Abstract
Telerehabilitation is an effective, accessible addition or alternative to conventional rehabilitation for fracture management, especially in older adults after hip fractures, leveraging video visits, mHealth apps, virtual reality (VR), and wearable sensors to deliver exercise, education, and monitoring at home with high satisfaction and adherence. Across non-surgical and surgical contexts, telemedicine shows feasibility and cost benefits, with mixed superiority but consistent non-inferiority for functional outcomes versus in-person care. In hip fracture populations, randomized and non-randomized studies indicate improvements in functional independence measure (FIM), Timed Up and Go test (TUG), Activities of Daily Living/Instrumental Activities of Daily Living (ADLs/IADLs), and quality of life, with some evidence for reduced anxiety and depression, while effects on mobility, pain, and adverse events remain uncertain overall. In patients with upper-limb fractures, telerehabilitation appears to improve function and pain, though strength gains may lag compared with in-person therapy in some trials; adjuncts like motor imagery and virtual reality may enhance outcomes and motivation. Application is facilitated by user-friendly platforms, caregiver involvement, and simple modalities such as structured phone follow-up. Limitations include small samples, heterogeneous protocols, scarce long-term data, and a predominance of non-inferiority or complementary designs, warranting larger, definitive trials. This technology can lead to improved patient management at home, effortlessly verifying treatment compliance, efficacy, and safety, while simultaneously reducing the need for hospitalization, promoting a more peaceful recovery. Here, we have undertaken a narrative review of the medical–scientific literature in this field.
1. Introduction
Increased life expectancy has led to an epidemiological shift in major chronic and degenerative diseases, with both their prevalence and incidence rising [1,2]. This increased prevalence and incidence has especially affected individuals over the age of 65, who are nowadays characterized by a greater degree of frailty than in previous periods [3,4]. This phenomenon has affected the whole Western world, and particularly countries with a significant proportion of elderly populations (e.g., Italy). Hip, femoral, and spinal fractures are examples of the consequences of this frailty and are an increasingly common occurrence among older adults in Italy [5,6,7].
Given the aging population, the global number of hip fractures is projected to increase from 1.26 million in 1990 to 4.5 million by 2050. The direct costs associated with this condition are and will be enormous, as the condition requires prolonged hospitalization and subsequent rehabilitation care [8,9]. Physicians specialized in Internal Medicine, Geriatrics, Orthopedics and Physical and Rehabilitation Medicine (PRM) are often consulted to evaluate patients with acute, subacute, or chronic manifestations and the outcomes of fractures of a different nature [10,11,12,13,14,15,16,17]. PRM specialists play an important role in the care of patients with fractures since therapeutic exercise and physical reconditioning have proved to be efficient in improving independence in Activities of Daily Living (ADL), cognitive functions, nutritional and physical conditions, balance, and muscle strength in the elderly with fractures. This should all be framed under a well-coordinated and designed Individual Rehabilitation Project (IRP) [18,19,20,21]. During the COVID-19 pandemic, healthcare costs further increased globally [22,23,24]. At the same time, the health crisis created during the pandemic, with the saturation of the healthcare system due to the number of hospitalizations of patients acutely suffering from severe respiratory illnesses, worsened the care of patients with fractures. Indeed, the difficult management of hospital beds and acute care in wards led, for example, to the deferral of fracture interventions well beyond the limits recommended by guidelines [25,26,27], to the undertreatment of osteoporotic and frail patients at risk of fracture, and to the prolongation of transfers to rehabilitation clinics, resulting in significant overall difficulties in properly managing resources and processes for these types of patients [28,29,30].
Nonetheless, during the COVID-19 pandemic, there was a renewed emphasis on rational resource allocation and their use to relaunch healthcare systems that prioritize hospitals for patients requiring high-intensity care and severe acute conditions. Community-based care and home care were emphasized as alternatives for patients with less intensive needs [31,32]. These observations led to the idea of leveraging existing and emerging technologies (simulation programs, artificial intelligence, computer networks, and management systems with teleconsultation capabilities) to support patient care remotely via so-called “telemedicine” [33]. Telemedicine has emerged as a transformative method for delivering healthcare services, particularly in rehabilitation (i.e., telerehabilitation), reflecting its multifaceted benefits for a diverse population. One of the primary advantages of telerehabilitation is its capacity to eliminate geographical barriers associated with traditional rehabilitation methods. Patients in rural and underserved areas can access rehabilitation services without the burden of extensive travel, thus increasing the overall accessibility of care [34,35]. This enhanced accessibility is particularly pertinent as it not only improves patient engagement but also enhances adherence to IRPs, resulting in improved physical function and quality of life over the long term [36,37]. Additionally, a systematic review by Wang et al. indicates that telerehabilitation not only saves time but also leads to higher patient satisfaction due to reduced waiting times and the convenience of engaging in therapy from home [38]. Telerehabilitation appears to significantly reduce healthcare costs for both patients and providers, mitigating the need for transportation and thereby reducing travel-related expenses [39]. Beyond convenience and cost-effectiveness, telerehabilitation includes the flexibility to customize therapeutic interventions based on individual patient needs. Healthcare professionals can utilize various digital formats, such as video conferencing and dedicated rehabilitation applications, to tailor exercises according to patient capability and progress [40,41]. The incorporation of telerehabilitation has proven instrumental during the COVID-19 pandemic, where direct access to healthcare services was significantly hindered. As face-to-face assessments posed risks, telerehabilitation provided a means to continue essential therapeutic interventions while maintaining safety protocols. Research indicates that providers and patients reported positive experiences and outcomes during these challenging times, thus establishing telerehabilitation as a viable and effective alternative in the realm of healthcare delivery [42].
Given the increased frailty and risk of fractures, with the resulting increase in healthcare costs, telerehabilitation appears to be an effective means of reducing these risks. The aim of this narrative review is to offer a comprehensive analysis of the existing literature on the possible application of telemedicine, and, in particular, telerehabilitation, as a new, innovative tool for fracture management.
2. Use of Telemedicine in Clinical Conditions
Telemedicine and telerehabilitation have already been applied effectively in non-surgical conditions (Table 1). An important example of the use of telemedicine, implemented after the COVID-19 pandemic, concerns diabetes. Telemedicine offers important opportunities, both in hospital and community settings, to monitor patients with diabetes [43,44]. Through dedicated software, physicians can communicate with patients at home, asking for clinical information, verifying adherence to treatment, and monitoring glycemic control (blood sugar, glycated hemoglobin, and ketones) [45,46,47]. Many studies have shown the benefits of this approach on glycemic control, both in type 1 and type 2 diabetes [48,49].

Table 1.
Benefits of telemedicine and telerehabilitation in non-surgical conditions.
In addition to metabolic diseases, a recent trial on more than one hundred patients has verified the effects of telemedicine on patients suffering from respiratory diseases [50]. In terms of rehabilitation, a randomized controlled trial by Zanaboni et al. studied the remote supervision of patients with chronic obstructive pulmonary disease (COPD) followed for two years [51]. In a multicenter, single-blind, randomized, controlled clinical trial by Hansen et al., patients were assigned to either a 10-week telemedicine pulmonary re-educational treatment (60 min three times a week) or a conventional, non-telemedicine pulmonary re-educational treatment (90 min twice a week); results showed no superiority of remote treatment and supervision over conventional re-educational [52]. Another study on 65 patients by Cameron-Tucker et al. also showed no substantial benefit [53] and even a publication by Berkhof et al. did not report positive effects [54].
On the contrary, the group of Nagatomi et al. praised telemedicine as a tool to better follow fragile patients with heart failure, in a safer way and at their home [55]. Cardiac telemetry has long been used in cardiology departments and in rehabilitation units for patients suffering from heart failure and post-cardiac surgery. The expansion of the monitoring systems, placing them at a more considerable distance from the bed-monitoring stations in the open space of the department, offers several advantages. First, it enhances patient comfort. Second, in certain studies, such as the one conducted by Wita et al. [56] or in large trials (such as the BEAT-HF or the CardioBBEAT) [57,58], it has been demonstrated to reduce the likelihood of rehospitalizations for these patients. Regarding rehabilitation, prominent cardiology scientific societies, such as the European Society of Cardiology, have proposed the potential of telerehabilitation with patient supervision, ensuring the absence of adverse events. This underscores the safety of the protocols employed in this delicate patient group, while leaving the crucial task of precisely determining its clinical benefits to future trials [59].
In the oncology field, to reduce burdensome travel and provide remote or telephone monitoring of patients, new devices have been developed. The implementation of mobile technologies, particularly through dedicated apps, is a subject of numerous studies in the medical literature. For instance, the eSMART study demonstrated the effectiveness of remote symptom monitoring [60]. In a randomized trial conducted by Galiano-Castillo et al., a telemonitoring program for breast cancer patients was evaluated. The study involved 81 patients and found that the telemonitoring program was beneficial in assessing the outcomes of the patients followed and useful for monitoring rehabilitation exercises and their impact on quality of life [61].
Telemonitoring has also proven effective in detecting blood glucose levels continuously over 24 h [62], the presence of malignant arrhythmias [63], and remotely viewing spirometry tracings from the doctor’s office [64,65,66]. Additionally, it can be used to monitor electrocardiograms [67].
3. Use of Telemedicine in Surgical Conditions
Telemedicine and telerehabilitation have also been studied in patients undergoing surgical procedures (Table 2).

Table 2.
Benefits of telemedicine and telerehabilitation in surgical conditions.
A randomized study conducted by Babar et al. compared patients who underwent in-person consultations with a specialist after surgery to those who received postoperative telematic monitoring by a urologist [68]. Shin et al. evaluated patients’ satisfaction with teleconsultation after undergoing pelvic floor reconstructive surgery [69]. In gynecological surgery, trials have explored the effects of telemedicine on monitoring patients’ disabling symptoms, including pain, sleep disturbances, stress, and other mental health disorders [70]. Additionally, they have investigated the impact of virtual visits compared to in-person consultations [71,72].
Bernason et al. conducted a clinical trial with cardiac surgery patients using telemonitoring to monitor their symptoms. The results were promising, with subjects returning to their preoperative levels of functioning within 3 to 6 months after coronary artery bypass grafting. Moreover, they increased their physical activity levels compared to their reported preoperative levels. Interestingly, cardiac rehabilitation is a class IA recommendation for patients with cardiovascular diseases [73]. Physical activity is the core component of a cardiac re-educational program [74]. However, many patients with cardiovascular diseases are failing to meet cardiac rehabilitation guidelines that recommend moderate-to-vigorous intensity physical activity. For this purpose, telerehabilitation interventions are effective at increasing minutes per week of moderate-to-vigorous intensity physical activity among patients in cardiac rehabilitation [75]. While existing studies in the literature have not demonstrated significant benefits in terms of outcomes, such as reduced anxiety, in patients undergoing aortic aneurysm surgery [76], remote telemonitoring of vital signs remains beneficial for early detection of red flags in these complex patients, even after surgery [77,78].
The positive impact of the telemedicine intervention is also described in thoracic surgery, in patients who have undergone lung transplantation [79,80], and in patients affected by lung cancer, treated surgically, also from a rehabilitation perspective [81,82].
4. Methods
The PubMed database was searched for clinical experiences and studies with the following key terms: “telerehabilitation,” “fractures,” in combination with the Boolean operator “AND”. The search was limited to studies on humans, encompassing all study types (e.g., case reports, clinical trials, reviews, and randomized controlled trials). The search was expanded through the bibliography of the retrieved texts. Considering this is a narrative review, a formal inclusion and exclusion criteria selection was not performed, but the search was maintained as broad as possible to achieve a comprehensive summary of current literature of the topic. Only papers written in English were considered.
Studies that did not mention a specific telerehabilitation methodology or that only talked about fractures from an orthopedic, geriatric or internistic points of view were not considered for the purpose of this narrative review.
A first check of the current literature was performed by M.M.V. and V.G., with a consensus subsequently formed by M.N., S.A., and L.C. Supervision of the overall screening, summary, and writing process was carried out by F.C.
5. Telerehabilitation in Patients with Fractures
Patients who suffer fractures can be followed up using telemedicine and telerehabilitation. Several medical literature studies have described the effectiveness of this approach. Telerehabilitation in fracture patients aligns with the findings of a study by Seron et al., which analyzed 53 studies spanning 14 prevalent musculoskeletal conditions. The most prevalent telerehabilitation interventions included therapeutic exercises, functional training, and education. The study revealed that telerehabilitation resulted in reduced hospital visits, decreased hospital costs, and improved functional outcomes [83]. In a multicenter randomized controlled trial, Li et al. investigated the impact of remote telerehabilitation, administered for six months, on a cohort of nearly eight hundred women at risk of osteoporotic fragility fractures. A key criterion for discontinuing the study is the occurrence of a fragility fracture [84].
In the prospective randomized ActiveFLS study, the intervention group included older adults who had sustained a hip fracture. The intervention consisted of a multicomponent approach, including personalized home exercises using an app called @ctive hip® (developer partner: SOCIOEMPRENDE SL, Valencia, Spain), an asynchronous mobile app installed on the patient’s smartphone, for three months. This was followed by nine months of exercises with Vivifrail®. (The Vivifrail® project is a program for the Promotion of Physical Exercise that is an international reference for community and hospital intervention for the prevention of frailty and falls in the elderly. Link: https://vivifrail.com/ (accessed on 20 July 2025). The Vivifrail® app is a tool for healthcare professionals and operators to prevent frailty and falls in the elderly. Developer: Miguel Eugenio Izquierdo Redín. Email: mikel.izquierdo@gmail.com. Nation: Spain. Link: https://play.google.com/store/apps/details?id=com.mikelizquierdo.vivifrail&hl=it (accessed on 20 July 2025)) [85]). See Figure 1.
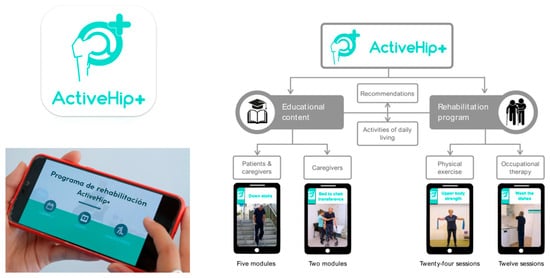
Figure 1.
ActiveHip+® is an educational and telerehabilitation program for patients who have undergone surgery after a hip fracture or require hip replacement. Left side of the figure: main features offered in both the health professionals’ environment and the patients’ and caregivers’ environment. Right side of the figure: ActiveHip+® content provided to patients and caregiver. For further details, see the dedicated section in the main text, go to the corresponding article by clicking on the following doi: https://doi.org/10.1002/nur.22218, or, or more information about the App, see the following link: https://play.google.com/store/apps/details?id=nabelia.active_hip (accessed on 20 July 2025). For more information about the project, please also visit the following link: https://www.activehipplus.com/?lang=en (accessed on 20 July 2025).
Mora-Traverso et al. implemented an mHealth telerehabilitation and health education program on the physical performance of patients with hip fractures and their family caregivers, based on the ActiveHip+® program. ActiveHip+® is an educational and telerehabilitation program designed for patients who have undergone surgery after a hip fracture or require hip replacement. It also provides specific training and counseling to their caregivers. The program’s primary objective is to enhance the functional recovery of patients who have undergone surgery, thereby promoting their independence in performing daily activities and ultimately improving their overall quality of life [86].
In 2018, Anton D et al. published a research paper on a telemonitoring system specifically designed for rehabilitation. They named it KiReS® (Kinect Telerehabilitation System). KiReS® is a Kinect-based telerehabilitation system that aims to enhance the rehabilitation experience for both physiotherapists and patients. The system offers an intuitive and motivating interface for patients, providing valuable feedback to improve the rehabilitation process. At the same time, it assists physiotherapists in designing, managing, and evaluating physiotherapy protocols and sessions, offering smart data and real-time monitoring. Kinect is an innovative developer kit for spatial processing, equipped with advanced models for computer vision and speech recognition, as well as advanced artificial intelligence sensors. It is a sensor capable of capturing three-dimensional body movements. Based on limb and body movements, specific commands are provided, whether it is a game or a video. KiReS® monitored patients while they performed the exercises prescribed in front of a Kinect device. The system provided a physiotherapist interface that assisted in creating exercises step-by-step. These exercises could be created from scratch or reused if they were already stored in the KiReS database [87] (Figure 2).
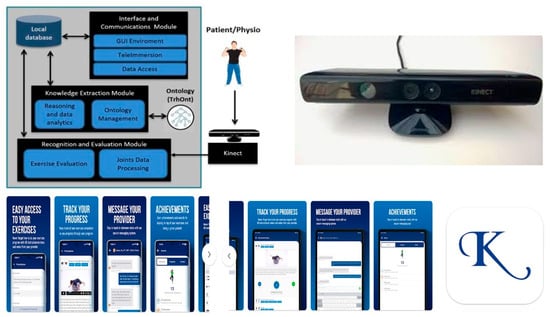
Figure 2.
KiReS® (Kinect Telerehabilitation System) is a Kinect-based telerehabilitation system that aims to improve the rehabilitation experience for both physiotherapists and patients. For further details, see the dedicated section in the main text, or go to the corresponding article by clicking on the following link: https://ieeexplore.ieee.org/document/6720717 (accessed on 20 July 2025). At the bottom of the figure are the various screens of the Kriz® physical therapy app (developer: PT Wired Inc.; email: vikram@ptwired.com. Greensboro, NC, USA). Patients can access their personalized home exercise program via mobile device, and using this app, they can access their personalized home exercise program with HD instructional videos. In the “Messages” tab, they can communicate securely with their Kriz® physical therapy provider. For more information about the App, visit the following link: https://play.google.com/store/apps/details?id=com.ptwired.krizpt (accessed on 20 July 2025).
In 2019, the results of an Israeli study were published. The study randomized patients over 60 years of age who had undergone surgical hip fractures into three equally sized groups to receive either standard treatment as a control group, conventional rehabilitation treatment with both a patient and a caregiver physically present, or telerehabilitation. Outcome measures include the Functional Independence Measure (FIM) for evaluation of ADL, SF-12 for evaluation of Health-related QOL, the Geriatric Depression Scale (GDS), and The Zarit Caregiver Burden Scale [88].
Chirayath et al. recently conducted a study to explore various modalities of managing patients with calcaneal fractures. The authors delved into the technological innovations in management and surgical treatment, as well as the rehabilitation aspects. The study focused on a re-educational program that incorporated virtual reality (VR) technology. VR is emerging as a promising tool in telerehabilitation, offering new opportunities for remote rehabilitation. Patients can access re-educational therapies and receive emotional support directly from home using VR headsets and other tools. VR allows for the creation of virtual environments that simulate real or imaginary scenarios, enabling patients to interact with them and perform specific rehabilitation exercises. This is particularly beneficial in rehabilitation settings where the patient and PRM specialist are physically present. Patients can still participate in re-educational sessions from home, reducing the need for travel. A “non-immersive” VR has also been developed, using monitors or wall projections to produce a 3D image. This type of VR does not completely eliminate the external environment, allowing patients to interact with a three-dimensional virtual environment in real time, with the impression of their own body [89,90,91]. Chirayath et al. integrated VR technology into the program to create a virtual environment tailored to the patient’s needs. This virtual environment encouraged active participation in exercises, potentially improving compliance and motivation during recovery. Additionally, the authors discussed the potential of sensor-based rehabilitation devices and telerehabilitation systems. These systems enable remote assessments and exercise guidance between patients and healthcare professionals [92] (Figure 3).
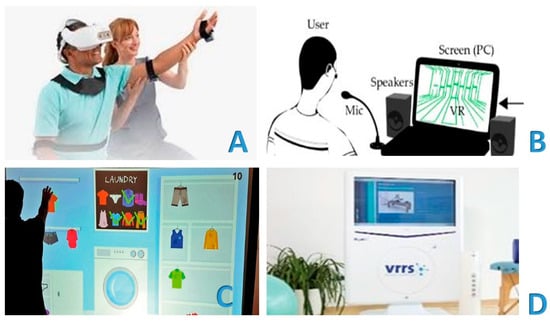
Figure 3.
Virtual reality is emerging as a promising tool in telerehabilitation, offering new opportunities for remote rehabilitation. Virtual reality allows for the creation of virtual environments that simulate real or imaginary scenarios, offering the patient the possibility of interacting with them and carrying out specific rehabilitation exercises, even in rehabilitation settings where there is the physical presence of both the patient and the physiatrist and physiotherapist (A). Patients can still participate in rehabilitation sessions from home, reducing the need for travel, with virtual reality (B). There is also a so-called “non-immersive” virtual reality, which uses monitors or wall projections to produce a 3D image. Therefore, the external environment is not completely eliminated, and the patient receives the impression of a three-dimensional virtual environment, interacting in real time with the representation of their own body (C). Figure 3D shows an example of virtual reality. It shows the Khymeia VRRS®—Virtual Reality Rehabilitation System—which is an internationally patented Class I certified medical device (D). VRRS® (Khymeia Group, Via San Marco 11/C 35129—Padova (PD)—Italy, VAT 03345930287, CCIAA 1998-64134. Email: info@khymeia.com. Link: https://khymeia.com/prodotti/ (accessed on 20 July 2025)) is an advanced, comprehensive, and clinically tested virtual reality system for rehabilitation. It is also characterized by its extreme ease of use, high customization capabilities, and complete automatic reporting. VRRS® is also designed as a “central hub” to which a series of specialized peripheral devices can be connected via a USB, fully synchronized and integrated with the system. VRRS®, with its exclusive magnetic kinematic acquisition system, is used as a clinical routine for the rehabilitation of a wide range of pathologies through numerous rehabilitation modules containing the largest library of clinically validated exercises available. The scientific paradigms on which the system is based are, in particular, those of “augmented feedback” and “motor imagery,” principles on which the consolidated experience of promoting motor and functional learning by the central nervous system is based. Augmented feedback, through exercises performed in a virtual environment, allows for the development of “awareness of results” and “awareness of the quality” of the movements performed. In this way, the central nervous system can activate a crucial “physiological learning” mechanism, which requires an increase in movement-specific information to produce a significant improvement in performance quality. For further information, along with the main text, please refer to the publication Pournajaf S, Giovanni Morone G, Goffredo M, Bonaiuti D, Franceschini M. “Virtual reality applied to rehabilitation: clinical evidence and future perspectives,” Italian Journal of Rehabilitation Medicine, Vol. 36, Number 3: 30–42, September 2021 (link: https://springerhealthplus.it/mr/archivio/realta-virtuale-applicata-alla-riabilitazione-evidenze-cliniche-e-prospettive-future/ (accessed on 20 July 2025)) and to the link for the VRRS® device: https://www.fisiomedlambrate.it/featured_post/realta-virtuale/ (accessed on 20 July 2025).
An equally recent Turkish study employing a method called Home-Based Real-Time Video Conferencing (HBRVC) telerehabilitation investigated a small group of patients with distal radius fractures. The study recruited patients between May 2022 and May 2023, with a specific sample of elderly subjects. All patients underwent volar plating due to a diagnosed and controlled radial fracture. The patients were included in a single-blind randomized study.
The study revealed a dual perspective, with both positive and negative aspects. On the one hand, the HBRVC telerehabilitation program appeared to be as effective as in-person rehabilitation in improving joint range of motion and reducing edema in patients undergoing volar plate fixation for a known fracture. On the other hand, the telerehabilitation method was found to be less effective in improving muscle function and strength compared to in-person rehabilitation [93]. Regarding upper limb fractures, da Silva et al. conducted a systematic review and found that telerehabilitation appears to have favorable effects on functional capacity and pain perception [94].
Kalayci et al. investigated the effects of motor imagery (MI) on distal radius fractures, seeking technological systems and re-educational interventions to enhance treatment effectiveness. MI involves mentally simulating a motor action without physically performing it. For instance, imagining performing a movement without actually moving the involved body part [95]. Techniques that exploit this principle have been used in various fields, such as rehabilitation and sports, to improve motor control and learning both in patients [96,97,98,99] and athletes [100,101]. MI has been shown to improve pain, function, range of motion, and muscle strength in musculoskeletal re-education [102,103], although studies on its effect on upper limb injuries are limited. In the study by Kalayci et al., a group of patients underwent MI in addition to traditional re-education, while another group received only traditional re-education (three times a week for 8 weeks). The study compared the two groups in terms of pain intensity, wrist function, muscle strength, and active range of motion. Statistically significant changes were observed in favor of the MI group in the Patient Rated Wrist Evaluation-function parameter, active range of motion in wrist extension, and hand grip strength. Interestingly, both groups showed improvements in their quality of life [104] (Figure 4).
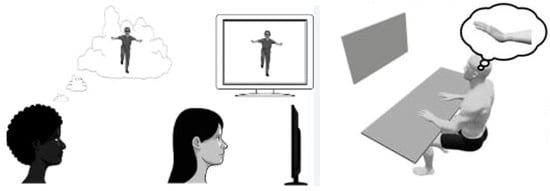
Figure 4.
Explanation of the mechanism underlying “motor imagery” used in telerehabilitation technologies. When a subject observes or imagines an action, their mirror neurons activate as if they were performing the action themselves, creating a sort of “internal emulation.” This process can be used to facilitate the recovery of motor functions lost, for example, following a stroke or other types of injury, by leveraging brain plasticity. Regarding the use of “motor imagery” in patients who have suffered a fracture, please refer to the main text.
Among the tools used for telerehabilitation, smartphone-based apps offer the convenience of remotely following re-educational programs, utilizing mobile technologies for patient care and monitoring. Rehabilitation platforms, dedicated software, and apps enable the development of personalized re-educational programs with these supportive features, along with user-friendly training videos. These systems, combined with the intervention of PRM specialists, primary care physicians, and physiotherapists, are user-friendly and facilitate understanding of correct training techniques, even allowing for self-training. Moreover, these systems facilitate data collection and monitoring of patient progress [105,106]. Chen et al. evaluated 31 apps, divided into four categories (smart intervention, angle measurement, smart monitoring, and rehabilitation games). These apps provided both guidance and training for patients’ home rehabilitation (compensating for the high costs and space limitations of traditional rehabilitation methods), as well as methods for evaluating outcomes and actively interacting with users for rehabilitation purposes. Indeed, the smart intervention category had the highest download rate on the app market. Angle measurement tools helped patients with distal radial fractures independently measure their joint angle to assess their rehabilitation status. Many of these apps have achieved good clinical validation over time [107].
Lastly, integrating wearable sensors and software algorithms during telerehabilitation enables the modulation of care for fracture patients, including home monitoring of bone load. Various authors have reported that data from inertial measurement units and pressure insoles in shoes can be fused into machine learning algorithms based on biomechanical data [108,109]. Nurse et al. studied an approach based on this type of technology on patients who had suffered tibial shaft fractures, using a significantly small sample (eight young participants, equally divided between men and women) trying to obtain useful information for remote monitoring of the load on the tibia bone with insoles using a pressure sensor [110].
6. Telerehabilitation in Patients with Fractures and Treated with Surgical Interventions
Telerehabilitation programs have also been used and are being studied in patients who have undergone surgical interventions due to fractures. In 2009, Eriksson et al. conducted a small study involving 22 subjects to test the benefits of remote physiotherapy after shoulder surgery. They evaluated clinical outcomes, such as pain (measured using a visual analog scale, VAS) and functional outcomes, including measuring the range of motion [111]: in this study, although with obvious limitations due to the small number of subjects, video monitoring of patients at home had advantages. Since, at least, the non-inferiority of this method had been highlighted at a distance in patients undergoing hip prosthesis for osteoarthritis [112], and since it also seemed to have economic benefits, as in Nelson’s study et al. [113], with data confirming such economic benefits for all orthopedic teleconsultations coming not only from the analysis of the Olsen group in the same year [114] but also from the study of Ohinmaa et al. almost two decades earlier [115], it was decided to extend these studies to the elderly population who had suffered fractures.
During the COVID-19 pandemic, Prieto-Moreno et al. launched a downloadable iPhone app to monitor the rehabilitation of elderly patients undergoing surgery. By 2022, the experience had proven satisfactory (in Spain, 85% app adoption was observed, with a small but significant percentage (64%) of 69 elderly patients recruited using the device), although the authors themselves recommended further data analysis through a specially designed randomized trial [116]. This trial (multicenter in three Spanish hospitals and open-label) was conducted shortly after, with equally valid and positive results, so much so that the App was then used in a greater number of hospitals and not only in Spain but also in Belgium and Portugal [117].
Other experiments aimed to extend the duration of observations. Wu et al. compared the effectiveness of a six-month home-based telerehabilitation program (double the duration of Prieto-Moreno et al. protocol) on a small group of elderly patients with hip fractures treated with total hip replacement. The telerehabilitation group used an Internet-based rehabilitation management system combined with conventional outpatient care. The telerehabilitation group showed a higher functional independence score (functional independence measure, FIM) compared to the control group. Interestingly, the system also allowed for other types of remote monitoring. It was equipped with a Bluetooth connection that enabled the functions of an electronic thermometer, an electronic blood pressure monitor, and an electronic blood glucose monitor by connecting these peripherals to the system [118].
Another study, also Chinese, studied the effects of remote monitoring via smartphone on 31 elderly patients who had suffered a hip fracture [119]. Zhang et al. investigated an internet-based telemedicine tool for remote monitoring and implementing re-educational programs in 29 patients after hip surgery, compared to telephone monitoring. The telerehabilitation management system showed promise in improving the functional recovery of the hip joint and enhancing the ability to perform daily activities and somatic integration to some extent [120].
Pliannuom et al. recently conducted a meta-analysis on PubMed, Embase, Cochrane, ProQuest, and CINAHL up to 3 January 2023. This comprehensive review included 16 studies. The study population consisted of adults aged 60 years and older who had undergone surgery for hip, femoral neck, intertrochanteric, or subtrochanteric fractures and were receiving post-operative care. The researchers assessed various functional outcomes, including the Timed Up and Go (TUG) test, Short Physical Performance Battery (SPPB), and FIM. Notably, these scores improved significantly after home telerehabilitation. Beyond the improvement in functional outcomes, the study also observed a notable increase in the subjects’ daily and instrumental activities of life (ADLs and IADLs). This improvement was accompanied by enhanced compliance with the telerehabilitation protocols. The follow-up modalities allowed for a higher adherence to the protocols, leading to a tendency towards more responsible self-care. Patients demonstrated more careful use and management of therapies, as well as a greater focus on their nutritional status. Among the various delivery methods, phone calls emerged as the most common. This finding raises questions about how these positive results can be achieved using relatively simple means, without necessarily resorting to sophisticated technology [121].
Telerehabilitation platforms are now also integrated with apps that, in addition to video calls, can provide valuable data on the exercises performed and the results achieved, both during each session and at the end of the observation period. This was demonstrated in a randomized trial conducted by a group from Shanghai, a renowned trauma center in China, on patients undergoing total hip replacement. The study compared the traditional physiotherapy approach with telerehabilitation. The traditional physiotherapy group received a standard re-educational intervention in the hospital for one month, followed by outpatient physiotherapy for the next two months. The telerehabilitation group was followed up through a proper mobile platform. Patients were assessed at baseline, four weeks, and twelve weeks after surgery using functional tests (TUG and Berg balance test) and self-administered questionnaires to assess quality of life (QoL), such as the Hip Disability and Osteoarthritis Outcome Score (HOOS) and Short Form 12 (SF-12). While there were no significant differences between the groups, the trial did highlight that the telerehabilitation method was non-inferior to the traditional one [122].
A recent Chinese review highlighted that numerous studies demonstrated the effectiveness of home-based telerehabilitation in promoting the recovery of hip joint function. This approach resulted in improvements in the QoL and, to some extent, psychological factors for elderly patients with hip fractures [123]. This focus on psychological factors and cognition has raised the interest of other authors. Pol et al. conducted a study that explored the benefits offered by telerehabilitation on these aspects and found cognitive positive results [124]. Fernández-González et al. conducted a study to assess the impact of the ActiveHip® application on the families and caregivers of patients with hip fractures. They used two mood assessment scales: the Hospital Anxiety and Depression Scale (HADS) and a functional assessment scale called the Physical Fitness with the International Fitness Scale (IFIS). While no statistically significant differences in care burden were found between the families of patients in the ActiveHip® group and the comparison group, a trend toward lower values was observed in the ActiveHip® group, as well as for anxiety and depression [125].
Mora-Traverso et al. reported similar findings regarding anxiety and depression in patients. The telerehabilitation group showed a greater reduction in the total HADS score and its subscales, including anxiety and depression, compared to the control group. Moreover, the differences between the telerehabilitation group and the control group were consistent at the 3-month follow-up for other self-perceived health indicators [126]. A recent meta-analysis evaluated 17 randomized controlled trials involving over 1500 patients [127]. This analysis separated the functional benefits from the psychological ones, specifically the relief of stress and anxiety. The results regarding the latter were reported with doubts. However, there was a difference in terms of functional benefit regarding the dedicated physical therapy scales. In contrast, a less recent study by Bramanti et al. reported positive aspects of telerehabilitation. Their review analyzed ten studies and found several advantages, including logistical advantages (follow-up of patients living in rural and/or disadvantaged areas) and a very positive psychological impact on the patients [128].
7. Discussion
Telemedicine and telerehabilitation emerge as promising tools that can significantly contribute to the management and rehabilitation of patients with fractures, particularly elderly individuals with hip fractures who show adequate functional independence, cognitive status, family or caregiver support, and access to technology. In this specific population, telerehabilitation seems feasible and can deliver multidisciplinary IRPs encompassing physical therapy, occupational therapy, and caregiver education remotely through videoconferencing or digital platforms [129,130]. Evidence from randomized and non-randomized trials support then notion that in selected patients, telerehabilitation yields functional outcomes (e.g., FIM, TUG) that are comparable to or slightly better than conventional home based in person rehabilitation, with high patient and caregiver satisfaction and good adherence [106,129]. QoL and self-efficacy also improve, and caregiver burden is not increased [130,131]. However, systematic reviews and meta-analyses indicate that the effect of telerehabilitation on mobility, adverse events, and pain is uncertain, with no clinically meaningful differences compared to standard care [132]. Telerehabilitation may improve patients’ confidence in ADLs and reduce barriers to access, especially where in-person services are limited. The most suitable telemedicine platforms for elderly patients with hip fractures are those that provide secure, user-friendly videoconferencing, structured exercise modules, and remote monitoring capabilities. The medical literature supports the use of Web-based platforms specifically designed for telerehabilitation, such as the ActiveHip® protocol, which delivers multidisciplinary rehabilitation (physical and occupational therapy, caregiver education) through a dedicated website accessible by patients and caregivers. This approach has demonstrated feasibility and effectiveness in this population, provided there is caregiver support and internet access [129,130].
Smartphone-based mHealth platforms [e.g., Caspar Health e-system® (Caspar Health is a company of GOREHA GmbH. Location: Neue Schönhauser Str. 20. 10178 Berlin. Email: patientenbetreuung@caspar-clinic.de. Link: https://www.caspar-health.de/en (accessed on 20 July 2025)) and SMPT® (SMPT® is an artificial intelligence (AI)-based platform that digitizes and improves the perioperative process and post-operative patient monitoring visits to control their status. Location: Passeig Bonanova, 10, Sarrià-Sant Gervasi, Barcelona 08022, Spain. Email: Link to the site: https://smtp.health/ (accessed on 20 July 2025))] are tools that offer exercise guidance, real-time feedback, and communication with therapists. These platforms are effective for home-based occupational therapy and physical rehabilitation, with high acceptability and satisfaction among older adults after hip fracture surgery [119,133]. Off-the-shelf videoconferencing applications [e.g., Zoom® (Founder: Eric Yuan; CEO: Eric Yuan (June 2011–); CTO: Xuedong Huang. Founded: 2011, San Jose, CA, USA. Headquarters: San Jose, CA, USA. Link to the Italian site: https://www.zoom.com/it (accessed on 20 July 2025)) and FaceTime® (Developer: Apple; iOS Operating System: macOS, iPadOS. Proprietary License: non-free license. Website: www.apple.com/it (accessed on 20 July 2025))] used on tablets or smartphones, combined with tailored exercise programs and regular therapist follow-up, have been shown to be feasible and acceptable for post-acute telerehabilitation in older adults, including those with fractures [134]. Key features that enhance suitability include intuitive interfaces, clear audiovisual quality, secure data handling, and the ability to involve caregivers in sessions. Platforms should be selected based on patient and caregiver familiarity, local technical support, and integration with existing clinical workflows [130,134] (Figure 5).
Figure 5.
Telerehabilitation uses various technological tools. Smartphone-based rehabilitation offers the possibility of remotely following rehabilitation programs, leveraging mobile technologies for patient care and monitoring. Rehabilitation using platforms, dedicated programs, and apps allows for the development of an individualized training plan with these types of support, along with user-friendly training videos. These, combined with the intervention of the physiatrist, primary care physician, and therapist, are very easy to use and facilitate understanding of correct training techniques, even allowing for self-training. These systems also allow for data collection and monitoring of patient therapy progress, evaluating exercises, and discussing results between the physiatrist, therapist, and patient. For further information, see the main text, the link below (https://apps.apple.com/it/app/caspar-health/id1222630969 (accessed on 20 July 2025)), and the following link: https://play.google.com/store/apps/details?id=com.casparhealth.android.patient&hl=it (accessed on 20 July 2025). Link to the site: https://www.caspar-health.de/en (accessed on 20 July 2025).
The use of telemedicine and telerehabilitation is becoming more prominent in the management of fracture patients treated with surgical interventions, particularly following hip and knee arthroplasty. The medical literature demonstrates that telerehabilitation is generally non-inferior to conventional in-person rehabilitation in terms of functional outcomes, patient-reported outcomes, and safety for patients after total hip or knee arthroplasty, as well as after hip fracture surgery [132,135]. Telerehabilitation can support recovery of mobility, ADLs, and QoL, and may also improve psychological factors such as anxiety and depression in older adults after hip fracture [126,129,136]. Patient, caregiver and operator satisfaction with telerehabilitation is high, and it offers advantages in accessibility, cost, and continuity of care, especially for those with limited access to in-person services [131,137,138]. Telemedicine also facilitates remote monitoring, follow-up, and early identification of complications, which can contribute to patient safety and potentially reduce healthcare utilization [139,140]. However, the evidence for telerehabilitation’s superiority over conventional rehabilitation is limited; most studies show equivalence rather than clear benefit, and some outcomes (e.g., mobility, adverse events) remain uncertain or show only small differences [132,135]. Barriers include technology access, risk of injury without direct supervision, and reduced patient-provider interaction, but these can be mitigated by user-centered program design and clinician support [141].
8. Limitations of the Examined Scientific Studies
Several limitations emerged in the scientific medical literature reviewed. Several studies did not examine the long-term outcomes of treatments using various telemedicine methods (e.g., Kalaycı et al.) [104]. Other studies did not assess participant compliance, which is an important measure of whether these methods are not only well accepted by patients but also used effectively. Further studies are certainly needed to explore the potential additional benefits of increasing the number of sessions using a given technology (e.g., for MI). Several studies examined only a few patients (about twenty per comparison group or even fewer, as in the case of Nurse’s work) [110] or a small sample of the study population. For this purpose, studies with a larger sample size could provide more robust information on the effectiveness of a treatment using a specific telerehabilitation protocol. Since these are sometimes not pure clinical studies but rather studies involving experimental technologies and engineering approaches (for example, studies on sensors for load monitoring in patients treated for tibial or calcaneal fractures and subsequently subjected to a rehabilitation program with the concomitant use of insoles or load sensors, as in the case of Nurse’s work) [110], estimating the sample size capable of generating adequate statistical power is particularly complex. In these studies, unlike medical studies, the methodology for calculating the hypotheses a priori and determining the sample size appears to be linked to a greater number of variables, which are sometimes not well-defined or change over the course of the study itself. There are also several practical difficulties in conducting these studies, especially on geriatric patients, who, especially after the stress of hospitalization and ongoing acute and chronic conditions, may lack the strength to participate fully in a study, declining the invitation or compromising the results due to suboptimal compliance [142]. However, one study in this regard suggested that caregiver support can aid in monitoring and even significantly increase adherence to this type of rehabilitation in these patients. The role of caregivers in this setting is further emphasized in a recent study by Ariza-Vega et al. [143] of the same year. Some studies either lack a comparison group or present the two rehabilitation modalities—remote and conventional outpatient—as overlapping and complementary: telerehabilitation is thus presented as a complementary treatment to standard physiotherapy sessions. Many studies therefore explore the benefits of combining telerehabilitation with standard orthopedic and physical therapy care, while superiority studies with significant data are lacking (non-inferiority studies prevail). A greater number of comparison and superiority studies would be desirable.
9. Future Perspectives
Current telerehabilitation applications include systems ranging from videophones to (expensive) fully immersive virtual reality systems with haptic interfaces. Naturally, for mobile phones, future prospects are geared towards lowering costs and using increasingly faster data transmission speeds, moving from low-bandwidth to broadband, fiber optics, and, in the future, a mixed model of fiber optics (FTTH), 5G, and satellite internet, with the goal of achieving extremely high speeds and a very high data throughput (with a target of 1 Gbit/s).
Future hopes for virtual reality systems also lie in lower costs. The advent of the European Recovery and Resilience Plan (PNRR) raises the question of the ever-increasing need for digital healthcare data, and this ambitious goal also brings with it challenges that must be addressed in the near future. Several obstacles have been identified to the establishment and advancement of telerehabilitation within the various healthcare systems and the platforms currently in use. There are also professional issues related to the intrinsic practical approach of certain treatments, authorization laws, professional skills development, the disability of the patient to be treated remotely and with which methods, reimbursement, and the scarcity of online assessment and treatment tools and outcome data. These are undoubtedly complex issues that are currently being addressed not only by the healthcare world but also by the commercial and political spheres.
Data is still insufficient to conclusively define whether telerehabilitation is better or worse than traditional rehabilitation. In this regard, while this review aimed to provide an overview of the various technologies and methods used in the rehabilitation field to remotely follow patients, future studies based on clinical experience and with strict methodologies should be focused on in order to assess telerehabilitation’s potential versus conventional rehabilitation.
10. Conclusions
Telerehabilitation appears as a feasible, non-inferior, and cost-effective addition or alternative to conventional rehabilitation for patients with fracture. Telerehabilitation seems particularly effective in older adults after hip fractures, improving functional outcomes (e.g., FIM, TUG), ADLs/IADLs, QoL, and adherence when supported by user-friendly platforms and caregiver involvement. These methods appear to offer many advantages when used in frail, older adults who, after a fracture and hospitalization, would have difficulty returning to the clinic for follow-up visits. At the same time, older adults with increased self-sufficiency issues after a fracture and without a strong family network or regular caregivers at home could benefit from remote rehabilitation programs, monitoring themselves and saving on the cost of equipment, travel, and in-person professionals. Superiority over in-person care is still difficult to demonstrate, and effects on pain, mobility, and adverse events remain uncertain. Wider adoption should prioritize secure videoconferencing, structured therapeutic exercise, remote monitoring, and integration into IRPs, while addressing barriers such as digital access, safety without direct supervision, and sustained patient–operator engagement. Future research requires adequately powered, longer-term comparative trials to define optimal candidates, protocols, and technologies (including VR, MI, sensors, and wearables) and to clarify the psychological impacts, safety, and cost-effectiveness across fracture types and settings.
Author Contributions
Conceptualization, N.M. and V.M.M.; methodology, N.M.; investigation, G.V. and V.M.M.; data curation, N.M.; writing—original draft preparation, G.V., A.S., and V.M.M.; writing—review and editing, N.M., C.L., and C.F.; supervision, C.L. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. All authors of this article declare that they have never received compensation and/or grants, in the past or currently, from the companies involved in the development of the technologies and applications mentioned in this research work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Randomized controlled trials: RCTs; chronic obstructive pulmonary disease: COPD; European Society of Cardiology: ESC; Kinect Telerehabilitation System: KiReS; Geriatric Depression Scale: GDS; virtual reality: VR; Home-Based Real-Time Video Conferencing telerehabilitation: HBRVC telerehabilitation; information technology: IT; inertial measurement units: IMUs; Hip Disability and Osteoarthritis Outcome Score: HOOS; Short Form 12: SF-12; quality of life: QOL; computerized tomography: CT; Visual Analogue Scale: VAS; range of motion: ROM; total hip replacement: THR; functional independence measure: FIM; Timed Up and Go test: TUG test; Short Physical Performance Battery: SPPB; Activities of Daily Living: ADL; Instrumental Activities of Daily Living: IADL; Hospital Anxiety and Depression Scale: HADS; Physical Fitness with the International Fitness Scale: IFIS; motor imagery: MI; Evidence-Based Medicine: EBM; artificial intelligence: AI; fiber optics: FTTH; National Recovery and Resilience Plan: NRRP (in Italian, Piano di Ripresa e Resilienza: PNRR).
References
- Zazzara, M.B.; Vetrano, D.L.; Carfì, A.; Onder, G. Frailty and Chronic Disease. Panminerva Med. 2020, 61, 486–492. [Google Scholar] [CrossRef]
- Onder, G.; Vetrano, D.L.; Marengoni, A.; Bell, J.S.; Johnell, K.; Palmer, K. Accounting for Frailty When Treating Chronic Diseases. Eur. J. Intern. Med. 2018, 56, 49–52. [Google Scholar] [CrossRef]
- De Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; De Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14, 982. [Google Scholar] [CrossRef]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in Older Persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Di Giovanni, P.; Di Martino, G.; Zecca, I.A.; Porfilio, I.; Romano, F.; Staniscia, T. Incidence of Hip Fracture and 30-day Hospital Readmissions in a Region of Central Italy from 2006 to 2015. Geriatr. Gerontol. Int 2019, 19, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Aicale, R. Proximal Femoral Fractures in the Elderly: A Few Things to Know, and Some to Forget. Medicina 2022, 58, 1314. [Google Scholar] [CrossRef] [PubMed]
- Alito, A.; Fenga, D.; Portaro, S.; Leonardi, G.; Borzelli, D.; Sanzarello, I.; Calabrò, R.S.; Milone, D.; Tisano, A.; Leonetti, D. Early Hip Fracture Surgery and Rehabilitation. How to Improve Functional Quality Outcomes. A Retrospective Study. Folia Medica 2023, 65, 879–884. [Google Scholar] [CrossRef]
- Veronese, N.; Maggi, S. Epidemiology and Social Costs of Hip Fracture. Injury 2018, 49, 1458–1460. [Google Scholar] [CrossRef]
- Castelli, A.; Daidone, S.; Jacobs, R.; Kasteridis, P.; Street, A.D. The Determinants of Costs and Length of Stay for Hip Fracture Patients. PLoS ONE 2015, 10, e0133545. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Custodero, C.; Maggi, S.; Polidori, M.C.; Veronese, N.; Ferrucci, L. A Multidimensional Approach to Frailty in Older People. Ageing Res. Rev. 2020, 60, 101047. [Google Scholar] [CrossRef]
- Unnanuntana, A.; Kuptniratsaikul, V.; Srinonprasert, V.; Charatcharoenwitthaya, N.; Kulachote, N.; Papinwitchakul, L.; Wattanachanya, L.; Chotanaphuti, T. A Multidisciplinary Approach to Post-Operative Fragility Hip Fracture Care in Thailand—A Narrative Review. Injury 2023, 54, 111039. [Google Scholar] [CrossRef]
- Manocchio, N.; Faraci, S.; Vita, G.; Silvestri, S.; Cicchi, L.; Ljoka, C.; Foti, C. Dislocation/Fracture of Proximal 5th Metatarsal: New Protocol with Self-Assessment Scale and Specific Plantar Orthosis. Muscle Ligaments Tendons J. 2025, 15, 11. [Google Scholar] [CrossRef]
- Rudy, M.D.; Grant, P.J. The Patient with Hip Fracture. Med. Clin. North Am. 2024, 108, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, A.S.; Gorczyca, J.T. Orthopedic Surgery and the Geriatric Patient. Clin. Geriatr. Med. 2019, 35, 65–92. [Google Scholar] [CrossRef]
- Piccione, F.; Maccarone, M.C.; Cortese, A.M.; Rocca, G.; Sansubrino, U.; Piran, G.; Masiero, S. Rehabilitative Management of Pelvic Fractures: A Literature-Based Update. Eur. J. Transl. Myol. 2021, 31, 9933. [Google Scholar] [CrossRef]
- Lena, F.; Etoom, M.; Al-Wardat, M.; Modugno, N. Osteoporotic Fracture and Conservative Management in Parkinson’s Disease and Pisa Syndrome: Case Report. J. Bodyw. Mov. Ther. 2021, 25, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Schäfer, L.; Simeone, F.; Vaish, A.; Bhadani, J.S.; Vaishya, R. Management of Distal Femoral Non-Union: A Systematic Review. JOIO 2024, 58, 1686–1723. [Google Scholar] [CrossRef]
- UEMS-PRM Section and Board; Zampolini, M.; Selb, M.; Boldrini, P.; Branco, C.A.; Golyk, V.; Hu, X.; Kiekens, C.; Negrini, S.; Nulle, A.; et al. The Individual Rehabilitation Project as the Core of Person-Centered Rehabilitation: The Physical and Rehabilitation Medicine Section and Board of the European Union of Medical Specialists Framework for Rehabilitation in Europe. Eur. J. Phys. Rehabil. Med. 2022, 58, 503–510. [Google Scholar] [CrossRef]
- Fairhall, N.J.; Dyer, S.M.; Mak, J.C.; Diong, J.; Kwok, W.S.; Sherrington, C. Interventions for Improving Mobility after Hip Fracture Surgery in Adults. Cochrane Database Syst. Rev. 2022, 2022, CD001704. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; Ferdinandi, V.; Cicchi, L.; Foti, C. Commentary on “The Learning Rehabilitation System: Strengthening an Intersectoral Strategy to Improve Functioning of an Ageing Population” by Bickenbach et Al. Health Policy 2025, 155, 105303. [Google Scholar] [CrossRef]
- Elboim-Gabyzon, M.; Andrawus Najjar, S.; Shtarker, H. Effects of Transcutaneous Electrical Nerve Stimulation (TENS) on Acute Postoperative Pain Intensity and Mobility after Hip Fracture: A Double-Blinded, Randomized Trial. CIA 2019, 14, 1841–1850. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Giannouchos, T.V.; Pavli, A.; Tsonou, P.; Dedoukou, X.; Tseroni, M.; Papadima, K.; Hatzigeorgiou, D.; Sipsas, N.V.; Souliotis, K. Costs Associated with COVID-19 in Healthcare Personnel in Greece: A Cost-of-Illness Analysis. J. Hosp. Infect. 2021, 114, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Orrù, G.; Littera, R.; Firinu, D.; Chessa, L.; Cossu, G.; Primavera, D.; Del Giacco, S.; Tramontano, E.; Manocchio, N.; et al. Comparing the Responses of Countries and National Health Systems to the COVID-19 Pandemic: A Critical Analysis with a Case-Report Series. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7868–7880. [Google Scholar] [CrossRef]
- Zeitouny, S.; Cheung, D.C.; Bremner, K.E.; Pataky, R.E.; Pequeno, P.; Matelski, J.; Peacock, S.; Del Giudice, M.E.; Lapointe-Shaw, L.; Tomlinson, G.; et al. The Impact of the Early COVID-19 Pandemic on Healthcare System Resource Use and Costs in Two Provinces in Canada: An Interrupted Time Series Analysis. PLoS ONE 2023, 18, e0290646. [Google Scholar] [CrossRef]
- Cianferotti, L.; Porcu, G.; Ronco, R.; Adami, G.; Alvaro, R.; Bogini, R.; Caputi, A.P.; Frediani, B.; Gatti, D.; Gonnelli, S.; et al. The Integrated Structure of Care: Evidence for the Efficacy of Models of Clinical Governance in the Prevention of Fragility Fractures after Recent Sentinel Fracture after the Age of 50 Years. Arch. Osteoporos. 2023, 18, 109. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; Buttarelli, L.; Giordan, L.; Sorbino, A.; Foti, C. Early Motor and Respiratory Re-Education in Patients Hospitalized for COVID-19. Adv. Rehabil. 2025, 39, 29–45. [Google Scholar] [CrossRef]
- Farrow, L.; Hall, A.; Wood, A.D.; Smith, R.; James, K.; Holt, G.; Hutchison, J.; Myint, P.K. Quality of Care in Hip Fracture Patients: The Relationship Between Adherence to National Standards and Improved Outcomes. J. Bone Jt. Surg. 2018, 100, 751–757. [Google Scholar] [CrossRef]
- Miranda, I.; Ferrás-Tarragó, J.; Colado, J.; Sangüesa-Nebot, M.J.; Doménech, J. Impacto de la pandemia por COVID-19 y el confinamiento estricto de la población en la incidencia de fractura de cadera en España. Una revisión sistemática. Rev. Española Geriatría Gerontol. 2023, 58, 101380. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Sangüesa-Nebot, M.J.; González, A.; Doménech, J. Impact of Strict Population Confinement on Fracture Incidence during the COVID-19 Pandemic. Experience from a Public Health Care Department in Spain. J. Orthop. Sci. 2022, 27, 677–680. [Google Scholar] [CrossRef]
- Dallari, D.; Zagra, L.; Cimatti, P.; Guindani, N.; D’Apolito, R.; Bove, F.; Casiraghi, A.; Catani, F.; D’Angelo, F.; Franceschini, M.; et al. Early Mortality in Hip Fracture Patients Admitted during First Wave of the COVID-19 Pandemic in Northern Italy: A Multicentre Study. J. Orthop. Traumatol. 2021, 22, 15. [Google Scholar] [CrossRef]
- Supady, A.; Curtis, J.R.; Abrams, D.; Lorusso, R.; Bein, T.; Boldt, J.; Brown, C.E.; Duerschmied, D.; Metaxa, V.; Brodie, D. Allocating Scarce Intensive Care Resources during the COVID-19 Pandemic: Practical Challenges to Theoretical Frameworks. Lancet Respir. Med. 2021, 9, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Melman, G.J.; Parlikad, A.K.; Cameron, E.A.B. Balancing Scarce Hospital Resources during the COVID-19 Pandemic Using Discrete-Event Simulation. Health Care Manag. Sci. 2021, 24, 356–374. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for Healthcare: Capabilities, Features, Barriers, and Applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Farrokhi, N.; Sarzaeem, M.M.; Feizi, D. Feasibility and Acceptability of a Telerehabilitation Intervention on Patients Undergoing Total Knee Arthroplasty in Iran: Randomised Controlled Trial Protocol. BMJ Open 2024, 14, e083784. [Google Scholar] [CrossRef]
- Solomon, R.M.; Dhakal, R.; Halpin, S.J.; Hariharan, R.; O’Connor, R.J.; Allsop, M.; Sivan, M. Telerehabilitation for Individuals with Spinal Cord Injury in Low-and Middle-Income Countries: A Systematic Review of the Literature. Spinal Cord. 2022, 60, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Baffert, S.; Hadouiri, N.; Fabron, C.; Burgy, F.; Cassany, A.; Kemoun, G. Economic Evaluation of Telerehabilitation: Systematic Literature Review of Cost-Utility Studies. JMIR Rehabil. Assist. Technol. 2023, 10, e47172. [Google Scholar] [CrossRef] [PubMed]
- Albahrouh, S.I.; Buabbas, A.J. Physiotherapists’ Perceptions of and Willingness to Use Telerehabilitation in Kuwait during the COVID-19 Pandemic. BMC Med. Inf. Decis. Mak. 2021, 21, 122. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; He, C. Research Progress and Hot Topics in Telerehabilitation for Hip or Knee Arthroplasty. Orthop. Surg. 2025, 17, 677–693. [Google Scholar] [CrossRef]
- Shambushankar, A.K.; Jose, J.; Gnasekaran, S.; Kaur, G. Cost-Effectiveness of Telerehabilitation Compared to Traditional In-Person Rehabilitation: A Systematic Review and Meta-Analysis. Cureus 2025, 17, e79028. [Google Scholar] [CrossRef]
- Nicolas, B.; Leblong, E.; Fraudet, B.; Gallien, P.; Piette, P. Telerehabilitation Solutions in Patient Pathways: An Overview of Systematic Reviews. Digit. Health 2024, 10, 20552076241294110. [Google Scholar] [CrossRef]
- Tarakci, E.; Tarakci, D.; Hajebrahimi, F.; Budak, M. Supervised Exercises versus Telerehabilitation. Benefits for Persons with Multiple Sclerosis. Acta Neuro Scand. 2021, 144, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Buabbas, A.J.; Albahrouh, S.E.; Alrowayeh, H.N.; Alshawaf, H. Telerehabilitation during the COVID-19 Pandemic: Patients and Physical Therapists’ Experiences. Med. Princ. Pr. 2022, 31, 156–164. [Google Scholar] [CrossRef]
- Rosta, L.; Menyhart, A.; Mahmeed, W.A.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; Cosentino, F.; et al. Telemedicine for Diabetes Management during COVID-19: What We Have Learnt, What and How to Implement. Front. Endocrinol. 2023, 14, 1129793. [Google Scholar] [CrossRef] [PubMed]
- De Kreutzenberg, S.V. Telemedicine for the Clinical Management of Diabetes; Implications and Considerations After COVID-19 Experience. High. Blood Press. Cardiovasc. Prev. 2022, 29, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.J.; Tarkington, P.E.; Bosworth, H.B.; Jeffreys, A.S.; Coffman, C.J.; Maciejewski, M.L.; Steinhauser, K.; Smith, V.A.; Dar, M.S.; Fredrickson, S.K.; et al. Effect of a Comprehensive Telehealth Intervention vs Telemonitoring and Care Coordination in Patients With Persistently Poor Type 2 Diabetes Control: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 943. [Google Scholar] [CrossRef]
- Agarwal, P.; Mukerji, G.; Desveaux, L.; Ivers, N.M.; Bhattacharyya, O.; Hensel, J.M.; Shaw, J.; Bouck, Z.; Jamieson, T.; Onabajo, N.; et al. Mobile App for Improved Self-Management of Type 2 Diabetes: Multicenter Pragmatic Randomized Controlled Trial. JMIR Mhealth Uhealth 2019, 7, e10321. [Google Scholar] [CrossRef]
- Esposito, S.; Sambati, V.; Fogliazza, F.; Street, M.E.; Principi, N. The Impact of Telemedicine on Pediatric Type 1 Diabetes Management: Benefits, Challenges, and Future Directions. Front. Endocrinol. 2024, 15, 1513166. [Google Scholar] [CrossRef]
- Eberle, C.; Stichling, S. Clinical Improvements by Telemedicine Interventions Managing Type 1 and Type 2 Diabetes: Systematic Meta-Review. J. Med. Internet Res. 2021, 23, e23244. [Google Scholar] [CrossRef]
- Eberle, C.; Stichling, S. Effect of Telemetric Interventions on Glycated Hemoglobin A1c and Management of Type 2 Diabetes Mellitus: Systematic Meta-Review. J. Med. Internet Res. 2021, 23, e23252. [Google Scholar] [CrossRef]
- Cox, N.S.; McDonald, C.F.; Mahal, A.; Alison, J.A.; Wootton, R.; Hill, C.J.; Zanaboni, P.; O’Halloran, P.; Bondarenko, J.; Macdonald, H.; et al. Telerehabilitation for Chronic Respiratory Disease: A Randomised Controlled Equivalence Trial. Thorax 2022, 77, 643–651. [Google Scholar] [CrossRef]
- Zanaboni, P.; Dinesen, B.; Hoaas, H.; Wootton, R.; Burge, A.T.; Philp, R.; Oliveira, C.C.; Bondarenko, J.; Tranborg Jensen, T.; Miller, B.R.; et al. Long-Term Telerehabilitation or Unsupervised Training at Home for Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2023, 207, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.; Bieler, T.; Beyer, N.; Kallemose, T.; Wilcke, J.T.; Østergaard, L.M.; Frost Andeassen, H.; Martinez, G.; Lavesen, M.; Frølich, A.; et al. Supervised Pulmonary Tele-Rehabilitation versus Pulmonary Rehabilitation in Severe COPD: A Randomised Multicentre Trial. Thorax 2020, 75, 413–421. [Google Scholar] [CrossRef]
- Cameron-Tucker, H.; Wood-Baker, R.; Joseph, L.; Walters, J.; Schuz, N.; Walters, E.H. A Randomized Controlled Trial of Telephone-Mentoring with Home-Based Walking Preceding Rehabilitation in COPD. COPD 2016, 11, 1991–2000. [Google Scholar] [CrossRef]
- Berkhof, F.F.; Van Den Berg, J.W.K.; Uil, S.M.; Kerstjens, H.A.M. Telemedicine, the Effect of Nurse-initiated Telephone Follow up, on Health Status and Health-care Utilization in COPD Patients: A Randomized Trial. Respirology 2015, 20, 279–285. [Google Scholar] [CrossRef]
- Nagatomi, Y.; Ide, T.; Higuchi, T.; Nezu, T.; Fujino, T.; Tohyama, T.; Nagata, T.; Higo, T.; Hashimoto, T.; Matsushima, S.; et al. Home-based Cardiac Rehabilitation Using Information and Communication Technology for Heart Failure Patients with Frailty. ESC Heart Fail. 2022, 9, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Wita, M.; Orszulak, M.; Szydło, K.; Wróbel, W.; Filipecki, A.; Simionescu, K.; Sanecki, K.; Uchwat, U.; Wybraniec, M.; Tabor, Z.; et al. The Usefulness of Telemedicine Devices in Patients with Severe Heart Failure with an Implanted Cardiac Resynchronization Therapy System during Two Years of Observation. Kardiol. Pol. 2022, 80, 41–48. [Google Scholar] [CrossRef]
- The BEAT-HF Research Group; Black, J.T.; Romano, P.S.; Sadeghi, B.; Auerbach, A.D.; Ganiats, T.G.; Greenfield, S.; Kaplan, S.H.; Ong, M.K. A Remote Monitoring and Telephone Nurse Coaching Intervention to Reduce Readmissions among Patients with Heart Failure: Study Protocol for the Better Effectiveness After Transition—Heart Failure (BEAT-HF) Randomized Controlled Trial. Trials 2014, 15, 124. [Google Scholar] [CrossRef]
- On behalf of the CardioBBEAT Investigators; Hofmann, R.; Völler, H.; Nagels, K.; Bindl, D.; Vettorazzi, E.; Dittmar, R.; Wohlgemuth, W.; Neumann, T.; Störk, S.; et al. First Outline and Baseline Data of a Randomized, Controlled Multicenter Trial to Evaluate the Health Economic Impact of Home Telemonitoring in Chronic Heart Failure—CardioBBEAT. Trials 2015, 16, 343. [Google Scholar] [CrossRef]
- Lundgren, K.M.; Langlo, K.A.R.; Salvesen, Ø.; Zanaboni, P.; Cittanti, E.; Mo, R.; Ellingsen, Ø.; Dalen, H.; Aksetøy, I.A. Feasibility of Telerehabilitation for Heart Failure Patients Inaccessible for Outpatient Rehabilitation. ESC Heart Fail. 2023, 10, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Real Time Remote Symptom Monitoring during Chemotherapy for Cancer: European Multicentre Randomised Controlled Trial (eSMART). BMJ 2021, 374, n2116. [CrossRef]
- Galiano-Castillo, N.; Cantarero-Villanueva, I.; Fernández-Lao, C.; Ariza-García, A.; Díaz-Rodríguez, L.; Del-Moral-Ávila, R.; Arroyo-Morales, M. Telehealth System: A Randomized Controlled Trial Evaluating the Impact of an Internet-based Exercise Intervention on Quality of Life, Pain, Muscle Strength, and Fatigue in Breast Cancer Survivors. Cancer 2016, 122, 3166–3174. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chan, C.K.Y.; Chua, S.S.; Ng, C.J.; Paraidathathu, T.; Lee, K.K.C.; Lee, S.W.H. Telemonitoring and Team-Based Management of Glycemic Control on People with Type 2 Diabetes: A Cluster-Randomized Controlled Trial. J. Gen. Intern. Med. 2020, 35, 87–94. [Google Scholar] [CrossRef]
- For the IN-TIME Study Group; Geller, J.C.; Lewalter, T.; Bruun, N.E.; Taborsky, M.; Bode, F.; Nielsen, J.C.; Stellbrink, C.; Schön, S.; Mühling, H.; et al. Implant-Based Multi-Parameter Telemonitoring of Patients with Heart Failure and a Defibrillator with vs. without Cardiac Resynchronization Therapy Option: A Subanalysis of the IN-TIME Trial. Clin. Res. Cardiol. 2019, 108, 1117–1127. [Google Scholar] [CrossRef]
- Thee, S.; Stahl, M.; Fischer, R.; Sutharsan, S.; Ballmann, M.; Müller, A.; Lorenz, D.; Urbanski-Rini, D.; Püschner, F.; Amelung, V.E.; et al. A Multi-Centre, Randomized, Controlled Trial on Coaching and Telemonitoring in Patients with Cystic Fibrosis: ConneCT CF. BMC Pulm. Med. 2021, 21, 131. [Google Scholar] [CrossRef]
- Deschildre, A.; Béghin, L.; Salleron, J.; Iliescu, C.; Thumerelle, C.; Santos, C.; Hoorelbeke, A.; Scalbert, M.; Pouessel, G.; Gnansounou, M.; et al. Home Telemonitoring (Forced Expiratory Volume in 1 s) in Children with Severe Asthma Does Not Reduce Exacerbations. Eur. Respir. J. 2012, 39, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Kapus, K.; Rárosi, F.; Novák, Z.; Peták, F.; Tolnai, J. Monitoring Respiratory Function with Telemedicine Devices in Asthmatic Children. Front. Med. 2025, 12, 1604909. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, E.; Jasionowska, A.; Banaszak-Bednarczyk, M.; Gwilkowska, J.; Piotrowicz, R. ECG Telemonitoring during Home-Based Cardiac Rehabilitation in Heart Failure Patients. J. Telemed. Telecare 2012, 18, 193–197. [Google Scholar] [CrossRef]
- Babar, M.; Zhu, D.; Loloi, J.; Laudano, M.; Ohmann, E.; Abraham, N.; Small, A.C.; Watts, K.L. Comparison of Patient Satisfaction and Safety Outcomes for Postoperative Telemedicine vs Face-to-Face Visits in Urology: Results of the Randomized Evaluation and Metrics Observing Telemedicine Efficacy (REMOTE) Trial. Urol. Pract. 2022, 9, 371–378. [Google Scholar] [CrossRef]
- Shin, C.; Allen, A.Z.; Zhu, D.; Tellechea, L.; Watts, K.L.; Abraham, N.E. Patient Satisfaction and Savings, and Clinical Outcomes of Televisits in Female Pelvic Medicine and Reconstructive Surgery at an Urban Academic Center. Neurourol. Urodyn. 2021, 40, 1834–1844. [Google Scholar] [CrossRef]
- Sohl, S.J.; Strahley, A.E.; Tooze, J.A.; Levine, B.J.; Kelly, M.G.; Wheeler, A.; Evans, S.; Danhauer, S.C. Qualitative Results from a Randomized Pilot Study of eHealth Mindful Movement and Breathing to Improve Gynecologic Cancer Surgery Outcomes. J. Psychosoc. Oncol. 2024, 42, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Lua-Mailland, L.L.; Nowacki, A.S.; Paraiso, M.F.R.; Park, A.J.; Wallace, S.L.; Ferrando, C.A. Virtual Compared With In-Office Postoperative Visits After Urogynecologic Surgery: A Randomized Controlled Trial. Obstet. Gynecol. 2024, 144, 562–572. [Google Scholar] [CrossRef]
- Lee, D.D.; Arya, L.A.; Andy, U.U.; Harvie, H.S. Video Virtual Clinical Encounters Versus Office Visits for Postoperative Care After Pelvic Organ Prolapse Surgery: A Randomized Clinical Trial. Female Pelvic Med. Reconstr. Surg. 2021, 27, 432–438. [Google Scholar] [CrossRef]
- Barnason, S.; Zimmerman, L.; Nieveen, J.; Schulz, P.; Miller, C.; Hertzog, M.; Tu, C. Influence of a Symptom Management Telehealth Intervention on Older Adults’ Early Recovery Outcomes after Coronary Artery Bypass Surgery. Heart Lung 2009, 38, 364–376. [Google Scholar] [CrossRef]
- Ferrara, P.E. The Optimization of the Post-Rehabilitation Process Heart Surgery: Our New Proposal Physiotherapy Record. Anatol. J. Cardiol. 2024, 28, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Bilbrey, T.; Martin, J.; Zhou, W.; Bai, C.; Vaswani, N.; Shah, R.; Chokshi, S.; Chen, X.; Bhusri, S.; Niemi, S.; et al. A Dual-Modality Home-Based Cardiac Rehabilitation Program for Adults With Cardiovascular Disease: Single-Arm Remote Clinical Trial. JMIR Mhealth Uhealth 2024, 12, e59098. [Google Scholar] [CrossRef]
- Nilsson, O.; Stenman, M.; Letterstål, A.; Hultgren, R. One-Year Results of an eHealth Intervention on Anxiety in Patients Undergoing Abdominal Aortic Aneurysm Surgery: Randomized Clinical Trial. BJS Open 2024, 9, zrae144. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Dupain, M.; Vu, A.; Jaffe, A.; Smith, K.; Fonda, H.; Dalman, R. Agreement Between Activity-Monitoring Devices During Home Rehabilitation: A Substudy of the AAA STOP Trial. J. Aging Phys. Act. 2014, 22, 87–95. [Google Scholar] [CrossRef]
- Farias, F.A.C.D.; Dagostini, C.M.; Bicca, Y.D.A.; Falavigna, V.F.; Falavigna, A. Remote Patient Monitoring: A Systematic Review. Telemed. E-Health 2020, 26, 576–583. [Google Scholar] [CrossRef] [PubMed]
- DeVito Dabbs, A.; Song, M.K.; Myers, B.A.; Li, R.; Hawkins, R.P.; Pilewski, J.M.; Bermudez, C.A.; Aubrecht, J.; Begey, A.; Connolly, M.; et al. A Randomized Controlled Trial of a Mobile Health Intervention to Promote Self-Management After Lung Transplantation. Am. J. Transplant. 2016, 16, 2172–2180. [Google Scholar] [CrossRef]
- Sengpiel, J.; Fuehner, T.; Kugler, C.; Avsar, M.; Bodmann, I.; Boemke, A.; Simon, A.; Welte, T.; Gottlieb, J. Use of Telehealth Technology for Home Spirometry after Lung Transplantation: A Randomized Controlled Trial. Prog. Transpl. 2010, 20, 310–317. [Google Scholar] [CrossRef]
- Ha, D.M.; Comer, A.; Dollar, B.; Bedoy, R.; Ford, M.; Gozansky, W.S.; Zeng, C.; Arch, J.J.; Leach, H.J.; Malhotra, A.; et al. Telemedicine-Based Inspiratory Muscle Training and Walking Promotion with Lung Cancer Survivors Following Curative Intent Therapy: A Parallel-Group Pilot Randomized Trial. Support. Care Cancer 2023, 31, 546. [Google Scholar] [CrossRef]
- Li, G.; Zhou, X.; Deng, J.; Wang, J.; Ai, P.; Zeng, J.; Ma, X.; Liao, H. Digital Therapeutics–Based Cardio-Oncology Rehabilitation for Lung Cancer Survivors: Randomized Controlled Trial. JMIR Mhealth Uhealth 2025, 13, e60115. [Google Scholar] [CrossRef]
- Seron, P.; Oliveros, M.-J.; Gutierrez-Arias, R.; Fuentes-Aspe, R.; Torres-Castro, R.C.; Merino-Osorio, C.; Nahuelhual, P.; Inostroza, J.; Jalil, Y.; Solano, R.; et al. Effectiveness of Telerehabilitation in Physical Therapy: A Rapid Overview. Phys. Ther. 2021, 101, pzab053. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liang, Q.; Yang, W.-J.; Zi, R.; Wu, X.; Du, C.; Jiang, Y. Effects of Tele-Exercise Rehabilitation Intervention on Women at High Risk of Osteoporotic Fractures: Study Protocol for a Randomised Controlled Trial. BMJ Open 2022, 12, e064328. [Google Scholar] [CrossRef]
- Cedeno-Veloz, B.A.; Casadamon-Munarriz, I.; Rodríguez-García, A.; Lozano-Vicario, L.; Zambom-Ferraresi, F.; Gonzalo-Lázaro, M.; Hidalgo-Ovejero, Á.M.; Izquierdo, M.; Martínez-Velilla, N. Effect of a Multicomponent Intervention with Tele-Rehabilitation and the Vivifrail© Exercise Programme on Functional Capacity after Hip Fracture: Study Protocol for the ActiveFLS Randomized Controlled Trial. JCM 2023, 13, 97. [Google Scholar] [CrossRef]
- Mora-Traverso, M.; Molina-Garcia, P.; Prieto-Moreno, R.; Borges-Cosic, M.; Cruz Guisado, V.; Del Pino Algarrada, R.; Moreno-Ramírez, P.; Gomez-Jurado, G.; Gomez Tarrias, C.; Hidalgo Isla, M.; et al. An m-Health Telerehabilitation and Health Education Program on Physical Performance in Patients with Hip Fracture and Their Family Caregivers: Study Protocol for the ActiveHip+ Randomized Controlled Trial. Res. Nurs. Health 2022, 45, 287–299. [Google Scholar] [CrossRef]
- Anton, D.; Berges, I.; Bermúdez, J.; Goñi, A.; Illarramendi, A. A Telerehabilitation System for the Selection, Evaluation and Remote Management of Therapies. Sensors 2018, 18, 1459. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, Y.; Maeir, T.; Karni, S.; Eisenberg, M.E.; Liebergall, M.; Schwartz, I.; Kaufman, Y. Effectiveness of a Tele-Rehabilitation Intervention to Improve Performance and Reduce Morbidity for People Post Hip Fracture—Study Protocol for a Randomized Controlled Trial. BMC Geriatr. 2019, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Aydin, M.; Candelise, C.; Coda, N.; Morone, G.; Antonucci, G.; Marinozzi, F.; Bini, F.; Paolucci, S.; Tieri, G. The Michelangelo Effect: Art Improves the Performance in a Virtual Reality Task Developed for Upper Limb Neurorehabilitation. Front. Psychol. 2021, 11, 611956. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.N.; Foreman, N.; Tlauka, M. Transfer of Spatial Information from a Virtual to a Real Environment in Physically Disabled Children. Disabil. Rehabil. 1996, 18, 633–637. [Google Scholar] [CrossRef]
- Lorusso, M.L.; Travellini, S.; Giorgetti, M.; Negrini, P.; Reni, G.; Biffi, E. Semi-Immersive Virtual Reality as a Tool to Improve Cognitive and Social Abilities in Preschool Children. Appl. Sci. 2020, 10, 2948. [Google Scholar] [CrossRef]
- Chirayath, A.; Dhaniwala, N.; Kawde, K. A Comprehensive Review on Managing Fracture Calcaneum by Surgical and Non-Surgical Modalities. Cureus 2024, 16, e54786. [Google Scholar] [CrossRef] [PubMed]
- Horoz, L.; Çiğdem Karaçay, B.; Ceylan, İ.; Fevzi Çakmak, M. Is Home-Based Real-Time Video Conferencing Telerehabilitation as Effective as Conventional Face-to-Face Rehabilitation in Patients with Operated for Distal Radius Fracture? A Single-Blind, Randomized Prospective Study. Turk. J. Phys. Med. Rehab 2024, 70, 506–516. [Google Scholar] [CrossRef]
- Grivicich Da Silva, G.; Xavier De Araújo, F.; Andriola, A.H.; Pereira, G.C.; Silva, M.F. Telerehabilitation on the Physical and Functional Capacity of Traumatic Fractures of the Upper Limbs: A Systematic Review with Meta-Analysis. Int. J. Telerehab 2025, 17, 6667. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Kanosue, K. Changes in Brain Activity during Action Observation and Motor Imagery: Their Relationship with Motor Learning. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 234, pp. 189–204. ISBN 978-0-12-811825-2. [Google Scholar]
- Limakatso, K.; Madden, V.J.; Manie, S.; Parker, R. The Effectiveness of Graded Motor Imagery for Reducing Phantom Limb Pain in Amputees: A Randomised Controlled Trial. Physiotherapy 2020, 109, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Borges, L.R.; Santiago, L.; Lucena, L.; Lindquist, A.R.; Ribeiro, T. Motor Imagery for Gait Rehabilitation after Stroke. Cochrane Database Syst. Rev. 2020, 9, CD013019. [Google Scholar] [CrossRef]
- Bowering, K.J.; O’Connell, N.E.; Tabor, A.; Catley, M.J.; Leake, H.B.; Moseley, G.L.; Stanton, T.R. The Effects of Graded Motor Imagery and Its Components on Chronic Pain: A Systematic Review and Meta-Analysis. J. Pain. 2013, 14, 3–13. [Google Scholar] [CrossRef]
- Cuomo, G.; Maglianella, V.; Ghanbari Ghooshchy, S.; Zoccolotti, P.; Martelli, M.; Paolucci, S.; Morone, G.; Iosa, M. Motor Imagery and Gait Control in Parkinson’s Disease: Techniques and New Perspectives in Neurorehabilitation. Expert. Rev. Neurother. 2022, 22, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Ae, K.; Soma, H.; Miyata, K.; Kajita, K.; Kawamura, T. Motor Imagery Ability in Baseball Players with Throwing Yips. PLoS ONE 2023, 18, e0292632. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Brass, M. How Kinesthetic Motor Imagery Works: A Predictive-Processing Theory of Visualization in Sports and Motor Expertise. J. Physiol. 2015, 109, 53–63. [Google Scholar] [CrossRef]
- Breckenridge, J.D.; Ginn, K.A.; Wallwork, S.B.; McAuley, J.H. Do People With Chronic Musculoskeletal Pain Have Impaired Motor Imagery? A Meta-Analytical Systematic Review of the Left/Right Judgment Task. J. Pain 2019, 20, 119–132. [Google Scholar] [CrossRef]
- Pastora-Bernal, J.M.; Estebanez-Pérez, M.J.; Lucena-Anton, D.; García-López, F.J.; Bort-Carballo, A.; Martín-Valero, R. The Effectiveness and Recommendation of Motor Imagery Techniques for Rehabilitation after Anterior Cruciate Ligament Reconstruction: A Systematic Review. JCM 2021, 10, 428. [Google Scholar] [CrossRef]
- Kalaycı, M.G.; Analay Akbaba, Y.; Güven, M.F. The Effect of Motor Imagery on Functionality, Pain, Kinesiophobia, and Quality of Life in Patients with Distal Radius Fractures: A Randomized Controlled Double-Blind Study. J. Hand Ther. 2025, S0894113025000456, in press. [Google Scholar] [CrossRef]
- Madanian, S.; Nakarada-Kordic, I.; Reay, S.; Chetty, T. Patients’ Perspectives on Digital Health Tools. PEC Innov. 2023, 2, 100171. [Google Scholar] [CrossRef]
- Muñoz-Tomás, M.T.; Burillo-Lafuente, M.; Vicente-Parra, A.; Sanz-Rubio, M.C.; Suarez-Serrano, C.; Marcén-Román, Y.; Franco-Sierra, M.Á. Telerehabilitation as a Therapeutic Exercise Tool versus Face-to-Face Physiotherapy: A Systematic Review. IJERPH 2023, 20, 4358. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Lin, X.; Han, Z.; Feng, Z.; Hua, X.; Chen, D.; Xu, X.; Zhang, Y.; Wang, G.; et al. Intelligent Rehabilitation Assistance Tools for Distal Radius Fracture: A Systematic Review Based on Literatures and Mobile Application Stores. Comput. Math. Methods Med. 2020, 2020, 7613569. [Google Scholar] [CrossRef]
- Matijevich, E.S.; Scott, L.R.; Volgyesi, P.; Derry, K.H.; Zelik, K.E. Combining Wearable Sensor Signals, Machine Learning and Biomechanics to Estimate Tibial Bone Force and Damage during Running. Hum. Mov. Sci. 2020, 74, 102690. [Google Scholar] [CrossRef] [PubMed]
- Elstub, L.J.; Nurse, C.A.; Grohowski, L.M.; Volgyesi, P.; Wolf, D.N.; Zelik, K.E. Tibial Bone Forces Can Be Monitored Using Shoe-Worn Wearable Sensors during Running. J. Sports Sci. 2022, 40, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Nurse, C.A.; Rodzak, K.M.; Volgyesi, P.; Noehren, B.; Zelik, K.E. Using Fitness Tracker Data to Overcome Pressure Insole Wear Time Challenges for Remote Musculoskeletal Monitoring. Sensors 2024, 24, 7717. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Lindström, B.; Gard, G.; Lysholm, J. Physiotherapy at a Distance: A Controlled Study of Rehabilitation at Home after a Shoulder Joint Operation. J. Telemed. Telecare 2009, 15, 215–220. [Google Scholar] [CrossRef]
- Nelson, M.; Bourke, M.; Crossley, K.; Russell, T. Telerehabilitation Is Non-Inferior to Usual Care Following Total Hip Replacement—A Randomized Controlled Non-Inferiority Trial. Physiotherapy 2020, 107, 19–27. [Google Scholar] [CrossRef]
- Nelson, M.; Russell, T.; Crossley, K.; Bourke, M.; McPhail, S. Cost-Effectiveness of Telerehabilitation versus Traditional Care after Total Hip Replacement: A Trial-Based Economic Evaluation. J. Telemed. Telecare 2021, 27, 359–366. [Google Scholar] [CrossRef]
- Buvik, A.; Bergmo, T.S.; Bugge, E.; Smaabrekke, A.; Wilsgaard, T.; Olsen, J.A. Cost-Effectiveness of Telemedicine in Remote Orthopedic Consultations: Randomized Controlled Trial. J. Med. Internet Res. 2019, 21, e11330. [Google Scholar] [CrossRef] [PubMed]
- Ohinmaa, A.; Vuolio, S.; Haukipuro, K.; Winblad, I. A Cost-Minimization Analysis of Orthopaedic Consultations Using Videoconferencing in Comparison with Conventional Consulting. J. Telemed. Telecare 2002, 8, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Moreno, R.; Estévez-López, F.; Molina-Garcia, P.; Mora-Traverso, M.; Deschamps, K.; Claeys, K.; De Buyser, J.; Ariza-Vega, P. ActiveHip+: A Feasible mHealth System for the Recovery of Older Adults after Hip Surgery during the COVID-19 Pandemic. Digit. Health 2022, 8, 205520762211396. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Moreno, R.; Mora-Traverso, M.; Estévez-López, F.; Molina-Garcia, P.; Ortiz-Piña, M.; Salazar-Graván, S.; Cruz-Guisado, V.; Gago, M.L.; Martín-Matillas, M.; Ariza-Vega, P. Effects of the ActiveHip+ mHealth Intervention on the Recovery of Older Adults with Hip Fracture and Their Family Caregivers: A Multicentre Open-Label Randomised Controlled Trial. eClinicalMedicine 2024, 73, 102677. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.; Zhang, Y.; Peng, B.; Xu, W. Clinical Effectiveness of Home-Based Telerehabilitation Program for Geriatric Hip Fracture Following Total Hip Replacement. Orthop. Surg. 2023, 15, 423–431. [Google Scholar] [CrossRef]
- Li, C.T.; Hung, G.K.; Fong, K.N.; Gonzalez, P.C.; Wah, S.; Tsang, H.W. Effects of Home-Based Occupational Therapy Telerehabilitation via Smartphone for Outpatients after Hip Fracture Surgery: A Feasibility Randomised Controlled Study. J. Telemed. Telecare 2022, 28, 239–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, Z.; Li, S.; Xu, W. Effect of Home-based Telerehabilitation on the Postoperative Rehabilitation Outcome of Hip Fracture in the Aging Population. Orthop. Surg. 2022, 14, 1768–1777. [Google Scholar] [CrossRef]
- Pliannuom, S.; Pinyopornpanish, K.; Buawangpong, N.; Wiwatkunupakarn, N.; Mallinson, P.A.C.; Jiraporncharoen, W.; Angkurawaranon, C. Characteristics and Effects of Home-Based Digital Health Interventions on Functional Outcomes in Older Patients With Hip Fractures After Surgery: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2024, 26, e49482. [Google Scholar] [CrossRef]
- Zhou, Y.; Lyu, Y.; Wang, Q.; Ma, Y.; Huang, L.; Zhang, X. Mobile-Based in-Home Telerehabilitation Compared with in-Hospital Face-to-Face Rehabilitation for Elderly Patients after Total Hip Arthroplasty in China’s Level 1 Trauma Center: A Noninferiority Randomized Controlled Trial. Front. Surg. 2025, 11, 1536579. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Zhang, R.; He, C. Emerging Trends and Prospects in Telerehabilitation for Hip Fracture: Bibliometric and Visualization Study. Digit. Health 2024, 10, 20552076241255465. [Google Scholar] [CrossRef]
- Pol, M.C.; Ter Riet, G.; Van Hartingsveldt, M.; Kröse, B.; Buurman, B.M. Effectiveness of Sensor Monitoring in a Rehabilitation Programme for Older Patients after Hip Fracture: A Three-Arm Stepped Wedge Randomised Trial. Age Ageing 2019, 48, 650–657. [Google Scholar] [CrossRef]
- Fernández-González, M.; Lozano-Lozano, M.; Martín-Martín, L.; Ortiz-Piña, M.; Martín-Matillas, M.; Ariza-Vega, P. Is a Telerehabilitation Programme for Older Adults with Hip Fracture Associated with Burden of Family Caregivers Who Provide Support? Digit. Health 2023, 9, 20552076231213574. [Google Scholar] [CrossRef]
- Mora-Traverso, M.; Prieto-Moreno, R.; Molina-Garcia, P.; Salas-Fariña, Z.; Martín-Martín, L.; Martín-Matillas, M.; Ariza-Vega, P. Effects of the @ctivehip Telerehabilitation Program on the Quality of Life, Psychological Factors and Fitness Level of Patients with Hip Fracture. J. Telemed. Telecare 2024, 30, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zeng, W.; Lu, L.; Yuan, J.; Yan, Z.; Wang, J. Effectiveness of Telerehabilitation in Postoperative Outcomes in Patients on Hip Fracture Surgery: A Meta-Analysis of Randomized Controlled Trials. BMC Sports Sci. Med. Rehabil. 2025, 17, 130. [Google Scholar] [CrossRef]
- Bramanti, A.; Ciurleo, R.; Vecchione, C.; Turolla, A.; Piramide, N.; Ciccarelli, M.; Piramide, E.; Garofano, M. Telerehabilitation: A Solution for Patients after Hip Fracture? Transl. Med. @ UniSa 2024, 26, 3–37. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Piña, M.; Molina-Garcia, P.; Femia, P.; Ashe, M.C.; Martín-Martín, L.; Salazar-Graván, S.; Salas-Fariña, Z.; Prieto-Moreno, R.; Castellote-Caballero, Y.; Estevez-Lopez, F.; et al. Effects of Tele-Rehabilitation Compared with Home-Based in-Person Rehabilitation for Older Adult’s Function after Hip Fracture. IJERPH 2021, 18, 5493. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Piña, M.; Salas-Fariña, Z.; Mora-Traverso, M.; Martín-Martín, L.; Galiano-Castillo, N.; García-Montes, I.; Cantarero-Villanueva, I.; Fernández-Lao, C.; Arroyo-Morales, M.; Mesa-Ruíz, A.; et al. A Home-based Tele-rehabilitation Protocol for Patients with Hip Fracture Called @ctivehip. Res. Nurs. Health 2019, 42, 29–38. [Google Scholar] [CrossRef]
- Amin, J.; Ahmad, B.; Amin, S.; Siddiqui, A.A.; Alam, M.K. Rehabilitation Professional and Patient Satisfaction with Telerehabilitation of Musculoskeletal Disorders: A Systematic Review. BioMed Res. Int. 2022, 2022, 7366063. [Google Scholar] [CrossRef]
- Tsuge, T.; Yamamoto, N.; Taito, S.; Miura, T.; Shiratsuchi, D.; Yorifuji, T. Efficacy of Telerehabilitation for Patients after Hip Fracture Surgery: A Systematic Review and Meta-Analysis. J. Telemed. Telecare 2025, 31, 174–183. [Google Scholar] [CrossRef]
- Lin, W.-T.M.; Lin, B.-S.; Lee, I.-J.; Lee, S.-H. Development of a Smartphone-Based mHealth Platform for Telerehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Crotty, M.; Killington, M.; Van Den Berg, M.; Morris, C.; Taylor, A.; Carati, C. Telerehabilitation for Older People Using Off-the-Shelf Applications: Acceptability and Feasibility. J. Telemed. Telecare 2014, 20, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Pastora-Bernal, J.M.; Martín-Valero, R.; Barón-López, F.J.; Estebanez-Pérez, M.J. Evidence of Benefit of Telerehabitation After Orthopedic Surgery: A Systematic Review. J. Med. Internet Res. 2017, 19, e142. [Google Scholar] [CrossRef] [PubMed]
- Bedra, M.; Finkelstein, J. Feasibility of Post-Acute Hip Fracture Telerehabilitation in Older Adults. Stud. Health Technol. Inf. 2015, 210, 469–473. [Google Scholar]
- Dillon, M.; Ridgewell, E.; Clarke, L.; Bishop, K.; Kumar, S. Exploration of the Barriers and Facilitators Influencing Use of Telehealth for Orthotic/Prosthetic Services in the United States of America: An Orthotist/Prosthetists Perspective. PLoS ONE 2024, 19, e0309194. [Google Scholar] [CrossRef]
- Phuphanich, M.E.; Sinha, K.R.; Truong, M.; Pham, Q.G. Telemedicine for Musculoskeletal Rehabilitation and Orthopedic Postoperative Rehabilitation. Phys. Med. Rehabil. Clin. North. Am. 2021, 32, 319–353. [Google Scholar] [CrossRef]
- Wongworawat, M.D.; Capistrant, G.; Stephenson, J.M. The Opportunity Awaits to Lead Orthopaedic Telehealth Innovation: AOA Critical Issues. J. Bone Jt. Surg. 2017, 99, e93. [Google Scholar] [CrossRef]
- Kamecka, K.; Rybarczyk-Szwajkowska, A.; Staszewska, A.; Engelseth, P.; Kozlowski, R. Process of Posthospital Care Involving Telemedicine Solutions for Patients after Total Hip Arthroplasty. IJERPH 2021, 18, 10135. [Google Scholar] [CrossRef]
- Ouendi, N.; Avril, E.; Dervaux, B.; Pudlo, P.; Wallard, L. Telerehabilitation in the Remote Care of Patients’ Post-Orthopaedic Surgery: Benefits and Limitations for Patients. J. Telemed. Telecare, 2025; ahead of print. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Gregersen, M.; Pallesen, S.H.; Damsgaard, E.M. A Group-Based Real-Time Videoconferencing Telerehabilitation Programme in Recently Discharged Geriatric Patients: A Feasibility Study. Eur. Geriatr. Med. 2021, 12, 801–808. [Google Scholar] [CrossRef]
- Ariza-Vega, P.; Prieto-Moreno, R.; Castillo-Pérez, H.; Martínez-Ruiz, V.; Romero-Ayuso, D.; Ashe, M.C. Family Caregivers’ Experiences with Tele-Rehabilitation for Older Adults with Hip Fracture. JCM 2021, 10, 5850. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).