Efficacy of Therapies for Solar Urticaria: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Data Extraction
Definition and Classification of Primary Outcome
2.3. Quality Assessment
2.4. Data Analysis and Synthesis
3. Results
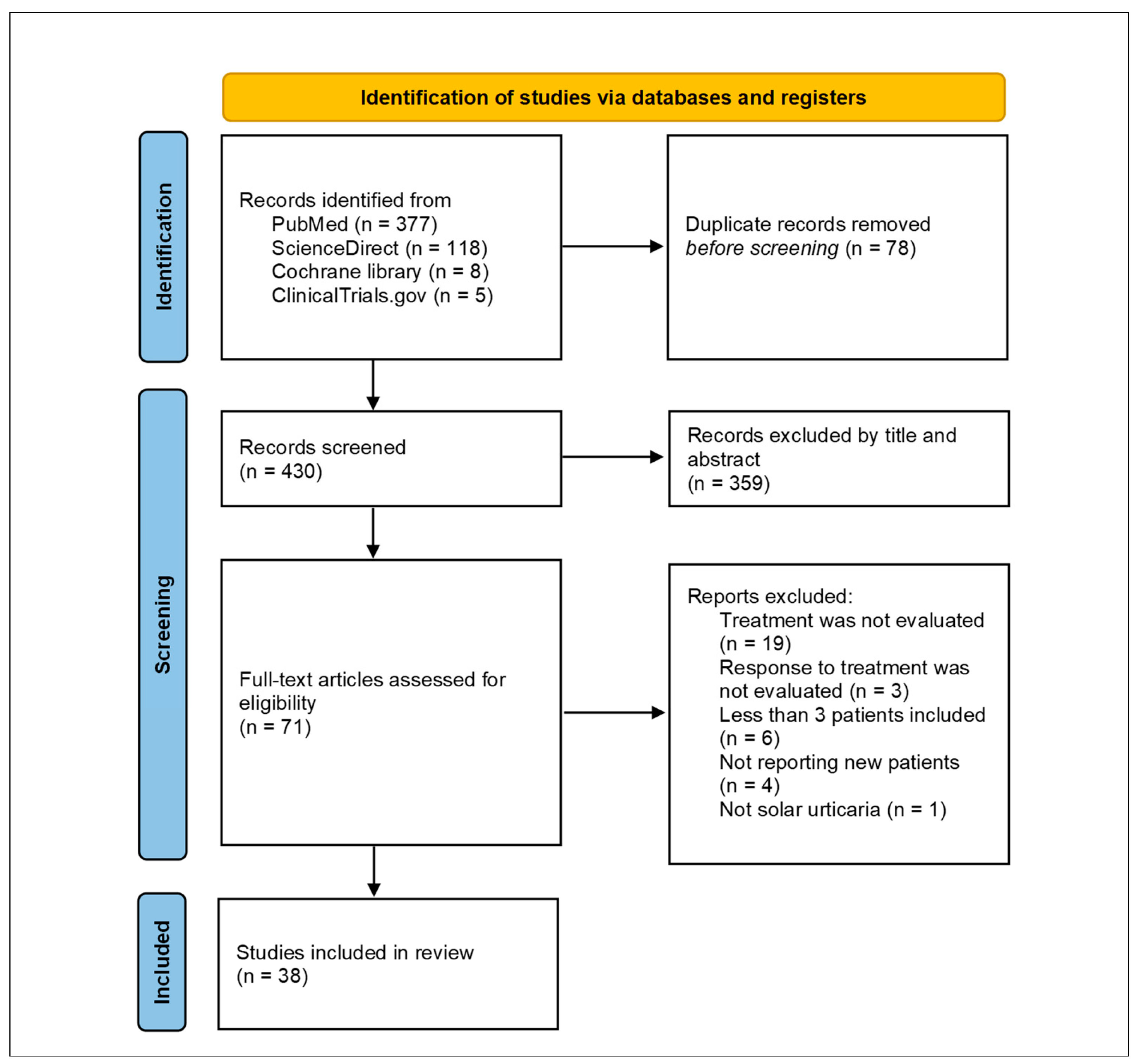
3.1. Study Selection
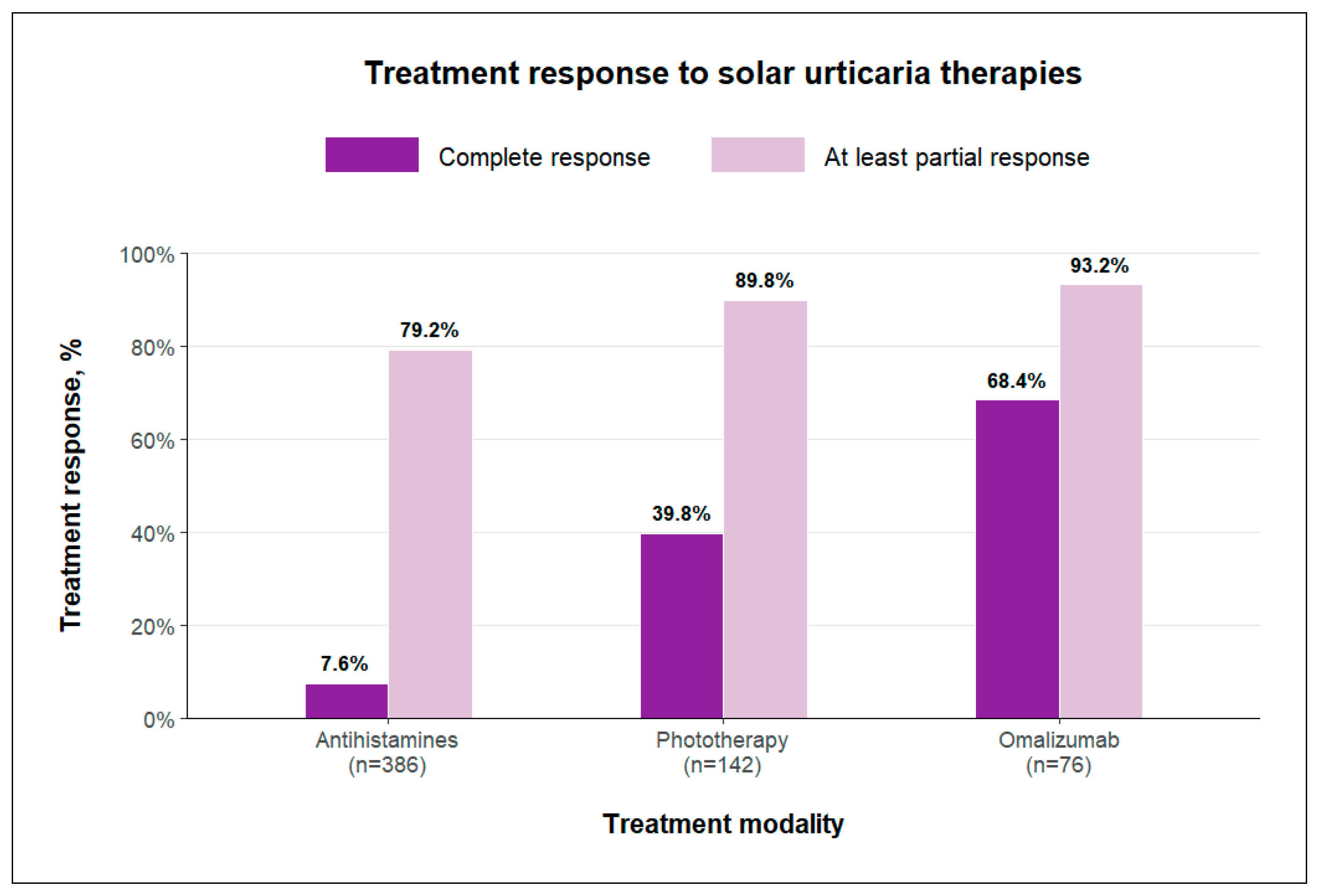
3.2. Treatment Response Evaluation
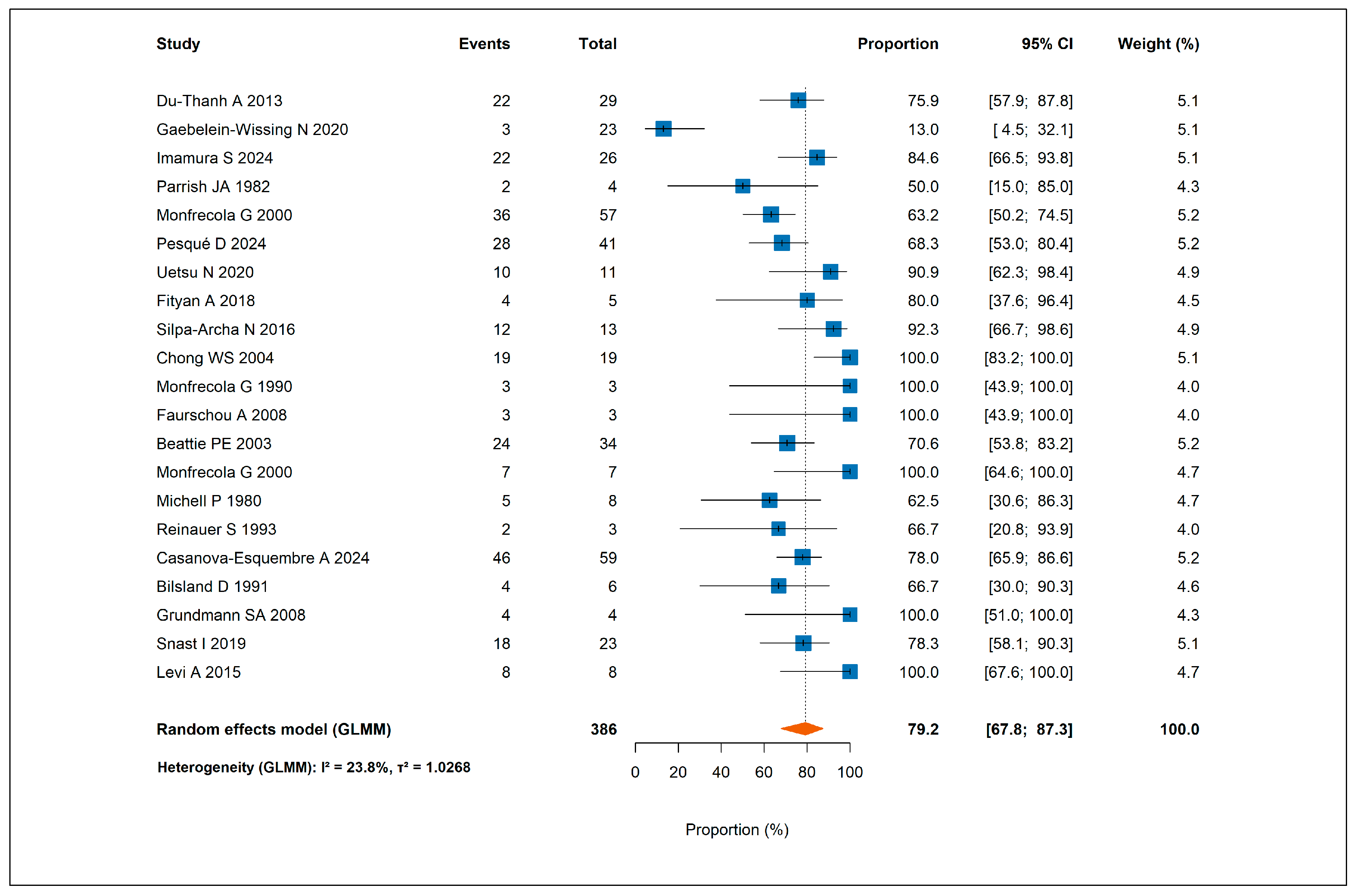
3.2.1. Response to Antihistamines
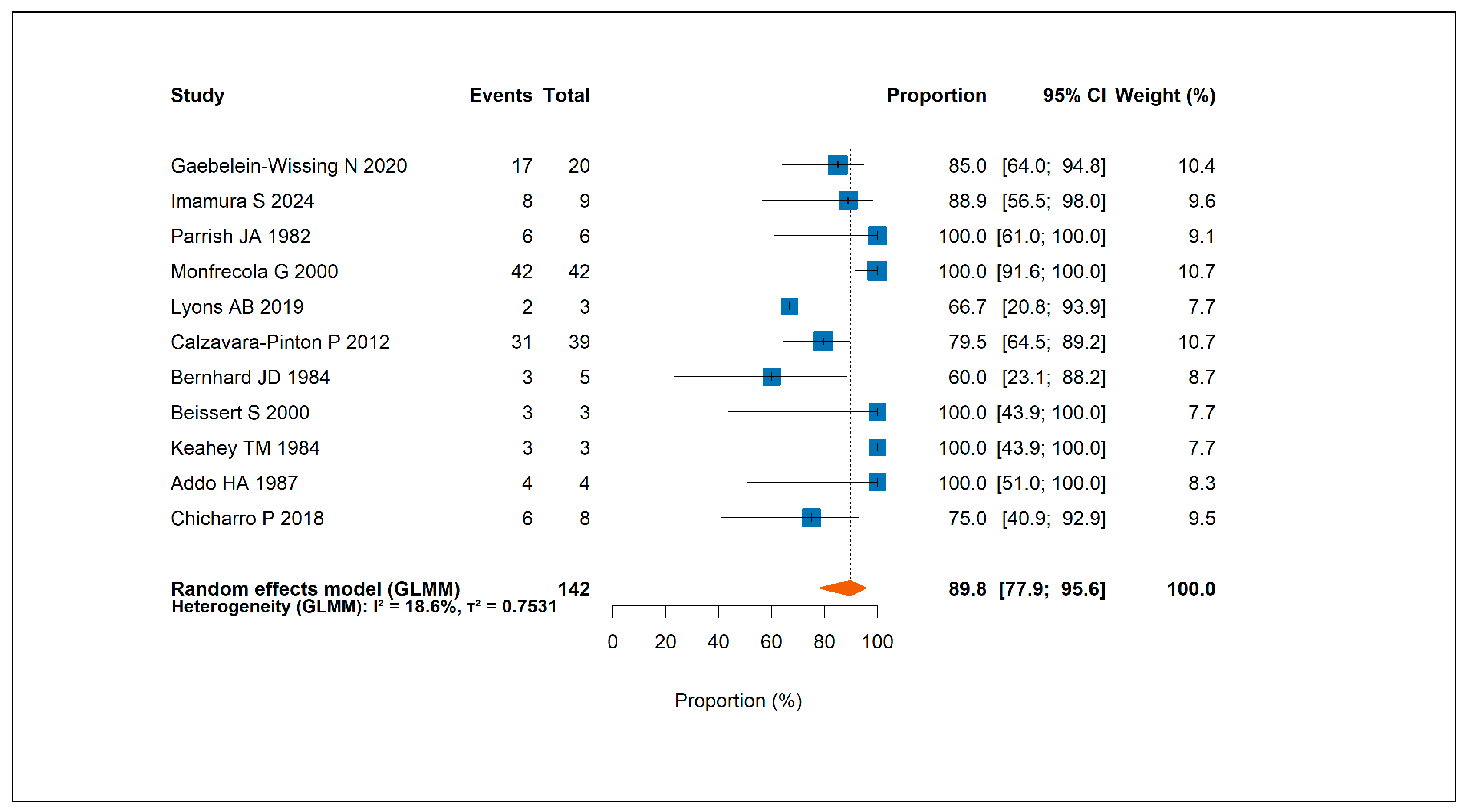
3.2.2. Response to Phototherapy
3.2.3. Response to Omalizumab
3.2.4. Other Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UVA | Ultraviolet A |
| VL | Visible light |
| LRAs | Leukotriene receptor antagonists |
| NB-UVB | Narrowband UVB |
| IVIG | Intravenous immunoglobulin |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCTs | Randomized controlled trials |
| JBI | Joanna Briggs Institute |
References
- Botto, N.C.; Warshaw, E.M. Solar urticaria. J. Am. Acad. Dermatol. 2008, 59, 909–920, quiz 921–922. [Google Scholar] [CrossRef]
- Haylett, A.K.; Koumaki, D.; Rhodes, L.E. Solar urticaria in 145 patients: Assessment of action spectra and impact on quality of life in adults and children. Photodermatol. Photoimmunol. Photomed. 2018, 34, 262–268. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Lang, D.M.; Khan, D.A.; Craig, T.; Dreyfus, D.; Hsieh, F.; Sheikh, J.; Weldon, D.; Zuraw, B.; Bernstein, D.I.; et al. The diagnosis and management of acute and 7chronic urticaria: 2014 update. J. Allergy Clin. Immunol. 2014, 133, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Du-Thanh, A.; Debu, A.; Lalheve, P.; Guillot, B.; Dereure, O.; Peyron, J.L. Solar urticaria: A time-extended retrospective series of 61 patients and review of literature. Eur. J. Dermatol. 2013, 23, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.; Enk, C.D. Treatment of solar urticaria using antihistamine and leukotriene receptor antagonist combinations tailored to disease severity. Photodermatol. Photoimmunol. Photomed. 2015, 31, 302–306. [Google Scholar] [CrossRef]
- Beissert, S.; Ständer, H.; Schwarz, T. UVA rush hardening for the treatment of solar urticaria. J. Am. Acad. Dermatol. 2000, 42, 1030–1032. [Google Scholar] [CrossRef]
- Addo, H.A.; Sharma, S.C. UVB phototherapy and photochemotherapy (PUVA) in the treatment of polymorphic light eruption and solar urticaria. Br. J. Dermatol. 1987, 116, 539–547. [Google Scholar] [CrossRef]
- Snast, I.; Kremer, N.; Lapidoth, M.; Enk, C.D.; Tal, Y.; Rosman, Y.; Confino-Cohen, R.; Hodak, E.; Levi, A. Omalizumab for the Treatment of Solar Urticaria: Case Series and Systematic Review of the Literature. J. Allergy Clin. Immunol. Pract. 2018, 6, 1198–1204.e3. [Google Scholar] [CrossRef]
- Boontaveeyuwat, E.; Willis, F.; Fassihi, H.; Sarkany, R.P.E. Successful serial plasmapheresis for solar urticaria, a case report and literature review. J. Dermatolog. Treat. 2024, 35, 2350229. [Google Scholar] [CrossRef]
- Edström, D.W.; Ros, A.M. Cyclosporin A therapy for severe solar urticaria. Photodermatol. Photoimmunol. Photomed. 1997, 13, 61–63. [Google Scholar] [CrossRef]
- Maksimovic, L.; Frémont, G.; Jeanmougin, M.; Dubertret, L.; Viguier, M. Solar urticaria successfully treated with intravenous immunoglobulins. Dermatology 2009, 218, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Matthew, S.; Edoardo, A. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Habibi, N.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Hasanoff, S.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The revised JBI critical appraisal tool for the assessment of risk of bias for quasi-experimental studies. JBI Evid. Synth. 2024, 22, 378–388. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Gaebelein-Wissing, N.; Ellenbogen, E.; Lehmann, P. Solar urticaria: Clinic, diagnostic, course and therapy management in 27 patients. J. Dtsch. Dermatol. Ges. 2020, 18, 1261–1268. [Google Scholar] [CrossRef]
- Imamura, S.; Oda, Y.; Fukumoto, T.; Mizuno, M.; Suzuki, M.; Washio, K.; Nishigori, C.; Fukunaga, A. Solar urticaria: Clinical characteristics, treatment effectiveness, long-term prognosis, and QOL status in 29 patients. Front. Med. 2024, 11, 1328765. [Google Scholar] [CrossRef]
- Monfrecola, G.; Masturzo, E.; Riccardo, A.M.; Balato, F.; Ayala, F.; Di Costanzo, M.P. Solar urticaria: A report on 57 cases. Am. J. Contact Dermat. 2000, 11, 89–94. [Google Scholar] [CrossRef]
- Uetsu, N.; Nomura, Y.; Matsuyama, Y.; Okamoto, H. Characteristics and clinical significance of augmentation spectra in solar urticaria. J. Dermatol. 2020, 47, 369–377. [Google Scholar] [CrossRef]
- Fityan, A.; McGibbon, D.; Fassihi, H.; Sarkany, R.S. Paediatric solar urticaria: A case series. Br. J. Dermatol. 2018, 178, 1453–1454. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Wongpraparut, C.; Leenutaphong, V. Analysis of solar urticaria in Thai patients. Asian Pac. J. Allergy Immunol. 2016, 34, 146–152. [Google Scholar] [CrossRef]
- Chong, W.S.; Khoo, S.W. Solar urticaria in Singapore: An uncommon photodermatosis seen in a tertiary dermatology center over a 10-year period. Photodermatol. Photoimmunol. Photomed. 2004, 20, 101–104. [Google Scholar] [CrossRef]
- Monfrecola, G.; Nappa, P.; Pini, D. Solar urticaria in the visible spectrum successfully treated with astemizole. Dermatologica 1990, 180, 154–156. [Google Scholar] [CrossRef]
- Grundmann, S.A.; Ständer, S.; Luger, T.A.; Beissert, S. Antihistamine combination treatment for solar urticaria. Br. J. Dermatol. 2008, 158, 1384–1386. [Google Scholar] [CrossRef]
- Beattie, P.E.; Dawe, R.S.; Ibbotson, S.H.; Ferguson, J. Characteristics and prognosis of idiopathic solar urticaria: A cohort of 87 cases. Arch. Dermatol. 2003, 139, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Reinauer, S.; Leenutaphong, V.; Hölzle, E. Fixed solar urticaria. J. Am. Acad. Dermatol. 1993, 29, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Snast, I.; Lapidoth, M.; Uvaidov, V.; Enk, C.D.; Mazor, S.; Hodak, E.; Levi, A. Real-life experience in the treatment of solar urticaria: Retrospective cohort study. Clin. Exp. Dermatol. 2019, 44, e164–e170. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.B.; Peacock, A.; Zubair, R.; Hamzavi, I.H.; Lim, H.W. Successful treatment of solar urticaria with UVA1 hardening in three patients. Photodermatol. Photoimmunol. Photomed. 2019, 35, 193–195. [Google Scholar] [CrossRef]
- Chicharro, P.; Rodríguez-Jiménez, P.; Capusan, T.M.; Herrero-Moyano, M.; de Argila, D. Induction of Light Tolerance Using Narrowband UV-B in Solar Urticaria. Actas Dermosifiliogr. 2018, 109, 888–892. [Google Scholar] [CrossRef]
- Morgado-Carrasco, D.; Giácaman-Von Der Weth, M.; Fustá-Novell, X.; Podlipnik, S.; Pérez-Ferriols, A.; Aguilera, P. Clinical response and long-term follow-up of 20 patients with refractory solar urticaria under treatment with omalizumab. J. Am. Acad. Dermatol. 2023, 88, 1110–1111. [Google Scholar] [CrossRef]
- Moncourier, M.; Assikar, S.; Matei, I.; Souyri, N.; Couture, M.; Rigot, E.; Delauménie, S.; Bédane, C. Visible light-induced solar urticaria is improved by omalizumab. Photodermatol. Photoimmunol. Photomed. 2016, 32, 314–316. [Google Scholar] [CrossRef]
- Sahuquillo-Torralba, A.; Rodríguez-Serna, M.; Botella Estrada, R. Effectiveness in the treatment of solar urticaria with omalizumab: Report of 7 cases. Med. Clin. 2018, 151, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Adamski, H.; Bedane, C.; Bonnevalle, A.; Thomas, P.; Peyron, J.L.; Rouchouse, B.; Cambazard, F.; Jeanmougin, M.; Viguier, M. Solar urticaria treated with intravenous immunoglobulins. J. Am. Acad. Dermatol. 2011, 65, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Hurabielle, C.; Bedane, C.; Avenel-Audran, M.; Adamski, H.; Aubin, F.; Jeanmougin, M.; Marguery, M.C.; Peyron, J.L.; Poreaux, C.; Schmutz, J.L.; et al. No major effect of cyclosporin A in patients with severe solar urticaria: A french retrospective case series. Acta Derm. Venereol. 2015, 95, 1030–1031. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jiménez, P.; Chicharro, P.; Pérez-Plaza, A.; de Argila, D. Response to Omalizumab in Solar Urticaria: Report of 3 Cases. Actas Dermosifiliogr. 2017, 108, e53–e55. [Google Scholar] [CrossRef]
- Leenutaphong, V.; Hölzle, E.; Plewig, G.; Kutkuhn, B.; Grabensee, B. Plasmapheresis in solar urticaria. Dermatologica 1991, 182, 35–38. [Google Scholar] [CrossRef]
- Parrish, J.A.; Jaenicke, K.F.; Morison, W.L.; Momtaz, K.; Shea, C. Solar urticaria: Treatment with PUVA and mediator inhibitors. Br. J. Dermatol. 1982, 106, 575–580. [Google Scholar] [CrossRef]
- Faurschou, A.; Wulf, H.C. Synergistic effect of broad-spectrum sunscreens and antihistamines in the control of idiopathic solar urticaria. Arch. Dermatol. 2008, 144, 765–769. [Google Scholar] [CrossRef]
- Monfrecola, G.; Masturzo, E.; Riccardo, A.M.; Del Sorbo, A. Cetirizine for solar urticaria in the visible spectrum. Dermatology 2000, 200, 334–335. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Zane, C.; Rossi, M.; Sala, R.; Venturini, M. Narrowband ultraviolet B phototherapy is a suitable treatment option for solar urticaria. J. Am. Acad. Dermatol. 2012, 67, e5–e9. [Google Scholar] [CrossRef]
- Bernhard, J.D.; Jaenicke, K.; Momtaz-T, K.; Parrish, J.A. Ultraviolet A phototherapy in the prophylaxis of solar urticaria. J. Am. Acad. Dermatol. 1984, 10, 29–33. [Google Scholar] [CrossRef]
- Keahey, T.M.; Lavker, R.M.; Kaidbey, K.H.; Atkins, P.C.; Zweiman, B. Studies on the mechanism of clinical tolerance in solar urticaria. Br. J. Dermatol. 1984, 110, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Aubin, F.; Avenel-Audran, M.; Jeanmougin, M.; Adamski, H.; Peyron, J.L.; Marguery, M.C.; Léonard, F.; Puyraveau, M.; Viguier, M. Omalizumab in patients with severe and refractory solar urticaria: A phase II multicentric study. J. Am. Acad. Dermatol. 2016, 74, 574–575. [Google Scholar] [CrossRef]
- Aubin, F.; Porcher, R.; Jeanmougin, M.; Léonard, F.; Bedane, C.; Moreau, A.; Schmutz, J.L.; Marguery, M.C.; Adamski, H.; Viguier, M. Severe and refractory solar urticaria treated with intravenous immunoglobulins: A phase II multicenter study. J. Am. Acad. Dermatol. 2014, 71, 948–953.e1. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, M.; Recalcati, S.; Piccinno, R. Oral polypodium leucotomos extract photoprotective activity in 57 patients with idiopathic photodermatoses. G. Ital. Dermatol. Venereol. 2011, 146, 85–87. [Google Scholar]
- Michell, P.; Hawk, J.L.; Shafrir, A.; Corbett, M.F.; Magnus, I.A. Assessing the treatment of solar urticaria. The dose-response as a quantifying approach. Dermatologica 1980, 160, 198–207. [Google Scholar] [CrossRef]
- Bilsland, D.; Ferguson, J. A comparison of cetirizine and terfenadine in the management of solar urticaria. Photodermatol. Photoimmunol. Photomed. 1991, 8, 62–64. [Google Scholar]
- Casanova-Esquembre, A.; Lorca-Spröhnle, J.; Peñuelas-Leal, R.; Pérez-Ferriols, A. Solar Urticaria and Omalizumab: A Retrospective Case-Control Study and Follow-Up. Actas Dermosifiliogr. 2024, 115, 931–932. [Google Scholar] [CrossRef]
- Pesqué, D.; Ciudad, A.; Andrades, E.; Soto, D.; Gimeno, R.; Pujol, R.M.; Giménez-Arnau, A.M. Solar Urticaria: An Ambispective Study in a Long-term Follow-up Cohort with Emphasis on Therapeutic Predictors and Outcomes. Acta Derm. Venereol. 2024, 104, adv25576. [Google Scholar] [CrossRef]
- Stern, R.S.; PUVA Follow-Up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef]
- Stern, R.S.; PUVA Follow up Study. The risk of melanoma in association with long-term exposure to PUVA. J. Am. Acad. Dermatol. 2001, 44, 755–761. [Google Scholar] [CrossRef]
- Hershkovitz, Y.; Khanimov, I.; Rubin, L.; Dranitzki, Z.; Talmon, A.; Ribak, Y.; Shamriz, O.; Levi, A.; Tal, Y. Successful ligelizumab treatment of severe refractory solar urticaria. J. Allergy Clin. Immunol. Pract. 2023, 11, 2576–2577. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Country | Study Design | No of Patients Included | Type of Treatment |
|---|---|---|---|---|
| Du-Thanh et al., 2013 [4] | France | Case series | 29 | Antihistamines |
| Gaebelein-Wissing et al., 2020 [16] | Germany | Case series | 23 | Antihistamines Phototherapy Omalizumab |
| Imamura et al., 2024 [17] | Japan | Case series | 26 | Antihistamines Phototherapy |
| Parrish et al., 1982 [37] | USA | Open-label interventional study | 8 | Antihistamines Phototherapy |
| Monfrecola et al., 2000 [18] | Italy | Case series | 57 | Antihistamines Phototherapy |
| Pesqué et al., 2024 [49] | Spain | Cohort study | 41 | Antihistamines Omalizumab |
| Uetsu et al., 2020 [19] | Japan | Case series | 11 | Antihistamines |
| Fityan et al., 2018 [20] | UK | Case series | 5 | Antihistamines |
| Silpa-Archa et al., 2016 [21] | Thailand | Case series | 13 | Antihistamines |
| Chong et al., 2004 [22] | Singapore | Case series | 19 | Antihistamines |
| Monfrecola et al., 1990 [23] | Italy | Case series | 3 | Antihistamines |
| Grundmann et al., 2008 [24] | Germany | Case series | 4 | Antihistamines + LRA |
| Faurschou et al., 2008 [38] | Denmark | Open-label interventional study | 3 | Antihistamines |
| Beattie et al., 2003 [25] | UK | Case series | 34 | Antihistamines |
| Monfrecola et al., 2000 [39] | Italy | Open-label interventional study | 7 | Antihistamines |
| Michell et al., 1980 [46] | UK | Randomized clinical trial | 8 | Antihistamines |
| Reinauer et al., 1993 [26] | Germany | Case series | 3 | Antihistamines |
| Casanova-Esquembre et al., 2024 [48] | Spain | Case-control study | 59 | Antihistamines Omalizumab |
| Bilsland et al., 1991 [47] | UK | Randomized clinical trial | 6 | Antihistamines |
| Snast et al., 2019 [27] | Israel | Case series | 23 | Antihistamines + LRA Omalizumab |
| Levi et al., 2015 [5] | Israel | Case series | 8 | Antihistamines + LRA |
| Lyons et al., 2019 [28] | USA | Case series | 3 | Phototherapy |
| Calzavara-Pinton et al., 2012 [40] | Italy | Open-label interventional study | 39 | Phototherapy |
| Bernhard et al., 1984 [41] | USA | Open-label interventional study | 5 | Phototherapy |
| Beissert et al., 2000 [6] | Germany | Case series | 3 | Phototherapy |
| Keahey et al., 1984 [42] | USA | Open-label interventional study | 3 | Phototherapy |
| Addo et al., 1987 [7] | UK | Open-label interventional study | 4 | Phototherapy |
| Chicharro et al., 2018 [29] | Spain | Case series | 8 | Phototherapy |
| Morgado-Carrasco et al., 2023 [30] | Spain | Case series | 20 | Omalizumab |
| Moncourier et al., 2016 [31] | France | Case series | 4 | Omalizumab |
| Aubin et al., 2016 [43] | France | Open-label interventional study | 10 | Omalizumab |
| Sahuquillo-Torralba et al., 2018 [32] | Spain | Case series | 7 | Omalizumab |
| Rodríguez-Jiménez et al., 2017 [35] | Spain | Case series | 3 | Omalizumab |
| Adamski et al., 2011 [33] | France | Case series | 7 | IVIG |
| Aubin et al., 2014 [44] | France | Open-label interventional study | 9 | IVIG |
| Hurabielle et al., 2015 [34] | France | Case series | 11 | Cyclosporine |
| Leenutaphong et al., 1991 [36] | Germany | Case series | 3 | Plasmapheresis |
| Caccialanza et al., 2011 [45] | Italy | Open-label interventional study | 4 | Polypodium leucotomos |
| Clinical Assessment | Phototesting | Both Clinical Assessment and Phototesting | Not Specified | |
|---|---|---|---|---|
| No of studies | 14 | 6 | 15 | 3 |
| No of patients | 251 | 30 | 165 | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engler Markowitz, M.; Noyman, Y.; Khanimov, I.; Zahavi, I.; Davidovici, B.; Kassem, R.; Mimouni, D.; Levi, A. Efficacy of Therapies for Solar Urticaria: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5736. https://doi.org/10.3390/jcm14165736
Engler Markowitz M, Noyman Y, Khanimov I, Zahavi I, Davidovici B, Kassem R, Mimouni D, Levi A. Efficacy of Therapies for Solar Urticaria: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(16):5736. https://doi.org/10.3390/jcm14165736
Chicago/Turabian StyleEngler Markowitz, Maya, Yehonatan Noyman, Israel Khanimov, Itay Zahavi, Batya Davidovici, Riad Kassem, Daniel Mimouni, and Assi Levi. 2025. "Efficacy of Therapies for Solar Urticaria: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 16: 5736. https://doi.org/10.3390/jcm14165736
APA StyleEngler Markowitz, M., Noyman, Y., Khanimov, I., Zahavi, I., Davidovici, B., Kassem, R., Mimouni, D., & Levi, A. (2025). Efficacy of Therapies for Solar Urticaria: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(16), 5736. https://doi.org/10.3390/jcm14165736






