395. [Google Scholar] [CrossRef]
- Schmolders, J.; Koob, S.; Schepers, P.; Kehrer, M.; Frey, S.P.; Wirtz, D.C.; Pennekamp, P.H.; Strauss, A.C. Silver-coated endoprosthetic replacement of the proximal humerus in case of tumour—Is there an increased risk of periprosthetic infection by using a trevira tube? Int. Orthop. 2017, 41, 423–428. [Google Scholar] [CrossRef]
- Gosheger, G. Silver-coated megaendoprostheses in a rabbit model—An analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556. [Google Scholar] [CrossRef]
- Morimoto, T.; Hirata, H.; Eto, S.; Hashimoto, A.; Kii, S.; Kobayashi, T.; Tsukamoto, M.; Yoshihara, T.; Toda, Y.; Mawatari, M. Development of Silver-Containing Hydroxyapatite-Coated Antimicrobial Implants for Orthopaedic and Spinal Surgery. Medicina 2022, 58, 519. [Google Scholar] [CrossRef]
- Le, L.-Q.R.V.; Lanzino, M.C.; Blum, M.; Höppel, A.; Al-Ahmad, A.; Killinger, A.; Gadow, R.; Rheinheimer, W.; Seidenstuecker, M. Copper-enriched hydroxyapatite coatings obtained by high-velocity suspension flame spraying. Effect of various gas parameters on biocompatibility. J. Mater. Sci. Mater. Med. 2024, 35, 70. [Google Scholar] [CrossRef]
- Akay, S.; Yaghmur, A. Recent Advances in Antibacterial Coatings to Combat Orthopedic Implant-Associated Infections. Molecules 2024, 29, 1172. [Google Scholar] [CrossRef]
- Fiore, M.; Sambri, A.; Zucchini, R.; Giannini, C.; Donati, D.M.; De Paolis, M. Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: A systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 201–220. [Google Scholar] [CrossRef]
- Khakzad, T.; Karczewski, D.; Thielscher, L.; Reiter, K.; Wittenberg, S.; Paksoy, A.; Flörcken, A.; Rau, D.; Märdian, S. Prosthetic Joint Infection in Mega-Arthroplasty Following Shoulder, Hip and Knee Malignancy—A Prospective Follow-Up Study. Life 2022, 12, 2134. [Google Scholar] [CrossRef]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure Mode Classification for Tumor Endoprostheses: Retrospective Review of Five Institutions and a Literature Review. J. Bone Jt. Surg. 2011, 93, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.K.J.; Pai, P.K.; Rajasubramanya, P. A Rare Case of Ewing’s-like Adamantinoma of Tibia Managed by Limb Salvage Surgery Using Long Segment Ilizarov Bone Transport: A Case Report and Review of Literature. J. Orthop. Case Rep. 2021, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Paraliticci, G.U.; Lin, P.P.; Lewis, V.O.; Yasko, A.W. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J. Shoulder Elb. Surg. 2009, 18, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Wilson, J.N.; Scales, J.T. Endoprosthetic replacement of the proximal humerus. J. Bone Jt. Surg. Br. Vol. 1987, 69, 656–661. [Google Scholar] [CrossRef]
- Evenhuis, R.; Bus, M.P.A.; Sellevold, S.; Dierselhuis, E.F.; Trikoupis, I.G.; Scorianz, M.; Walter, S.; Cabrolier, J.; Fiocco, M.; Van De Sande, M.A.J. Proximal humeral endoprosthetic reconstruction for tumour defects: Clinical outcomes of 165 patients from the MUTARS Orthopedic Registry Orthopedic Registry Europe (MORE). Bone Jt. Open 2025, 6, 715–723. [Google Scholar] [CrossRef]
- Labrum, J.T.; De Marinis, R.; Atwan, Y.; Marigi, E.M.; Houdek, M.T.; Barlow, J.D.; Morrey, M.E.; Sanchez-Sotelo, J.; Sperling, J.W. Reverse shoulder arthroplasty megaprosthesis for surgical management of severe proximal humeral bone loss. J. Shoulder Elb. Surg. 2024, 33, S64–S73. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. 2015, 10, 157. [Google Scholar] [CrossRef]
- Trovarelli, G.; Angelini, A.; Pala, E.; Cappellari, A.; Breda, A.; Ruggieri, P. Infection in orthopaedic oncology: Crucial problem in modern reconstructive techniques. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 271–278. [Google Scholar]
- Gosheger, G.; Hillmann, A.; Lindner, N.; Rödl, R.; Hoffmann, C.; Bürger, H.; Winkelmann, W. Soft Tissue Reconstruction of Megaprostheses Using a Trevira Tube. Clin. Orthop. 2001, 393, 264–271. [Google Scholar] [CrossRef]
- Pesare, E.; Vitiello, R.; Greco, T.; Solarino, G.; Maccauro, G.; Ziranu, A. Soft Tissue Reconstruction and Integration to Implant After Bone-Tumor Resection: A Current Concept Review. Curr. Oncol. 2024, 31, 7190–7203. [Google Scholar] [CrossRef] [PubMed]
- Gosheger, G.; Hardes, J.; Ahrens, H.; Gebert, C.; Winkelmann, W. Endoprosthetic replacement of the humerus combined with trapezius and latissimus dorsi transfer: A report of three patients. Arch. Orthop. Trauma Surg. 2005, 125, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Guo, W.; Yang, R.; Tang, S.; Ji, T. Synthetic Mesh Improves Shoulder Function After Intraarticular Resection and Prosthetic Replacement of Proximal Humerus. Clin. Orthop. 2015, 473, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Kaspiris, A.; Vasiliadis, E.; Pantazaka, E.; Lianou, I.; Melissaridou, D.; Savvidis, M.; Panagopoulos, F.; Tsalimas, G.; Vavourakis, M.; Kolovos, I.; et al. Current Progress and Future Perspectives in Contact and Releasing-Type Antimicrobial Coatings of Orthopaedic Implants: A Systematic Review Analysis Emanated from In Vitro and In Vivo Models. Infect. Dis. Rep. 2024, 16, 298–316. [Google Scholar] [CrossRef]
- Onorato, F.; Masoni, V.; Gagliardi, L.; Comba, L.C.; Rivera, F. What to Know about Antimicrobial Coatings in Arthroplasty: A Narrative Review. Medicina 2024, 60, 574. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Lu, Z.; Elbourne, A.; Vasilev, K.; Roohani, I.; Zreiqat, H.; Truong, V.K. Engineering antibacterial bioceramics: Design principles and mechanisms of action. Mater. Today Bio 2024, 26, 101069. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Asavamongkolkul, A.; Eckardt, J.J.; Eilber, F.R.; Dorey, F.J.; Ward, W.G.; Kelly, C.M.; Wirganowicz, P.Z.; Kabo, J.M. Endoprosthetic Reconstruction for Malignant Upper Extremity Tumors. Clin. Orthop. 1999, 360, 207–220. [Google Scholar] [CrossRef]
- Miwa, S.; Shirai, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Tada, K.; Kajino, Y.; Higuchi, T.; Abe, K.; Aiba, H.; et al. Risk factors for surgical site infection after malignant bone tumor resection and reconstruction. BMC Cancer 2019, 19, 33. [Google Scholar] [CrossRef]
- Puchner, S.E.; Varga, R.; Hobusch, G.M.; Kasparek, M.; Panotopoulos, J.; Lang, S.; Windhager, R.; Funovics, P.T. Long-term outcome following treatment of Adamantinoma and Osteofibrous dysplasia of long bones. Orthop. Traumatol. Surg. Res. 2016, 102, 925–932. [Google Scholar] [CrossRef]
- Theil, C.; Schneider, K.N.; Gosheger, G.; Dieckmann, R.; Deventer, N.; Hardes, J.; Schmidt-Braekling, T.; Andreou, D. Does the Duration of Primary and First Revision Surgery Influence the Probability of First and Subsequent Implant Failures after Extremity Sarcoma Resection and Megaprosthetic Reconstruction? Cancers 2021, 13, 2510. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).