The Role of the Oral Microbiome and Dental Caries in Respiratory Health: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Protocol
2.3. Eligibility Criteria
- Investigated associations between oral and respiratory diseases;
- Were published in English;
- Represented prospective case series, non-randomized controlled studies (NRS), or randomized controlled trials (RCTs).
- Lack of a clear association between oral and respiratory conditions;
- Non-English language publications;
- Case or clinical reports;
- Opinion papers, editorials, or narrative reviews;
- Absence of full-text availability;
- Duplicate publications.
2.4. Information Sources, Search Strategy, and Study Selection
2.5. Data Collection Process and, Data Items
2.6. Risk of Bias Assessment in Individual Studies
2.7. Quality Assessment
- 1
- Is there an adequate rationale for using a mixed-methods design to address the research question?
- 2
- Are the different components of the study effectively integrated to answer the research question?
- 3
- Are the outputs of the integration of qualitative and quantitative components adequately interpreted?
- 4
- Are divergences and inconsistencies between quantitative and qualitative results adequately addressed?
- 5
- Do the different components of the study adhere to the quality criteria of each methodological tradition involved?
3. Results
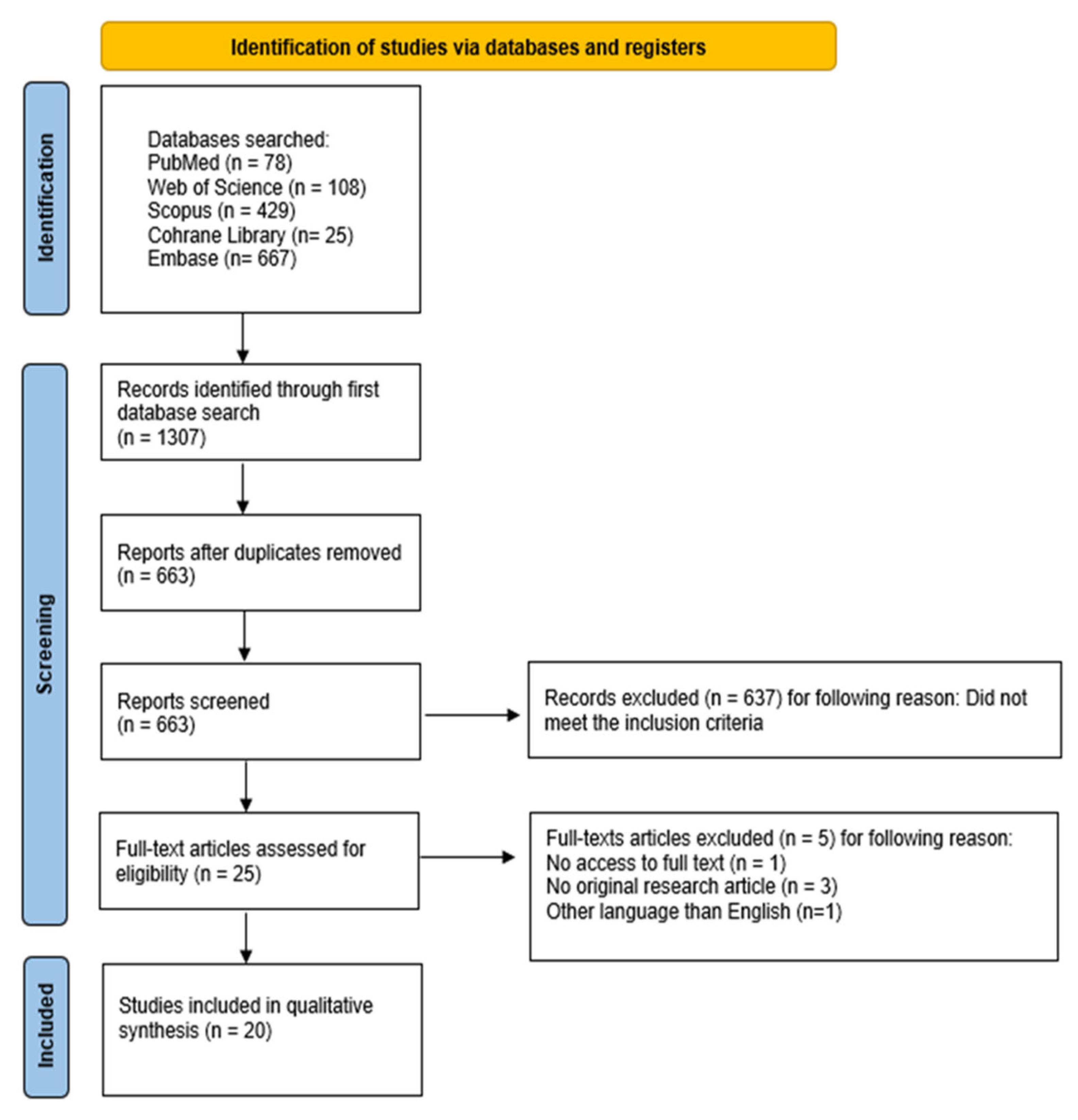
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Main Findings
3.3.1. Poor Oral Health and Increased Risk of Respiratory Infections
3.3.2. Oral Microbiome Dysbiosis as a Reservoir for Respiratory Pathogens
3.3.3. Periodontal Disease and Lung Disease: Microbiological and Clinical Link
3.3.4. Pharmacotherapy for Respiratory Diseases and Its Impact on the Oral Microbiome and Caries Development
3.3.5. Shared Colonization Between the Oral Cavity and Respiratory Tract
3.3.6. Oral Hygiene Interventions Reduce Respiratory Infection Risk
3.3.7. Hospital and ICU Outcomes
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dong, J.; Li, W.; Wang, Q.; Chen, J.; Zu, Y.; Zhou, X.; Guo, Q. Relationships Between Oral Microecosystem and Respiratory Diseases. Front. Mol. Biosci. 2022, 8, 718222. [Google Scholar] [CrossRef]
- Gupta, A.; Saleena, L.M.; Kannan, P.; Shivachandran, A. The Impact of Oral Diseases on Respiratory Health and the Influence of Respiratory Infections on the Oral Microbiome. J. Dent. 2024, 148, 105213. [Google Scholar] [CrossRef]
- Chestnutt, I.G. Is Dental Caries Associated with Lower Respiratory Tract Infections? Evid. Based Dent. 2020, 21, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.B.; Harner, A.; Buhay, M.; Firek, B.; Methé, B.; Morris, A.; Peck Palmer, O.M.; Promes, S.B.; Sherwin, R.L.; Southerland, L.; et al. The Salivary Microbiota of Patients with Acute Lower Respiratory Tract Infection–A Multicenter Cohort Study. PLoS ONE 2024, 19, e0290062. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The Role of Oral Microbiome in Respiratory Health and Diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Kim, K.H.; Lee, Y.M.; Ku, Y.; Seol, Y.J. Oral Microbiome and Host Health: Review on Current Advances in Genome-Wide Analysis. Appl. Sci. 2021, 11, 4050. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- ArumugaNainar, D.; Krishna Naik, V.; Ramadoss, R.; Balasundaram, A.; Victor, D.J. Evaluation of CD44 Antigen in Type 2 Diabetic Patients with Periodontitis: An Immunohistochemical Study. Dent. Med. Probl. 2024, 61, 225–231. [Google Scholar] [CrossRef]
- Narendran, N.; Shenoy, S.; Kodangala, S.; Vamsi, A.R.; Kamath, V. Assessment of Myocardial Strain in Hypertensive Patients with Periodontitis. Dent. Med. Probl. 2023, 60, 61–69. [Google Scholar] [CrossRef]
- Biria, M.; Sattari, M.; Iranparvar, P.; Eftekhar, L. Relationship between the Salivary Concentrations of Proteinase-3 and Interleukin-8 and Severe Early Childhood Caries. Dent. Med. Probl. 2023, 60, 577–582. [Google Scholar] [CrossRef]
- Albelali, A.; Wu, T.T.; Malmstrom, H.; Xiao, J. Early Childhood Caries Experience Associated with Upper Respiratory Infection in US Children: Findings from a Retrospective Cohort Study. J. Pediatr. Child. Health Care 2021, 6, 1044. [Google Scholar] [CrossRef] [PubMed]
- Mehtonen, I.T.; Rantala, A.K.; Hugg, T.T.; Jaakkola, M.S.; Jaakkola, J.J.K. Dental Caries Is Associated with Lower Respiratory Tract Infections: A Population-Based Cohort Study. Respir. Med. 2019, 158, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Kadowaki, T.; Tsukahara, H.; Yorifuji, T. Association between Dental Caries and Influenza Infection in Children: A Japanese Nationwide Population-Based Study. Children 2021, 8, 780. [Google Scholar] [CrossRef] [PubMed]
- Ucuncu, M.Y.; Topcuoglu, N.; Kulekci, G.; Ucuncu, M.K.; Erelel, M.; Gokce, Y.B. A Comparative Evaluation of the Effects of Respiratory Diseases on Dental Caries. BMC Oral Health 2024, 24, 13. [Google Scholar] [CrossRef]
- Liu, X.; Shi, F.; Zeng, J.; Bi, J.; Mo, C.; Chai, Y.; Wu, B.; Xu, S. Oral Microbiota and Respiratory Diseases: Advances and Perspectives. Clin. Microbiol. Rev. 2025, 38, e00150-24. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, S.; Li, K.Y.; Lo, E.C.M.; Gao, X. Association between Oral Health and Upper Respiratory Tract Infection among Children. Int. Dent. J. 2018, 68, 122–128. [Google Scholar] [CrossRef]
- WHO. Global Oral Health Status Report, 2025 Citation: World Health Organization. Tracking Progress on the Implementation of the Global Oral Health Action Plan 2023–2030: Baseline Report. 2025. Available online: https://www.who.int/publications/i/item/9789240106031 (accessed on 22 February 2025).
- Matys, J.; Kensy, J.; Gedrange, T.; Zawiślak, I.; Grzech-Leśniak, K.; Dobrzyński, M. A Molecular Approach for Detecting Bacteria and Fungi in Healthcare Environment Aerosols: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4154. [Google Scholar] [CrossRef]
- Pu, C.Y.; Seshadri, M.; Manuballa, S.; Yendamuri, S. The Oral Microbiome and Lung Diseases. Curr. Oral Health Rep. 2020, 7, 79–86. [Google Scholar] [CrossRef]
- Ramesh, D.; Jagadeeswari, S. Association of Recurrent Respiratory Infections with Poor Dental Hygiene. IOSR J. Dent. Med. Sci. (IOSR-JDMS) 2018, 17, 36–38. [Google Scholar] [CrossRef]
- Dobrzynski, M.; Pajaczkowska, M.; Nowicka, J.; Jaworski, A.; Kosior, P.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Bogucki, Z.A.; Filipiak, J.; et al. Study of Surface Structure Changes for Selected Ceramics Used in the CAD/CAM System on the Degree of Microbial Colonization, in Vitro Tests. Biomed. Res. Int. 2019, 2019, 9130806. [Google Scholar] [CrossRef]
- Elchaghaby, M.A.; Rashad, S.; Yousry, Y.M. Inhibitory Effect of Silver Nanoparticles Synthesized Using the Chamomile Extract against Streptococcus Mutans Cariogenic Pathogen. Dent. Med. Probl. 2023, 60, 483–488. [Google Scholar] [CrossRef]
- Mehdipour, A.; Fateh, R.; Fuladvand, F.; Aghaali, M.; Keykha, E.; Hadilou, M. Association between Sleep Pattern, Salivary Cariogenic Bacteria and Fungi Populations, PH and Buffering Capacity in Children: A Comparative Study. Dent. Med. Probl. 2024, 61, 217–224. [Google Scholar] [CrossRef]
- Matys, J.; Gedrange, T.; Dominiak, M.; Grzech-Leśniak, K. Quantitative Evaluation of Aerosols Produced in the Dental Office during Caries Treatment: A Randomized Clinical Trial. J. Clin. Med. 2023, 12, 4597. [Google Scholar] [CrossRef] [PubMed]
- Katebi, K.; Ashkannejhad, S.; Mahboobi, Z.; Faramarzi, E.; Sharififard, N. The Relationship between Dental Caries with Asthma, Disease Duration, and Type of Medications in the Azar Cohort Population. BMC Oral Health 2024, 24, 1511. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, Q.; Yan, G.; Yang, D. The Oral Microbiome and Salivary Proteins Influence Caries in Children Aged 6 to 8 Years. BMC Oral Health 2020, 20, 295. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Imai, K.; Iinuma, T.; Sato, S. Relationship between the Oral Cavity and Respiratory Diseases: Aspiration of Oral Bacteria Possibly Contributes to the Progression of Lower Airway Inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 224–230. [Google Scholar] [CrossRef]
- Matys, J.; Grzech-Leśniak, K. Dental Aerosol as a Hazard Risk for Dental Workers. Materials 2020, 13, 5109. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; Cruz, S.S.D.; Trindade, S.C.; Passos-Soares, J.S.; Carvalho-Filho, P.C.; Figueiredo, A.C.M.G.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis. 2020, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef]
- Kensy, J.; Dobrzyński, M.; Wiench, R.; Grzech-Leśniak, K.; Matys, J. Fibroblasts Adhesion to Laser-Modified Titanium Surfaces—A Systematic Review. Materials 2021, 14, 7305. [Google Scholar] [CrossRef] [PubMed]
- Małyszek, A.; Kiryk, S.; Kensy, J.; Kotela, A.; Michalak, M.; Kiryk, J.; Janeczek, M.; Matys, J.; Dobrzyński, M. Identification of Factors Influencing Fluoride Content in Tea Infusions: A Systematic Review. Appl. Sci. 2025, 15, 5974. [Google Scholar] [CrossRef]
- Rygas, J.; Matys, J.; Wawrzyńska, M.; Szymonowicz, M.; Dobrzyński, M. The Use of Graphene Oxide in Orthodontics—A Systematic Review. J. Funct. Biomater. 2023, 14, 500. [Google Scholar] [CrossRef]
- Rajewska, J.; Kowalski, J.; Matys, J.; Dobrzyński, M.; Wiglusz, R.J. The Use of Lactide Polymers in Bone Tissue Regeneration in Dentistry—A Systematic Review. J. Funct. Biomater. 2023, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The Influence of Fluoride Gels on the Physicochemical Properties of Tooth Tissues and Dental Materials—A Systematic Review. Gels 2024, 10, 98. [Google Scholar] [CrossRef]
- Dobrzyński, W.; Piszko, P.J.; Kiryk, J.; Kiryk, S.; Michalak, M.; Kotela, A.; Kensy, J.; Świenc, W.; Grychowska, N.; Matys, J.; et al. Dental Resin Composites Modified with Chitosan: A Systematic Review. Mar. Drugs 2025, 23, 199. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Bellissimo-Rodrigues, W.T.; Menegueti, M.G.; Gaspar, G.G.; Nicolini, E.A.; Auxiliadora-Martins, M.; Basile-Filho, A.; Martinez, R.; Bellissimo-Rodrigues, F. Effectiveness of a Dental Care Intervention in the Prevention of Lower Respiratory Tract Nosocomial Infections among Intensive Care Patients: A Randomized Clinical Trial. Infect. Control Hosp. Epidemiol. 2014, 35, 1342–1348. [Google Scholar] [CrossRef]
- Winning, L.; Moran, G.; McClory, M.; El Karim, I.; Lundy, F.T.; Patterson, C.C.; Linden, D.; Cullen, K.M.; Kee, F.; Linden, G.J. Subgingival Microbial Diversity and Respiratory Decline: A Cross-Sectional Study. J. Clin. Periodontol. 2023, 50, 921–931. [Google Scholar] [CrossRef]
- Rantala, A.K.; Mehtonen, I.T.; Jaakkola, M.S.; Näyhä, S.; Hugg, T.T.; Jaakkola, J.J.K. Early Respiratory Infections and Dental Caries in the First 27 Years of Life: A Population-Based Cohort Study. PLoS ONE 2016, 11, e0168141. [Google Scholar] [CrossRef] [PubMed]
- Shirazian, S.; Manifar, S.; Gharabaghi, M.A.; Keshvari, Z.; Bahrami, N. Comparison of Oral Disease Manifestation in Patients with Pulmonary Disease and Healthy Group. Minerva Pneumol. 2018, 57, 27–35. [Google Scholar] [CrossRef]
- Ploenes, T.; Pollok, A.; Jöckel, K.H.; Kampe, S.; Darwiche, K.; Taube, C.; Buer, J.; Aigner, C. The Pathological Oral Cavity as a Preventable Source of Postoperative Pneumonia in Thoracic Surgery: A Prospective Observational Study. J. Thorac. Dis. 2022, 14, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Arweiler, N.B.; Rahmel, V.; Alashkar Alhamwe, B.; Alhamdan, F.; Zemlin, M.; Boutin, S.; Dalpke, A.; Renz, H. Dental Biofilm and Saliva Microbiome and Its Interplay with Pediatric Allergies. Microorganisms 2021, 9, 1330. [Google Scholar] [CrossRef]
- Cherkasov, S.V.; Popova, L.Y.; Vivtanenko, T.V.; Demina, R.R.; Khlopko, Y.A.; Balkin, A.S.; Plotnikov, A.O. Oral Microbiomes in Children with Asthma and Dental Caries. Oral Dis. 2019, 25, 898–910. [Google Scholar] [CrossRef]
- Wang, D.H.; Tsai, F.T.; Tu, H.F.; Yang, C.C.; Hsu, M.L.; Huang, L.J.; Lin, C.T.; Hsu, W.E.; Lin, Y.C. Profiles of Oral Microbiome Associated with Nasogastric Tube Feeding. J. Oral Microbiol. 2023, 15, 2200898. [Google Scholar] [CrossRef]
- Al-Fahham, H.R.A.; Hussin, H.A.; Kareem, K.R.; Motaweq, Z.Y. Molecular Detection of FimH Gene Isolated from Klebsiella Pneumoniae Isolated from Oral Cavity Patients in AL-Najaf Province. Microb. Biosyst. 2025, 10, 255–262. [Google Scholar] [CrossRef]
- Cieplik, F.; Wiedenhofer, A.M.; Pietsch, V.; Hiller, K.A.; Hiergeist, A.; Wagner, A.; Baldaranov, D.; Linker, R.A.; Jantsch, J.; Buchalla, W.; et al. Oral Health, Oral Microbiota, and Incidence of Stroke-Associated Pneumonia—A Prospective Observational Study. Front. Neurol. 2020, 11, 528056. [Google Scholar] [CrossRef]
- Wang, D.-H.; Tsai, F.-T.; Tu, H.-F.; Yang, C.-C.; Hsu, M.-L.; Huang, L.-J.; Lin, C.-T.; Hsu, W.-E.; Lin, Y.-C. Effects of Nasogastric Tube on Oral Microbiome among Long-Term Care Patients. medRxiv 2022. [Google Scholar] [CrossRef]
- Bairappan, S.; Puranik, M.P.; Sowmya, K.R. Impact of Asthma and Its Medication on Salivary Characteristics and Oral Health in Adolescents: A Cross-Sectional Comparative Study. Spec. Care Dent. 2020, 40, 227–237. [Google Scholar] [CrossRef]
- Willis, J.R.; Saus, E.; Iraola-Guzmán, S.; Cabello-Yeves, E.; Ksiezopolska, E.; Cozzuto, L.; Bejarano, L.A.; Andreu-Somavilla, N.; Alloza-Trabado, M.; Blanco, A.; et al. Citizen-Science Based Study of the Oral Microbiome in Cystic Fibrosis and Matched Controls Reveals Major Differences in Diversity and Abundance of Bacterial and Fungal Species. J. Oral Microbiol. 2021, 13, 1897328. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.L.S.; Lima, F.P.; Machado, F.C.; Santos, S.d.S.; Malheiro, A.R.X.; Ataíde, L.A.; Figueiredo, A.C.L. Oral and Tracheal Microbiota of Pediatric and Adolescent Patients in an Intensive Care Unit. Spec. Care Dent. 2021, 41, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, F.; Duvivier, B.; Boutigny, H.; Roussel-Delvallez, M.; Chopin, C. Colonization of Dental Plaque: A Source of Nosocomial Infections in Intensive Care Unit Patients. Crit. Care Med. 1998, 26, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ortega, O.; Sakwinska, O.; Combremont, S.; Berger, B.; Sauser, J.; Parra, C.; Zarcero, S.; Nart, J.; Carrión, S.; Clavé, P. High Prevalence of Colonization of Oral Cavity by Respiratory Pathogens in Frail Older Patients with Oropharyngeal Dysphagia. Neurogastroenterol. Motil. 2015, 27, 1804–1816. [Google Scholar] [CrossRef]
- Fourrier, F.; Cau-Pottier, E.; Boutigny, H.; Roussel-Delvallez, M.; Jourdain, M.; Chopin, C. Effects of Dental Plaque Antiseptic Decontamination on Bacterial Colonization and Nosocomial Infections in Critically Ill Patients. Intensive Care Med. 2000, 26, 1239–1247. [Google Scholar] [CrossRef]
- Varzhapetian, S.; Makarenko, O.; Sydoryako, A.; Baleha, M.; Bunyatyan, K. Aerobic microflora in the pathogenesis of maxillary sinusitis after the treatment of caries complications. Georgian Med. News 2019, 289, 42–46. [Google Scholar]
- Rajab, B.; Laskin, D.M.; Abubaker, A.O. Odontogenic Infection Leading to Adult Respiratory Distress Syndrome. J. Oral Maxillofac. Surg. 2013, 71, 302–304. [Google Scholar] [CrossRef]
- Baker, J.L.; Hendrickson, E.L.; Tang, X.; Lux, R.; He, X.; Edlund, A.; McLean, J.S.; Shi, W. Klebsiella and Providencia Emerge as Lone Survivors Following Long-Term Starvation of Oral Microbiota. Proc. Natl. Acad. Sci. USA 2019, 116, 8499–8504. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Zhou, X. Lung Microbiome: New Insights into the Pathogenesis of Respiratory Diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Freeman, C.M.; McCloskey, L.; Falkowski, N.R.; Huffnagle, G.B.; Curtis, J.L. Bacterial Topography of the Healthy Human Lower Respiratory Tract. mBio 2017, 8, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Charlson, E.S.; Bittinger, K.; Chen, J.; Diamond, J.M.; Li, H.; Collman, R.G.; Bushman, F.D. Assessing Bacterial Populations in the Lung by Replicate Analysis of Samples from the Upper and Lower Respiratory Tracts. PLoS ONE 2012, 7, e0042786. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.N.; Alekseyenko, A.V.; Clemente, J.C.; Kulkarni, R.; Wu, B.; Chen, H.; Berger, K.I.; Goldring, R.M.; Rom, W.N.; Blaser, M.J.; et al. Enrichment of Lung Microbiome with Supraglottic Taxa Is Associated with Increased Pulmonary Inflammation. Microbiome 2013, 1, 19. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The Respiratory Tract Microbiome and Lung Inflammation: A Two-Way Street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef]
- Dickson, R.P.; Huffnagle, G.B. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog. 2015, 11, e1004923. [Google Scholar] [CrossRef]
- Ehrenzeller, S.; Klompas, M. Association Between Daily Toothbrushing and Hospital-Acquired Pneumonia A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2024, 184, 131–142. [Google Scholar] [CrossRef]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral Health and All-Cause, Cardiovascular Disease, and Respiratory Mortality in Older People in the UK and USA. Sci. Rep. 2021, 11, 16452. [Google Scholar] [CrossRef]
- Son, M.; Jo, S.; Lee, J.S.; Lee, D.H. Association between Oral Health and Incidence of Pneumonia: A Population-Based Cohort Study from Korea. Sci. Rep. 2020, 10, 9576. [Google Scholar] [CrossRef]
- Segal, L.N.; Clemente, J.C.; Tsay, J.C.J.; Koralov, S.B.; Keller, B.C.; Wu, B.G.; Li, Y.; Shen, N.; Ghedin, E.; Morris, A.; et al. Enrichment of the Lung Microbiome with Oral Taxa Is Associated with Lung Inflammation of a Th17 Phenotype. Nat. Microbiol. 2016, 1, 16031. [Google Scholar] [CrossRef]
- Campbell, C.D.; Gleeson, M.; Sulaiman, I. The Role of the Respiratory Microbiome in Asthma. Front. Allergy 2023, 4, 1120999. [Google Scholar] [CrossRef]
- Zimmerman, S.; Sloane, P.D.; Ward, K.; Wretman, C.J.; Stearns, S.C.; Poole, P.; Preisser, J.S. Effectiveness of a Mouth Care Program Provided by Nursing Home Staff vs. Standard Care on Reducing Pneumonia Incidence: A Cluster Randomized Trial. JAMA Netw. Open 2020, 3, e204321. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.U.J.; Paranhos, L.R.; Meneses-Santos, D.; Blumenberg, C.; Macedo, D.R.; Cardoso, S.V. Combination of Toothbrushing and Chlorhexidine Compared with Exclusive Use of Chlorhexidine to Reduce the Risk of Ventilator-Associated Pneumonia: A Systematic Review with Meta-Analysis. Clinics 2021, 76, e2659. [Google Scholar] [CrossRef] [PubMed]
- De Lacerda Vidal, C.F.; Vidal, A.K.D.L.; Monteiro, J.G.D.M.; Cavalcanti, A.; Henriques, A.P.T.; Oliveira, M.; Godoy, M.; Coutinho, M.; Sobral, P.D.; Vilela, C.A.; et al. Impact of Oral Hygiene Involving Toothbrushing versus Chlorhexidine in the Prevention of Ventilator-Associated Pneumonia: A Randomized Study. BMC Infect. Dis. 2017, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, C.; Lin, J.; Ng, L.; Needleman, I.; Walsh, T.; Li, C. Oral Care Measures for Preventing Nursing Home-Acquired Pneumonia. Cochrane Database Syst. Rev. 2022, 2022, CD012416. [Google Scholar] [CrossRef]
- Sozkes, S.; Sozkes, S. Use of Toothbrushing in Conjunction with Chlorhexidine for Preventing Ventilator-Associated Pneumonia: A Random-Effect Meta-Analysis of Randomized Controlled Trials. Int. J. Dent. Hyg. 2023, 21, 389–397. [Google Scholar] [CrossRef]
- Pragman, A.A.; Lyu, T.; Baller, J.A.; Gould, T.J.; Kelly, R.F.; Reilly, C.S.; Isaacson, R.E.; Wendt, C.H. The Lung Tissue Microbiota of Mild and Moderate Chronic Obstructive Pulmonary Disease. Microbiome 2018, 6, 7. [Google Scholar] [CrossRef]
- Tokarz, Z.; Krzysciak, P.; Wieczorek, A. Effectiveness of Methods for Removing the Candida Albicans Biofilm from the Dental Acrylic Surface. Dent. Med. Probl. 2023, 60, 665–671. [Google Scholar] [CrossRef]
- Jang, H.J.; Choi, J.Y.; Kim, K.; Yong, S.H.; Kim, Y.W.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; et al. Relationship of the Lung Microbiome with PD-L1 Expression and Immunotherapy Response in Lung Cancer. Respir. Res. 2021, 22, 322. [Google Scholar] [CrossRef]
- Campbell, S.; Gerasimidis, K.; Milling, S.; Dicker, A.J.; Hansen, R.; Langley, R.J. The Lower Airway Microbiome in Paediatric Health and Chronic Disease. Paediatr. Respir. Rev. 2024, 52, 31–43. [Google Scholar] [CrossRef]
- Caverly, L.J.; Huang, Y.J.; Sze, M.A. Past, Present, and Future Research on the Lung Microbiome in Inflammatory Airway Disease. Chest 2019, 156, 376–382. [Google Scholar] [CrossRef]
- Røsland, A.; Bertelsen, R.J.; Heinrich, J.; Lie, S.A.; Malinovschi, A.; Bunæs, D.F. Effect of Periodontal Therapy on Lung Function: A Twelve-Month Follow-up Intervention Study. Respir. Res. 2025, 26, 172. [Google Scholar] [CrossRef]
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Szopa, M.; Dobrzyński, M.; Gamian, A. High Doses of Tocopherol in the Prevention and Potentiation of Dioxin in Experimental Inflammation- Potential Application. Postep. Hig. I Med. Dosw. 2011, 65, 143–157. [Google Scholar] [CrossRef]
- Calkosinski, I.; Rosinczuk-Tonderys, J.; Dobrzynski, M.; Palka, L.; Bazan, J. Occurrence of Disseminated Intravascular Coagulation in 2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Induced Pneumonia in the Rat. Adv. Exp. Med. Biol. 2013, 788, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Branson, R.; Cawcutt, K.; Crist, M.; Eichenwald, E.C.; Greene, L.R.; Lee, G.; Maragakis, L.L.; Powell, K.; Priebe, G.P.; et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2022, 43, 687–713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Bronowicka-Szydełko, A.; Dzierzba, K.; Bazan, J.; Dobrzyński, M.; Majda, J.; Gamian, A. Effect of Tocopherol on Biochemical Blood Parameters in Pleuritis-Induced Rats Treated with 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Toxicol. Ind. Health 2015, 31, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Nishihama, S.; Kawada-Matsuo, M.; Le, M.N.-T.; Fujii, A.; Haruta, A.; Kajihara, T.; Hashimoto, Y.; Yoshikawa, M.; Aikawa, T.; Tsuga, K.; et al. Oral Colonization of Antimicrobial-Resistant Bacteria in Home Health Care Participants and Their Association with Oral and Systemic Status. Sci. Rep. 2025, 15, 5776. [Google Scholar] [CrossRef]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the Upper Respiratory Tract Microbiotas as the Source of the Lung and Gastric Microbiotas in Healthy Individuals. mBio 2015, 6, 1110–1128. [Google Scholar] [CrossRef]
- Sjögren, P.; Nilsson, E.; Forsell, M.; Johansson, O.; Hoogstraate, J. A Systematic Review of the Preventive Effect of Oral Hygiene on Pneumonia and Respiratory Tract Infection in Elderly People in Hospitals and Nursing Homes: Effect Estimates and Methodological Quality of Randomized Controlled Trials. J. Am. Geriatr. Soc. 2008, 56, 2124–2130. [Google Scholar] [CrossRef]
- Xiong, K.; Ao, K.; Wei, W.; Dong, J.; Li, J.; Yang, Y.; Tang, B.; Li, Y. Periodontitis Aggravates COPD through the Activation of Γδ T Cell and M2 Macrophage. Msystems 2024, 9, e00572-23. [Google Scholar] [CrossRef]
- He, Q.; Peng, Z.; He, C.; Zhang, C.; Hu, R. Effect of different mouthwashes on ventilator-related outcomes and mortality in intensive care unit patients: A network meta-analysis. Aust. Crit. Care 2025, 38, 101095. [Google Scholar] [CrossRef] [PubMed]

| Study | Aim of the Study | Materials and Methods | Results | Conclusions |
|---|---|---|---|---|
| Zhou 2018 [16] | To investigate the association between oral health and upper respiratory tract infection in children. | In 288 children aged 4 years, the DMFT and plaque index were assessed and a history of upper respiratory tract infections in the last 12 months was collected. | A significantly higher rate of dmft was observed in children who had no upper respiratory tract infections compared to children who had at least 3 infections. | Children with poorer oral health are less likely to experience upper respiratory tract infections. |
| Winning 2023 [42] | To investigate the relationship between subgingival microbial diversity associated with periodontitis and reduced respiratory function | Subgingival plaque swabs were collected from 507 men and their microbial diversity index (MDI) was assessed, and spirometry was performed. | An increase in MDI by 1 was associated with a 0.71% decrease in FEV1. | The diversity of subgingival microorganisms is associated with reduced respiratory function. |
| Rantala 2016 [43] | Assessment of the relationship between the occurrence of respiratory infections before the eruption of permanent teeth and the development of dental caries. | Information on the occurrence of respiratory infections was obtained from the medical records of 1623 patients, and then information on the number of dental fillings was obtained from the interview. | The average number of teeth with fillings was 1.4 higher in patients who experienced a respiratory infection requiring hospitalization before the age of 2 years. | The occurrence of respiratory infections in early childhood is associated with poorer condition of the permanent dentition. |
| Shirazian 2018 [44] | Assessment of oral diseases in patients with lung diseases compared to healthy individuals. | In 42 patients from the respiratory ward and healthy candidates, periodontal status, gingivitis, xerostomia, halitosis, dental caries, tongue coating and existence of oral lesion were assessed. | The incidence of gingivitis, periodontitis, halitosis and tongue coating in patients with lung diseases was significantly higher than in healthy individuals. | More attention needs to be paid to improving the oral health of patients suffering from lung diseases. |
| Ploenes 2022 [45] | To investigate the relationship between oral health and postoperative pneumonia. | 230 patients were assessed for the presence of caries and periodontal disease, number of lost teeth, and regularity of dental visits the day before thoracic surgery. | Patients with a higher frequency of dental caries who do not regularly visit the dentist have a significantly higher incidence of postoperative pneumonia. | Poor oral health is a risk factor for postoperative complications. |
| Arweiler 2021 [46] | To investigate the difference in salivary biofilm and dental plaque in children with respiratory allergies compared to healthy children. | RNA sequencing studies were performed on material collected from saliva and dental plaque of 46 pediatric patients from the allergy department and healthy candidates. | Bacteria in the saliva of healthy children are more diverse. In the dental plaque of children with allergies, an increase in the number of Fusobacterium nucleatum bacteria was noted compared to healthy children, while the number of Fusobacterium unclassified and Prevotella_6 unclassified decreased. | The study results indicate the possible involvement of dental biofilm in the development of allergies and asthma in children. |
| Bellissimo-Rodrigues 2014 [41] | To evaluate whether incorporating comprehensive dental care into ICU patients’ oral hygiene regimens can reduce the incidence of nosocomial lower respiratory tract infections (LRTIs), including ventilator-associated pneumonia (VAP). | Design: Observer-blind randomized clinical trial. Setting: General ICU at University Hospital, Ribeirão Preto Medical School, Brazil. Participants: 254 adult patients expected to stay ≥ 48 h in the ICU. Intervention:
Primary: Incidence of LRTI. Secondary: Mortality, antibiotic use, ICU stay duration, adverse events. | LRTI Incidence: Experimental group: 8.7% Control group: 18.1% Adjusted RR: 0.44 (95% CI, 0.20–0.96); p = 0.04 VAP Rate: Control: 16.5 per 1000 ventilator-days Experimental: 7.6 per 1000 ventilator-days (p < 0.05) Mortality: No significant difference (≈30% in both groups). Adverse Events: Mild (mucosal irritation, minor bleeding); more frequent in the intervention group but not severe. | Comprehensive dental care is safe and effective in preventing LRTIs among ICU patients. The intervention significantly reduces infection rates, particularly VAP. No major complications were associated with dental procedures. Further studies may be needed to assess mortality impact and cost-effectiveness in other settings. |
| Cherkasov 2019 [47] | To investigate whether differences exist in the dental plaque microbiota of asthmatic children with and without dental caries, using 16S rDNA sequencing. | Design: Observational, cross-sectional study using 16S rDNA sequencing. Participants: 18 asthmatic children (ages 3–6).
Analysis: DNA extracted from plaque. Sequencing performed on Illumina MiSeq (targeting V3–V4 region). Bioinformatic analysis included OTU clustering, alpha and beta diversity assessment, and taxonomic classification. | No significant differences in both groups Key microbial findings:
| The overall bacterial diversity and community structure of dental plaques in asthmatic children with and without caries were broadly similar. However, specific taxa, especially Veillonella, were significantly more abundant in caries-affected children, indicating a potential role in caries development. The study highlights that asthmatic children’s oral cavities may harbor opportunistic bacteria relevant to both caries and respiratory diseases, warranting further investigation into shared microbiome-pathogenesis pathways. |
| Wang 2023 [48] | To characterize changes in the oral (tongue dorsum) microbiome of older, long-term care patients receiving nutrition through nasogastric (NG) tubes, and to identify microbial taxa associated with potential aspiration pneumonia risk. | Design: Observational, cross-sectional study. Participants: 53 elderly patients in long-term care:
Microbial Analysis: 16S rRNA amplicon sequencing (V3–V4 regions); ASV-based bioinformatics Statistical Methods: Alpha/beta diversity metrics, hierarchical clustering, LEfSe to identify biomarkers, and co-occurrence network analysis | Diversity & Composition: Significant separation in microbiome composition between NG-tube and oral-feeding groups Longer duration of NG-tube placement correlated with distinct microbial profiles Biomarkers:
Pneumonia Association: Microbial signatures from NG-tube patients mirrored taxa associated with aspiration pneumonia events, supporting clinical relevance. | NG-tube feeding in older patients is associated with oral dysbiosis, shifting microbiome to a more pathogenic profile. Enrichment with opportunistic pathogens (Pseudomonas, Corynebacterium) and reduction of commensals may increase aspiration pneumonia risk. Microbiome data could inform targeted antimicrobial therapy prior to culture results. While causality cannot be claimed, findings are consistent across cultures and countries, suggesting predictable microbiome shifts linked to NG-tube placement. |
| Ucuncu 2024 [14] | To determine whether adults with asthma or COPD exhibit increased susceptibility to dental caries by analyzing saliva’s physical, chemical, and microbiological characteristics—focusing on the impact of respiratory disease medications on oral health. | Design: Cross-sectional comparative study. Participants (n = 104, aged 18–70): Asthma group (n = 41) Healthy controls (n = 21—inferred total) Oral Assessments: Dental caries via DMFT and DMFS indices Oral hygiene via Green & Vermillion Simplified OHI-S Saliva Analysis: Flow rate and buffering capacity Microbial counts: Streptococcus mutans, Lactobacillus casei, Staphylococcus aureus, and Candida albicans colony counts Caries Risk Profiling: Utilized the Cariogram software (https://cariogram.se; accessed on 1 January 2025) to integrate clinical and microbiologic risk factors | The respiratory disease groups (asthma + COPD) demonstrated significantly higher: DMFT, DMFS, and OHI-S scores compared to controls (p < 0.01) Cariogram-predicted caries risk also significantly elevated (p < 0.01), with no difference between asthma and COPD patients No significant difference in caries risk was found between COPD patients using two vs. three inhaled medications (p > 0.05) | Adults with asthma or COPD are more prone to dental caries, likely due to medication-induced changes in saliva and oral microbial environment. These individuals should adopt enhanced oral hygiene measures and undergo regular dental evaluations to mitigate heightened caries risk. |
| Al-Fahham 2025 [49] | To investigate the presence and virulence of Klebsiella pneumoniae in patients with oral cavity infections and to detect the fimH gene, which is associated with biofilm formation and pathogenicity. | Study Design: Cross-sectional, laboratory-based analysis. Samples: 150 oral swabs from patients (aged 7–65) with gingivitis, dental caries, and dental plaque, collected between September 2023–April 2024. Culture & Identification:
Biofilm Testing: Microtiter plate method (OD at 630 nm). Molecular Detection: PCR assay for fimH gene; electrophoresis for band size confirmation. | Bacterial Distribution:
High resistance to ticarcillin and piperacillin-tazobactam (88.8% each). Moderate resistance to trimethoprim-sulfamethoxazole (44.4%). No resistance to meropenem, imipenem, levofloxacin, ciprofloxacin, amikacin, or tobramycin. Biofilm Production: 85.7% of K. pneumoniae isolates were strong biofilm formers. 57.1% were encapsulated (capsule presence confirmed via Indian ink stain). Molecular Findings: fimH gene detected in 11/14 (78.8%) K. pneumoniae isolates. The gene is linked to type 1 fimbriae, which enhance adhesion and biofilm development. | There is a significant presence of antibiotic-resistant K. pneumoniae in the oral cavity of patients with dental infections in Al-Najaf. The fimH gene was prevalent in the majority of isolates, supporting its role in biofilm formation and virulence. |
| Cieplik 2020 [50] | To investigate associations between oral health, oral microbiota profiles, and the incidence of stroke-associated pneumonia (SAP) in patients admitted with acute stroke-like symptoms. | Design: Prospective observational cohort over 5 months (February–July 2018) Enrolled patients with stroke-like symptoms within 24 h of admission. Participants: 99 patients total—57 confirmed stroke, 42 stroke mimics. Timepoints: 1. Baseline (≤24 h): demographics, neurology, immunology (e.g., CRP), dental exam (DMFT), and microbiological sampling (saliva + subgingival plaque). 2. 48 h and 120 h: repeated immunology and microbiota sampling Microbial Analysis: Culture + 16S rRNA sequencing. Primary Outcome: Incidence of SAP in stroke patients, defined by clinical criteria. Exclusions: Intubated on admission, endocarditis prophylaxis, recent stroke (<1 month). | Incidence of SAP: 8 out of 57 stroke patients (14%) developed SAP. Risk Associations: SAP was significantly linked to older age, dysphagia, higher stroke severity (NIHSS), embolectomy, nasogastric tube placement, and elevated baseline CRP Oral Health: Trends towards more missing teeth and poorer oral hygiene in SAP patients, though differences were not statistically significant. DMFT scores similar across groups (~23–25). Microbiota Findings: No major differences in microbial composition between SAP and non–SAP groups. However, SAP patients exhibited ecological shifts over time—likely due to antibiotic usage. | SAP incidence (14%) in this stroke cohort aligns with previously reported ranges. Key SAP risk factors: age, dysphagia, stroke severity, nasogastric feeding, and inflammation (elevated CRP). Oral hygiene factors (e.g., tooth loss, plaque) showed a trend but did not reach statistical significance—likely due to small sample size. Oral microbiota composition wasn’t directly linked to SAP, but microbiome disruption post-SAP reflected antibiotic effects. Clinical recommendation: Larger studies needed to validate oral health strategies for SAP prevention and to assess implementing oral care protocols in stroke units. |
| Wang 2022 [51] | The aim of this study was to examine the impact of nasogastric tube feeding on the composition of the oral (tongue) microbiome in long-term care patients. | Tongue swab samples from 27 NG-tube-fed patients and 26 orally fed controls were analyzed using 16S rRNA next-generation sequencing to compare microbial composition. | NG-tube-fed patients had significantly altered oral microbiomes, with increased Gram-negative aerobes and higher levels of pneumonia-associated pathogens such as Corynebacterium and Pseudomonas, along with reduced levels of beneficial commensals like Streptococcus and Veillonella. | This study highlights distinct microbial changes in NG-tube-fed patients, offering important insights for improving oral care in long-term clinical settings. |
| Bairappan 2020 [52] | to investigate differences in salivary properties and oral health between adolescents with and without asthma, and to examine how asthma and its treatment influence the occurrence of dental caries | A cross-sectional study was conducted among 50 asthmatic and 50 non-asthmatic adolescents (12–15 years) in Bangalore. Salivary parameters and oral health were assessed using questionnaires, saliva analysis, and WHO 2013 criteria. Statistical tests were performed with significance set at p < 0.05. | Asthmatic adolescents had significantly more dental caries, gingival bleeding, and erosion than non-asthmatics. They also showed lower salivary flow, pH, buffering capacity, and higher levels of S. mutans and Lactobacilli. These factors were strongly linked to asthma severity and medication use. | Asthmatic adolescents demonstrated notably poorer salivary parameters and oral health. Both asthma and its treatment significantly influenced salivary function and increased the risk of dental caries in this group. |
| Willis 2021 [53] | The aim of this study was to characterize the composition of the oral microbiome in individuals with cystic fibrosis and to explore its potential role in respiratory health. | Oral rinse samples were obtained from 31 individuals with cystic fibrosis and matched controls in Spain. Bacterial and fungal communities were analyzed using 16S rRNA sequencing, culturing methods, and proteomics-based fungal identification. | The oral microbiome in CF patients showed reduced diversity, increased Candida albicans prevalence, and altered bacterial profiles linked to lung infections and oral diseases like caries and periodontitis. | This study offers an initial overview of the oral microbiome in cystic fibrosis, highlighting the need for further research into its connection with the lung microbiome. |
| Pinheiro 2021 [54] | The aim of this study was to investigate the composition of the oral and tracheal microbiota in children admitted to the pediatric intensive care unit. | An exploratory study was conducted among PICU patients aged 5 months to 13 years. Oral and tracheal samples were collected within the first 24–48 h of admission. Caries experience and oral hygiene were assessed using the DMFT/dmf and visual plaque index. | The mean DMFT/dmf score was 1.66, and the average visual plaque index was 43%. Klebsiella pneumoniae was the most frequently detected microorganism. Patients on mechanical ventilation had significantly higher rates of oral colonization by opportunistic pathogens compared to those breathing spontaneously. No significant association was found between plaque index or caries experience and changes in oral microbiota. | Children in intensive care are vulnerable to early colonization by respiratory and opportunistic pathogens, regardless of oral hygiene or dental condition. |
| Fourrier 1998 [55] | The influence of dental plaque decontamination on its colonization with aerobic bacteria of hospital infections in intensive care unit patients. | Sixty patients were divided into two groups. In the study group, a gel containing 0.2% CHX was applied to dental plaque three times daily. In both groups, bacterial cultures were performed from dental plaque, nasal swabs, tracheal aspirates, blood, and urine on days 0, 5, and 10, and weekly thereafter. | After 24 days, 50% of patients in the control group had at least one nosocomial pathogen in their dental plaque, compared with 28% in the study group. In tracheal aspirates, the figures were 45% and 28%, respectively. | The use of chlorhexidine gel may reduce the risk of hospital-acquired infections in intensive care unit patients. |
| Ortega 2015 [56] | Understanding the pathogenesis of aspiration pneumonia in different phenotypes of frail older patients with oropharyngeal dysphagia (OD). | Bacterial colonies of the oral/nasal cavity of 61 patients aged 70 years or older were assessed by PCR. | In the nasopharynx the predominant bacteria were: Moraxella, Corynebacterium, Staphylococcus, Streptococcus, and Alloiococcus, while in the oral cavity the predominant bacteria were: Veionella, Neisseria, Prevotella, Porphyromonas, Haemophilus, and streptococci. | Older, frail patients with OD have been found to have high oral bacterial concentrations and frequent colonization of the oral cavity with respiratory pathogens. |
| Fourrier 2000 [57] | Assessment of dental plaque colonization by aerobic bacteria and their association with nosocomial infections in intensive care unit patients. | Fifty-seven patients were examined every five days until discharge or death. The DMFT index and bacterial counts in dental plaque, nasal secretions, and tracheal aspirates were assessed. Cultures were performed on blood plates for 72 h. | The DMFT index remained stable throughout the stay. Aerobic bacterial colonies were present in 23% of patients on day 0 and in 46% of patients on day 10. Twenty-one patients developed nosocomial infections, of which six had the causative bacteria initially isolated from dental plaque. | Aerobic bacteria from dental plaque can be a significant cause of nosocomial infections in intensive care unit patients. |
| Varzhapetian 2019 [58] | Investigation of bacterial flora in iatrogenic maxillary sinusitis of dental origin. | From eight patients material was taken directly from the maxillary sinus wall during surgery. Cultures were incubated on 5% blood agar, boiled blood agar, Endo agar, and Chistovich’s medium up to 120 h. | Staphylococcus aureus (+) was identified in four patients. Additionally, staphylococcus epidermidis (+), Streptococcus pneumoniae (+), and Candida albikans were present in two patients each. | In iatrogenic maxillary sinusitis, which developed during the treatment of complications of maxillary caries, aerobic bacteria are cultured in 100.0% of patients and are represented by Gram-positive flora of Staphylococcus and Streptococcus (80.0%) and Candida fungi (20.0%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zygmunt, Ł.; Kiryk, S.; Wesołek, K.; Kiryk, J.; Nawrot-Hadzik, I.; Rybak, Z.; Sztyler, K.; Małyszek, A.; Matys, J.; Dobrzyński, M. The Role of the Oral Microbiome and Dental Caries in Respiratory Health: A Systematic Review. J. Clin. Med. 2025, 14, 7670. https://doi.org/10.3390/jcm14217670
Zygmunt Ł, Kiryk S, Wesołek K, Kiryk J, Nawrot-Hadzik I, Rybak Z, Sztyler K, Małyszek A, Matys J, Dobrzyński M. The Role of the Oral Microbiome and Dental Caries in Respiratory Health: A Systematic Review. Journal of Clinical Medicine. 2025; 14(21):7670. https://doi.org/10.3390/jcm14217670
Chicago/Turabian StyleZygmunt, Łukasz, Sylwia Kiryk, Kamil Wesołek, Jan Kiryk, Izabela Nawrot-Hadzik, Zbigniew Rybak, Klaudia Sztyler, Agata Małyszek, Jacek Matys, and Maciej Dobrzyński. 2025. "The Role of the Oral Microbiome and Dental Caries in Respiratory Health: A Systematic Review" Journal of Clinical Medicine 14, no. 21: 7670. https://doi.org/10.3390/jcm14217670
APA StyleZygmunt, Ł., Kiryk, S., Wesołek, K., Kiryk, J., Nawrot-Hadzik, I., Rybak, Z., Sztyler, K., Małyszek, A., Matys, J., & Dobrzyński, M. (2025). The Role of the Oral Microbiome and Dental Caries in Respiratory Health: A Systematic Review. Journal of Clinical Medicine, 14(21), 7670. https://doi.org/10.3390/jcm14217670









