Metastatic Potential of Very Small (≤2 cm) Renal Cell Carcinoma: Insights from a Single-Center Experience and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
3. Results
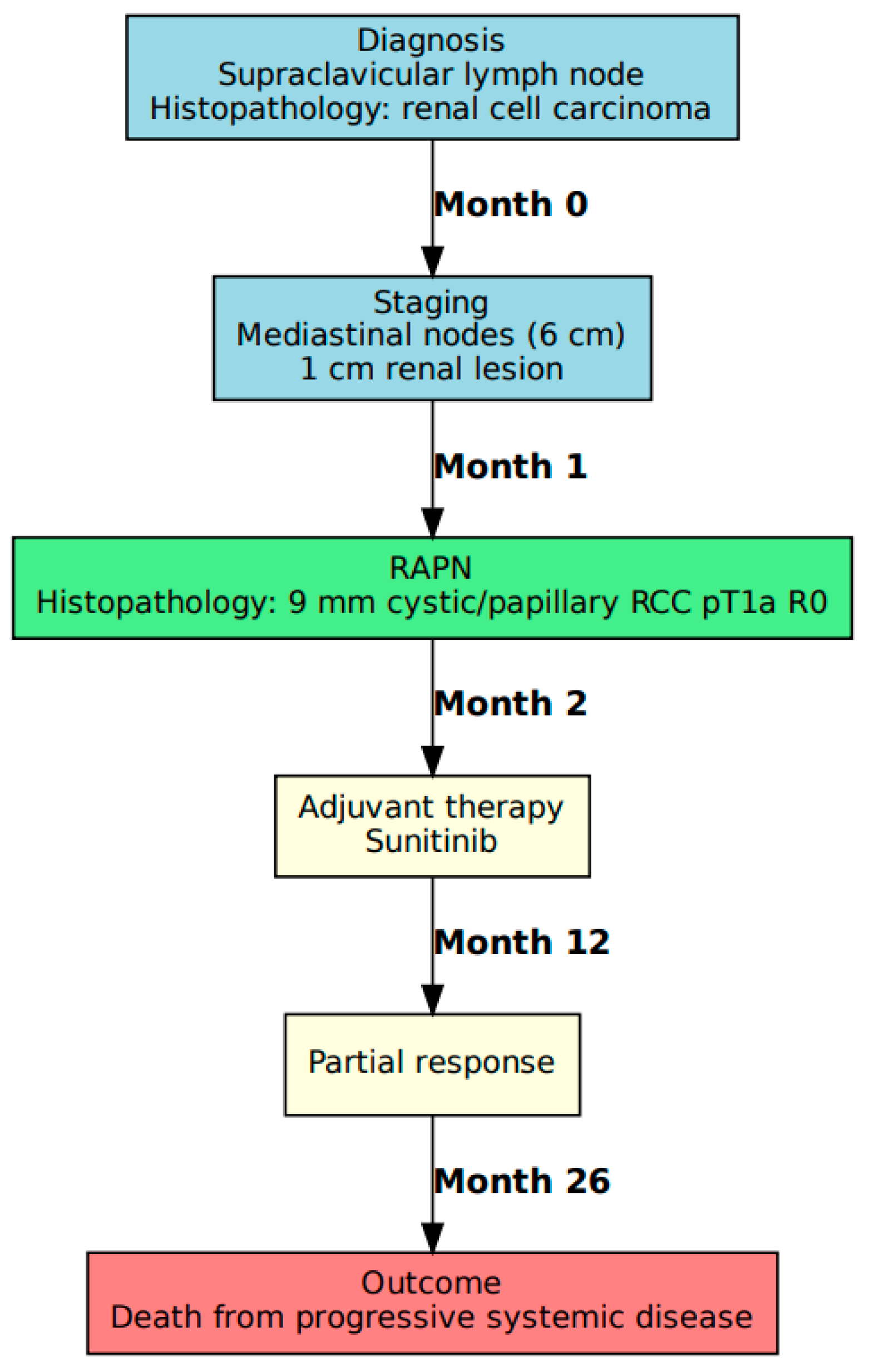
3.1. Case A: Presentation and Management
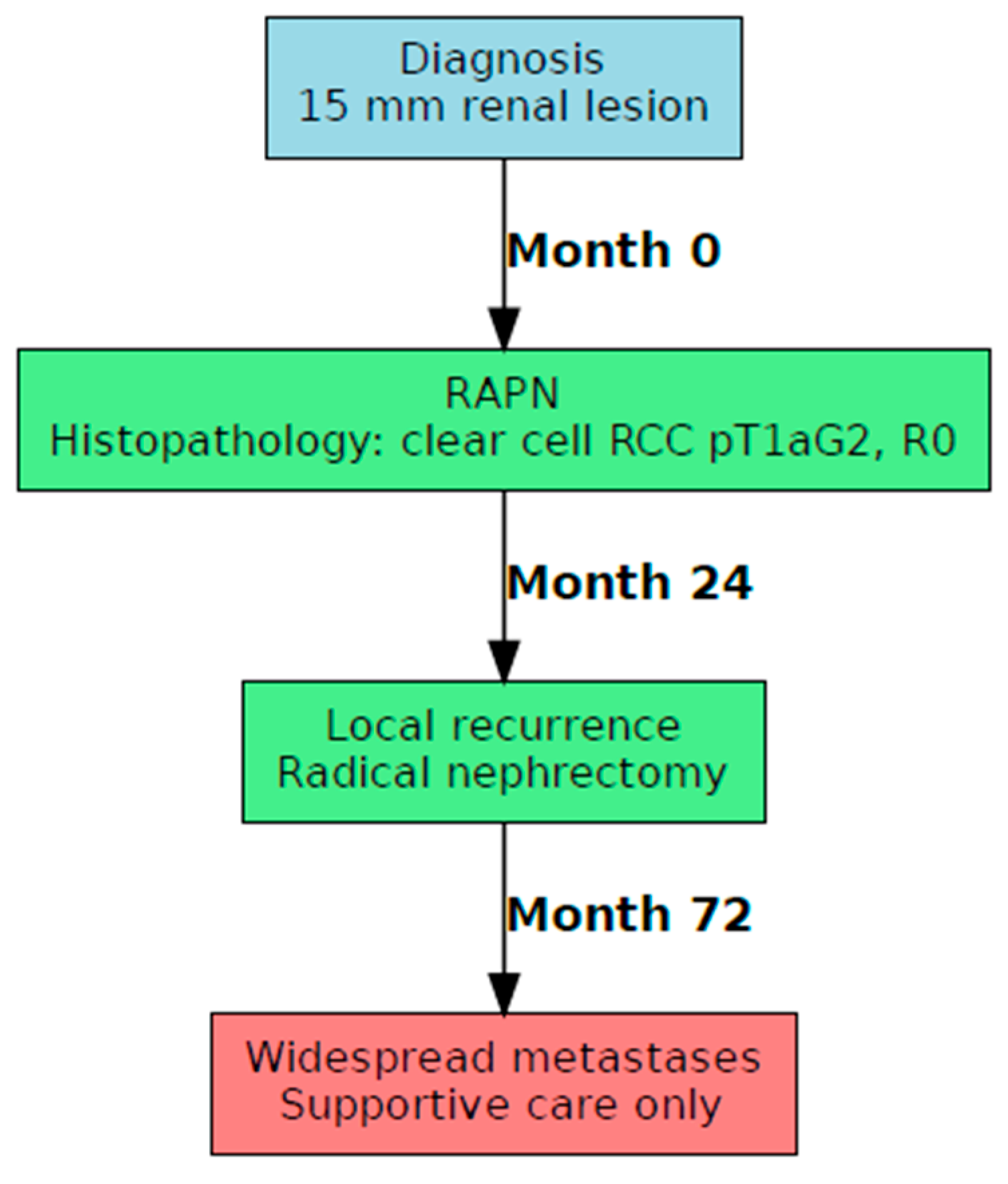
3.2. Case B: Presentation and Management
3.3. Review of the Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCC | Renal Cell Carcinoma |
| RAPN | Robotic-assisted partial nephrectomy |
| BSC | Best Supportive care |
| SM | Synchronous Metastases |
| SRMs | Small Renal Masses |
References
- Israel, G.M.; Bosniak, M.A. Pitfalls in renal mass evaluation and how to avoid them. Radiographics 2008, 28, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Ghanem, Y.A.; Albiges, L.; Bonn, S.; Campi, R.; Capitanio, U.; Dabestani, S.; Hora, M.; Klatte, T.; Kuusk, T.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2025 Update. Eur. Urol. 2025, 87, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.M.; Chan, D.Y.; Siegelman, S.S. Small renal cell carcinomas: Correlation of size with tumor stage, nuclear grade, and histologic subtype. AJR Am. J. Roentgenol. 2004, 182, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Kurta, J.M.; Kaag, M.; Tickoo, S.K.; Kundu, S.; Katz, D.; Nogueira, L.E.; Reuter, V.E.; Paul Russo, P. Tumor size is associated with malignant potential in renal cell carcinoma cases. J. Urol. 2009, 181, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Frank, I.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Weaver, A.L.; Zincke, H. Solid renal tumors: An analysis of pathological features related to tumor size. J. Urol. 2003, 170 Pt 1, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Sebastià, C.; Corominas, D.; Musquera, M.; Paño, B.; Ajami, T.; Nicolau, C. Active surveillance of small renal masses. Insights Imaging 2020, 11, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Remzi, M.; Ozsoy, M.; Klingler, H.C.; Susani, M.; Waldert, M.; Seitz, C.; Schmidbauer, J.; Marberger, M. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J. Urol. 2006, 176, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, M.C.; Kutikov, A.; Egleston, B.L.; Canter, D.J.; Viterbo, R.; Chen, D.Y.; Jewett, M.A.; Greenberg, R.E.; Uzzo, R.G. Small renal masses progressing to metastases under active surveillance: A systematic review and pooled analysis. Cancer 2012, 118, 997–1006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thompson, R.H.; Hill, J.R.; Babayev, Y.; Cronin, A.; Kaag, M.; Kundu, S.; Bernstein, M.; Coleman, J.; Dalbagni, G.; Touijer, K.; et al. Metastatic renal cell carcinoma risk according to tumor size. J. Urol. 2009, 182, 41–45. [Google Scholar] [CrossRef]
- Kunkle, D.A.; Crispen, P.L.; Li, T.; Uzzo, R.G. Tumor size predicts synchronous metastatic renal cell carcinoma: Implications for surveillance of small renal masses. J. Urol. 2007, 177, 1692–1696; discussion 1697. [Google Scholar] [CrossRef]
- Lughezzani, G.; Jeldres, C.; Isbarn, H.; Perrotte, P.; Shariat, S.F.; Sun, M.; Widmer, H.; Arjane, P.; Peloquin, F.; Pharand, D.; et al. Tumor Size is a Determinant of the Rate of Stage T1 Renal Cell Cancer Synchronous Metastasis. J. Urol. 2009, 182, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.M.; Gill, I.S. Effect of Renal Cancer Size on the Prevalence of Metastasis at Diagnosis and Mortality. J. Urol. 2009, 181, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Patard, J.J.; de Martino, M.; Bensalah, K.; Verhoest, G.; de La Taille, A.; Abbou, C.-C.; Allhoff, E.P.; Carrieri, G.; Riggs, S.B. Tumor size does not predict risk of metastatic disease or prognosis of small renal cell carcinomas. J. Urol. 2008, 179, 1719–1726. [Google Scholar] [CrossRef]
- Guðmundsson, E.; Hellborg, H.; Lundstam, S.; Erikson, S.; Ljungberg, B.; Swedish Kidney Cancer Quality Register Group. Metastatic potential in renal cell carcinomas ≤ 7 cm: Swedish Kidney Cancer Quality Register data. Eur. Urol. 2011, 60, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Pahernik, S.; Ziegler, S.; Roos, F.; Melchior, S.W.; Thüroff, J.W. Small renal tumors: Correlation of clinical and pathological features with tumor size. J. Urol. 2007, 178, 414–417; discussion 416–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.K.; Kim, K.; Kwak, C.; Kim, H.H.; Byun, S.S.; Lee, S.E.; Hong, S.K. Risk of metastasis for T1a renal cell carcinoma. World J. Urol. 2016, 34, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, N.J.; Mansour, A.M.; Hayn, M.H.; Schwaab, T. Is there a role for partial nephrectomy in patients with metastatic renal cell carcinoma? Urol. Oncol. 2013, 31, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Umbreit, E.C.; Shimko, M.S.; Childs, M.A.; Lohse, C.M.; Cheville, J.C.; Leibovich, B.C.; Blute, M.L.; Thompson, R.H. Metastatic potential of a renal mass according to original tumour size at presentation. BJU Int. 2012, 109, 190–194; discussion 194. [Google Scholar] [CrossRef] [PubMed]
- Ingimarsson, J.P.; Sigurdsson, M.I.; Hardarson, S.; Petursdottir, V.; Jonsson, E.; Einarsson, G.V.; Gudbjartsson, T. The impact of tumour size on the probability of synchronous metastasis and survival in renal cell carcinoma patients: A population-based study. BMC Urol. 2014, 14, 72, Erratum in BMC Urol. 2015, 15, 5. https://doi.org/10.1186/1471-2490-15-5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pahernik, S.; Huber, J.; Pfitzenmaier, J.; Haferkamp, A.; Hohenfellner, M. Small renal cell carcinoma: Oncological outcome with tumour size. Scand. J. Urol. Nephrol. 2011, 45, 432–435. [Google Scholar] [CrossRef]
- Steffens, S.; Junker, K.; Roos, F.C.; Janssen, M.; Becker, F.; Henn, D.; Wegener, G.; Siemer, S.; Hofmann, R.; Schrader, M.; et al. German Renal Tumor Network. Small renal cell carcinomas—How dangerous are they really? Results of a large multicenter study. Eur. J. Cancer 2014, 50, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Fischer, C.; Freese, R.; Altmannsberger, M.; Weidner, W. Nephron-sparing surgery for renal cell carcinoma—Is tumor size a suitable parameter for indication? Urology 1999, 54, 988. [Google Scholar] [CrossRef]
- Zastrow, S.; Phuong, A.; von Bar, I.; Novotny, V.; Hakenberg, O.W.; Wirth, M.P. Primary tumor size in renal cell cancer in relation to the occurrence of synchronous metastatic disease. Urol. Int. 2014, 92, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, A.; Palumbo, C.; Knipper, S.; Mistretta, F.A.; Rosiello, G.; Tian, Z.; St-Hilaire, P.A.; Shariat, S.F.; Saad, F.; Lavallée, L.; et al. Synchronous Metastasis Rates in T1 Renal Cell Carcinoma: A Surveillance, Epidemiology, and End Results Database-based Study. Eur. Urol. Focus 2021, 7, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Monda, S.M.; Lui, H.T.; Pratsinis, M.A.; Chandrasekar, T.; Evans, C.P.; Dall’Era, M.A. The Metastatic Risk of Renal Cell Carcinoma by Primary Tumor Size and Subtype. Eur. Urol. Open Sci. 2023, 52, 137–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kates, M.; Korets, R.; Sadeghi, N.; Pierorazio, P.M.; McKiernan, J.M. Predictors of locally advanced and metastatic disease in patients with small renal masses. BJU Int. 2012, 109, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Yonese, J.; Tsukamoto, T.; Maezawa, T.; Ishikawa, Y.; Fukui, I. Retroperitoneal cystic metastasis from a small clear cell renal carcinoma. Int. J. Urol. 2001, 8, 637–639. [Google Scholar] [CrossRef][Green Version]
- Aizawa, S.; Suzuki, M.; Kikuchi, Y.; Nikaido, T.; Matsumoto, K. Clinicopathological study on small renal cell carcinomas with metastases. Acta Pathol. Jpn. 1987, 37, 947–954. [Google Scholar] [CrossRef]
- Dong, A.; Yang, B.; Bai, Y.; Zuo, C. 68 Ga-FAPI-04 PET/CT in a Small Sarcomatoid Renal Cell Carcinoma with Widespread Metastases. Clin. Nucl. Med. 2023, 48, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Kuyava, J.M.; Grande, J.P.; Swetz, K.M. Metastatic renal cell carcinoma mimicking diverticulitis in a patient with chronic lymphocytic leukaemia. BMJ Case Rep. 2015, 2015, bcr2014206101. [Google Scholar] [CrossRef]
- Alam, R.; Tosoian, J.J.; Pierorazio, P.M.; Johnson, M.H. Distant Metastases From a Small Renal Cell Carcinoma: A Case Report. Urol. Case Rep. 2015, 4, 59–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Chalfin, H.J.; Gurda, G.T.; Hammers, H.J.; Netto, G.J.; Bivalacqua, T.J. Renal cell carcinoma presenting with brain metastasis from a 1.6 cm primary tumor. Can. J. Urol. 2013, 20, 6965–6967. [Google Scholar] [PubMed][Green Version]
- Curry, N.S.; Schabel, S.I.; Betsill, W.L., Jr. Small renal neoplasms: Diagnostic imaging, pathologic features, and clinical course. Radiology 1986, 158, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Suzuki, M.; Fujimura, T.; Fukuhara, H.; Enomoto, Y.; Nishimatsu, H.; Homma, Y. Distant metastasis of renal cell carcinoma with a diameter of 3 cm or less-which is aggressive cancer? J. Urol. 2010, 184, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Evins, S.C.; Varner, W. Renal adenoma—A misnomer. Urology 1979, 13, 85–86. [Google Scholar] [CrossRef]
- Masago, T.; Watanabe, T.; Nemoto, R. Small renal cell carcinoma with pancreas metastasis: A case report. Hinyokika Kiyo. Acta Urol. Jpn. 2011, 57, 607–610. [Google Scholar]
- Talamo, T.S.; Shonnard, J.W. Small renal adenocarcinoma with metastases. J. Urol. 1980, 124, 132–134. [Google Scholar] [CrossRef]
- Chow, W.H.; Devesa, S.S.; Warren, J.L.; Fraumeni, J.F., Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999, 281, 1628–1631. [Google Scholar] [CrossRef]
- Wunderlich, H.; Reichelt, O.; Schumann, S.; Schlichter, A.; Kosmehl, H.; Werner, W.; Vollandt, R.; Schubert, J. Nephron sparing surgery for renal cell carcinoma 4 cm. Or less in diameter: Indicated or under treated? J. Urol. 1998, 159, 1465. [Google Scholar] [CrossRef]
- Ku, J.H.; Moon, K.C.; Kwak, C.; Kim, H.H. Metachronous metastatic potential of small renal cell carcinoma: Dependence on tumor size. Urology 2009, 74, 1271–1275. [Google Scholar] [CrossRef]
- Jiang, W.; Shou, J.; Shi, H.; Wen, L.; Zhang, H.; Zheng, S.; Li, C.; Ma, J. Impact of Primary Tumor Size on Prognosis in Patients with Metastatic Renal Cell Carcinoma Receiving Cytoreductive Nephrectomy: A Population Study of a Chinese Center and the US SEER Database. Technol. Cancer Res. Treat. 2021, 20, 15330338211019507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tappero, S.; Barletta, F.; Piccinelli, M.L.; Cano Garcia, C.; Incesu, R.B.; Morra, S.; Scheipner, L.; Tian, Z.; Parodi, S.; Dell’Oglio, P.; et al. The Association Between Cytoreductive Nephrectomy and Overall Survival in Metastatic Renal Cell Carcinoma with Primary Tumor Size ≤ 4 cm. Eur. Urol. Focus 2023, 9, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, R.G.; Xie, W.; Becerra, M.F.; Silagy, A.W.; Attalla, K.; Sanchez, A.; Mano, R.; Marcon, J.; Blum, K.A.; Benfante, N.E.; et al. The Association Between Small Primary Tumor Size and Prognosis in Metastatic Renal Cell Carcinoma: Insights from Two Independent Cohorts of Patients Who Underwent Cytoreductive Nephrectomy. Eur. Urol. Oncol. 2020, 3, 47–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takao, S.; Ushijima, Y.; Motomura, Y.; Sakamoto, K.; Hirakawa, M.; Nishie, A.; Mimori, K.; Yamashita, Y.; Tsutsumi, T.; Ishigami, K. Radiology- and gene-based risk stratification in small renal cell carcinoma: A preliminary study. PLoS ONE 2021, 16, e0256471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moynihan, M.J.; Sullivan, T.B.; Burks, E.; Schober, J.; Calabrese, M.; Fredrick, A.; Kalantzakos, T.; Warrick, J.; Canes, D.; Raman, J.D.; et al. MicroRNA profile in stage I clear cell renal cell carcinoma predicts progression to metastatic disease. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 799.e11–799.e22. [Google Scholar] [CrossRef]
| Author | Type of Study | <1.0 | 1.1–2.0 | 2.1–3.0 | 3.1–4.0 | 4.1–5.0 | 5.1–6.0 | 6.1–7.0 |
|---|---|---|---|---|---|---|---|---|
| Remzi [7] | Single-center, retrospective | 2.4% | 8.4% | NR | NR | NR | ||
| Thompson [9] | Single-center, retrospective | 0% | 0% | 0.2% | 1.8% | 2.3% | 6.8% | 6.6% |
| Kunkle [10] | Single-center, retrospective | 0% | 0% | 10% | 12% | 21% | 28% | 39% |
| Lughezzani [11] | SEER database | 4.8% | 4.2% | 4.9% | 7.1% | 12.1% | 13.3% | 18.4% |
| Nguyen [12] | SEER database | 1.4% | 2.5% | 4.7% | 7.4% | 11.8% | 15.9% | 21.6% |
| Klatte [13] | Multi-center, retrospective | 7% | 6% | 5% | 8% | NR | NR | NR |
| Gudmundsson [14] | Population based | 0% | 4% | 5% | 10% | 14% | 15% | 28% |
| Pahernik [15] | Single-center, retrospective | 3% | 2.6% | 6.0% | NR | NR | NR | |
| Lee [16] | Single-center, retrospective | 0% | 0.5% | 1.2% | 1.4% | NR | NR | NR |
| Hellenthal [17] | Retrospective (SEER) | 4% | 4% | 5% | 7% | 11% | 13% | 18% |
| Umbreit [18] | Single-center, retrospective | 0% | 0% | 0.2% | 1.1% | 2.9% | 4.1% | 5.5% |
| Ingimarsson [19] | Population-based | 0% | 11% | 25% | ||||
| Pahernik [20] | Single-center, retrospective | 2.9% | 7.5% | 9.1% | NR | NR | NR | |
| Steffens [21] | German Renal Tumor Network | 2.3% | 2.4% | 3.0% | NR | NR | NR | |
| Miller [22] | Single-center, retrospective | 0% | 13.8% | NR | NR | NR | ||
| Zastrow [23] | Multi-center retrospective | 1.6% | 2.8% | 3.3% | 3.8% | 7.0% | ||
| Zu [23] | Single-center, retrospective | 2.1% | 1.6% | 0% | NR | NR | NR | |
| Pecoraro [24] | Retrospective (SEER) | NR | 0.7% | 1% | 1.9% | 3% | 5.1% | 7.4% |
| M Monda [25] | Retrospective (SEER) | 2.7% | 2.5% | 4% | 7.1% | 11.5% | 16.7% | |
| Kates [26] | Retrospective (SEER) | 2.4% | 4.3% | NR | NR | NR | NR | |
| Range | 0–7 | 0–6 | 0.2–10 | 1.1–13.8 | 2.3–21 | 4.1–28 | 5.5–39 | |
| Author | Presentation | Primary Tumor Size (mm) | Hystotype | Metastatic Site | Therapy | Outcome (mos) |
|---|---|---|---|---|---|---|
| Ishii [27] | Incidental | 20 | clear cell | hilar lymph nodes | open partial nephrectomy + INF | ANED (18) |
| Aizawa [28] | Incidental * | 20 | papillary | bone | BSC | DNED (4) |
| Dong [29] | Symptomatic | 18 | sarcomatoid clear cell | bones, lymph nodes, adrenals, and liver | Laparoscopic parzial nefrectomy | NR |
| Hwang [30] | Symptomatic | 18 | clear cell | sigmoid, liver, lung, etc. | BSC | DOD (3) |
| Alam [31] | Symptomatic | 16 | clear cell | bone | BSC | NR |
| Chalfin [32] | Symptomatic | 16 | clear cell | brain, lung | BSC | NR |
| Aizawa [28] | Symptomatic * | 15 | clear cell | bone | BSC | DOD (3) |
| Curry [33] | Symptomatic | 15 | tubular adenocarcinoma | supradiaphragmatic lymph nodes | radical nephrectomy | DOD (30) |
| Kume [34] | Symptomatic | 15 | clear cell + sarcomatoid | bone | embolization | DOD (27) |
| Kume [34] | Symptomatic | 13 | clear cell + sarcomatoid | bone | embolization | DOD (6) |
| Evins [35] | Symptomatic * | 13 | papillary | lungs, bone marrow, lymph nodes | orchiectomy + DES | DOD (6) |
| Masago [36] | Incidental | 13 | clear cell | pancreas | radical nephrectomy | ANED (12) |
| Aizawa [28] | Symptomatic * | 12 | sarcomatoid | chestwall | BSC | DOD (3) |
| Talamo [37] | Symptomatic * | 9 | clear cell | lungs, bones, lymph nodes | BSC | DOD (2) |
| Present case | Symptomatic | 9 | papillary | supradiaphragmatic lymph nodes | RAPN + targeted therapy | DOD (26) |
| Aizawa [28] | Symptomatic * | 8 | clear cell | bone | BSC | DOD (7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luciani, L.G.; Ceccato, T.; Cai, T.; Chiodini, S.; Botti, S.; Vattovani, V.; Puglisi, M.; Abramo, A.; Mattevi, D. Metastatic Potential of Very Small (≤2 cm) Renal Cell Carcinoma: Insights from a Single-Center Experience and Review of the Literature. J. Clin. Med. 2025, 14, 6781. https://doi.org/10.3390/jcm14196781
Luciani LG, Ceccato T, Cai T, Chiodini S, Botti S, Vattovani V, Puglisi M, Abramo A, Mattevi D. Metastatic Potential of Very Small (≤2 cm) Renal Cell Carcinoma: Insights from a Single-Center Experience and Review of the Literature. Journal of Clinical Medicine. 2025; 14(19):6781. https://doi.org/10.3390/jcm14196781
Chicago/Turabian StyleLuciani, Lorenzo Giuseppe, Tommaso Ceccato, Tommaso Cai, Stefano Chiodini, Simone Botti, Valentino Vattovani, Marco Puglisi, Andrea Abramo, and Daniele Mattevi. 2025. "Metastatic Potential of Very Small (≤2 cm) Renal Cell Carcinoma: Insights from a Single-Center Experience and Review of the Literature" Journal of Clinical Medicine 14, no. 19: 6781. https://doi.org/10.3390/jcm14196781
APA StyleLuciani, L. G., Ceccato, T., Cai, T., Chiodini, S., Botti, S., Vattovani, V., Puglisi, M., Abramo, A., & Mattevi, D. (2025). Metastatic Potential of Very Small (≤2 cm) Renal Cell Carcinoma: Insights from a Single-Center Experience and Review of the Literature. Journal of Clinical Medicine, 14(19), 6781. https://doi.org/10.3390/jcm14196781







