Blood Pressure Variability and Low-Grade Inflammation in Pediatric Patients with Primary Hypertension
Abstract
1. Introduction
- To compare different BPV indices between children with untreated PH and healthy children.
- To determine the relationship between BPV indices and inflammatory parameters in these groups of patients.
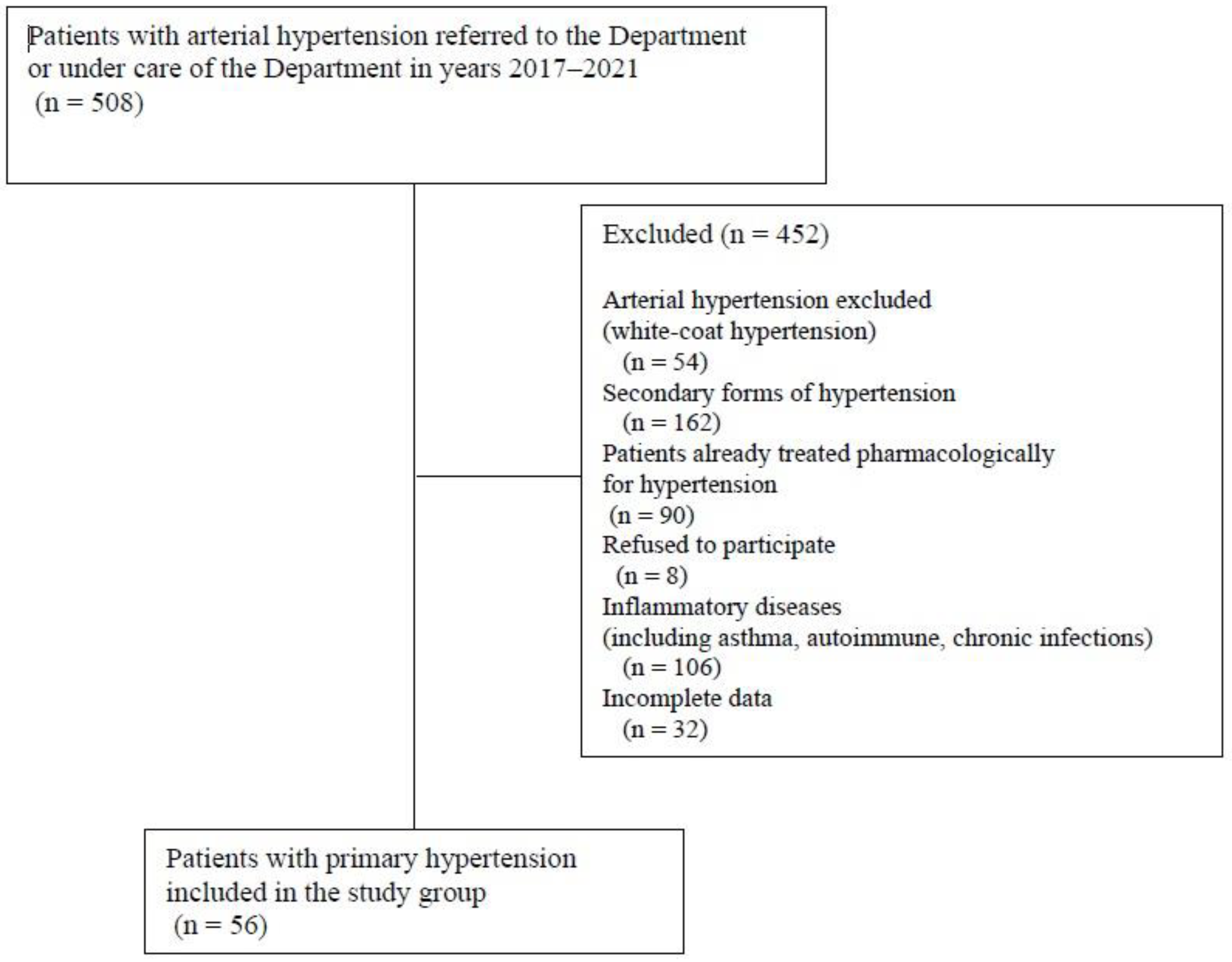
2. Materials and Methods
- BP variability—the standard deviation (SD) of systolic, diastolic, and mean blood pressure within 24 h (24 h SBPV, 24 h DBPV, 24 h MAPV).
- Systolic and diastolic blood pressure dipping (SBP DIP, DBP DIP)—the difference between daytime and nighttime blood pressure expressed in percentages of daytime blood pressure [%].
- Morning blood pressure surge was defined as the mean BP in the first 2 h after wake-up minus the mean BP 2 h before wake-up [mm Hg].
- PP/SBP ratio—PP/SBP during 24 h (24 h PP/SBP).
- Rate–pressure product (index)—heart rate multiplied by systolic blood pressure during 24 h (24 h RPI) [bpm·mm Hg].
- The variation in 24-h weighted blood pressure was defined as the mean of daytime and nighttime BP adjusted for the day and night period (the mean of the daytime and nighttime SDs, weighted for the duration of the daytime and nighttime periods) (24 h WSBPV, 24 h WDBV, 24 h WMAPV).
- Coefficient of variation (CoV) was defined as blood pressure standard deviation/divided by mean BP expressed in percentiles during 24 h (24 h CoVSBP, 24 h CoVDBP, 24 h CoVMAP) [%].
- Ambulatory arterial stiffness index (AASI) was defined as one minus the regression slope of DBP over SBP 24 h ABPM values [23].
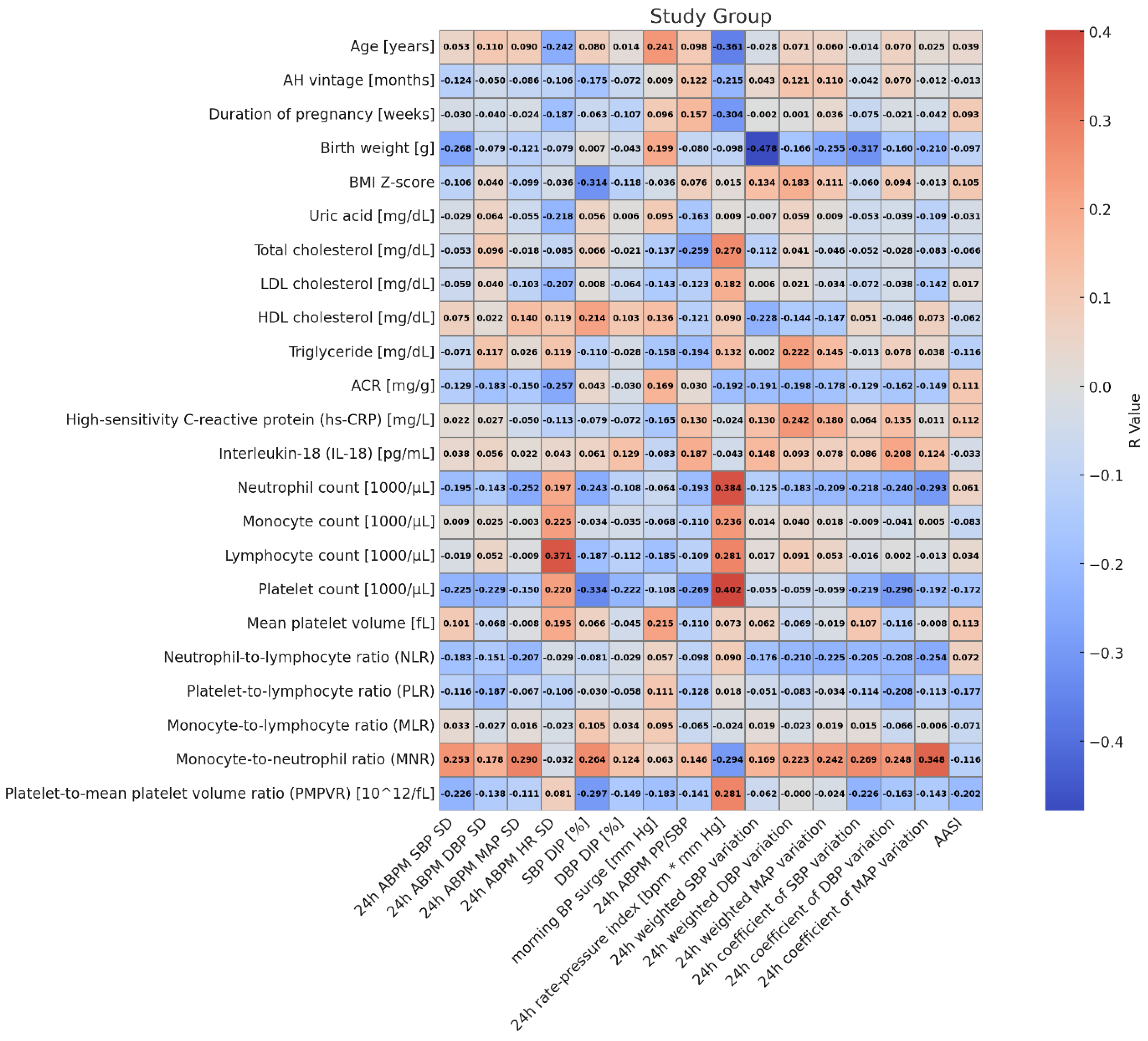
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Aune, D.; Huang, W.; Nie, J.; Wang, Y. Hypertension and the Risk of All-Cause and Cause-Specific Mortality: An Outcome-Wide Association Study of 67 Causes of Death in the National Health Interview Survey. BioMed Res. Int. 2021, 2021, 9376134. [Google Scholar] [CrossRef]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global Prevalence of Hypertension in Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Malhotra, M.; Banker, A.; Shete, S.; Hashmi, S.S.; Tyson, J.E.; Barratt, M.S.; Hecht, J.T.; Milewicz, D.M.; Boerwinkle, E. Essential hypertension vs. secondary hypertension among children. Am. J. Hypertens. 2015, 28, 73–80. [Google Scholar]
- Litwin, M.; Feber, J.; Niemirska, A.; Michałkiewicz, J. Primary hypertension is a disease of premature vascular aging associated with neuro-immuno-metabolic abnormalities. Pediatr. Nephrol. 2016, 31, 185–194. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Urbina, E.M.; Brady, T.M.; Baker-Smith, C.; Daniels, S.R.; Hayman, L.L.; Mitsnefes, M.; Tran, A.; Zachariah, J.P. Ambulatory Blood Pressure Monitoring in Children and Adolescents: 2022 Update: A Scientific Statement From the American Heart Association. Hypertension 2022, 79, e114–e124. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Bilo, G.; Kollias, A.; Pengo, M.; Ochoa, J.E.; Castiglioni, P.; Stergiou, G.S.; Mancia, G.; Asayama, K.; Asmar, R.; et al. Blood pressure variability: Methodological aspects, clinical relevance and practical indications for management—A European Society of Hypertension position paper ∗. J. Hypertens. 2023, 41, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Saputra, P.B.T.; Lamara, A.D.; Saputra, M.E.; Pasahari, D.; Kurniawan, R.B.; Farabi, M.J.A.; Multazam, C.; Oktaviono, Y.H.; Alkaff, F.F. Long-term systolic blood pressure variability independent of mean blood pressure is associated with mortality and cardiovascular events: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102343. [Google Scholar] [CrossRef]
- Parati, G.; Ochoa, J.E.; Lombardi, C.; Bilo, G. Assessment and management of blood-pressure variability. Nat. Rev. Cardiol. 2013, 10, 143–155. [Google Scholar] [CrossRef]
- Parati, G.; Ochoa, J.E.; Salvi, P.; Lombardi, C.; Bilo, G. Prognostic value of blood pressure variability and average blood pressure levels in patients with hypertension and diabetes. Diabetes Care 2013, 36 (Suppl. 2), S312–S324. [Google Scholar] [CrossRef]
- Feber, J.; Litwin, M. Blood pressure (BP) assessment-from BP level to BP variability. Pediatr. Nephrol. 2016, 31, 1071–1079. [Google Scholar] [CrossRef]
- Dziedzic-Jankowska, K.; Bujanowicz, A.; Szyszka, M.; Stelmaszczyk-Emmel, A.; Skrzypczyk, P. Subclinical inflammation in paediatric patients with primary hypertension and white coat hypertension. Pediatr. Med. Rodz. 2024, 20, 215–224. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Bujanowicz, A.; Ofiara, A.; Szyszka, M.; Pańczyk-Tomaszewska, M. 24-h central blood pressure and immune system activation in adolescents with primary hypertension—A preliminary study. Cent. Eur. J. Immunol. 2022, 47, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczyk, P.; Przychodzień, J.; Bombińska, M.; Kaczmarska, Z.; Mazur, M.; Pańczyk-Tomaszewska, M. Complete blood count-derived inflammatory markers in adolescents with primary arterial hypertension: A preliminary report. Cent. Eur. J. Immunol. 2018, 43, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczyk, P.; Zacharzewska, A.; Szyszka, M.; Ofiara, A.; Pańczyk-Tomaszewska, M. Arterial stiffness in children with primary hypertension is related to subclinical inflammation. Cent. Eur. J. Immunol. 2021, 46, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic-Jankowska, K.; Kołodziej, M.; Skrzypczyk, P. Association of Subclinical Inflammation Markers with Primary Hypertension in Children-A Systematic Review with Meta-Analysis. J. Clin. Med. 2025, 14, 2319. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Muddasani, V.; Peterson, C.; Sheibani, N.; Arkin, C.; Cheong, I.; Majersik, J.J.; Biffi, A.; Petersen, N.; Falcone, G.J.; et al. Baseline Serum Biomarkers of Inflammation and Subsequent Visit-to-Visit Blood Pressure Variability: A Post Hoc Analysis of MESA. Am. J. Hypertens. 2023, 36, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Bind, M.A.; Peters, A.; Koutrakis, P.; Coull, B.; Vokonas, P.; Schwartz, J. Quantile Regression Analysis of the Distributional Effects of Air Pollution on Blood Pressure, Heart Rate Variability, Blood Lipids, and Biomarkers of Inflammation in Elderly American Men: The Normative Aging Study. Environ. Health. Perspect. 2016, 124, 1189–1198. [Google Scholar] [CrossRef]
- Tatasciore, A.; Zimarino, M.; Renda, G.; Zurro, M.; Soccio, M.; Prontera, C.; Emdin, M.; Flacco, M.; Schillaci, G.; De Caterina, R. Awake blood pressure variability, inflammatory markers and target organ damage in newly diagnosed hypertension. Hypertens. Res. 2008, 31, 2137–2146. [Google Scholar] [CrossRef]
- Musiał, K.; Bargenda-Lange, A.; Mazurkiewicz, P.; Gaik, M.; Gralec, S.; Zwolińska, D. Lymphocyte to monocyte ratio and blood pressure variability in childhood hypertension-a pilot study. Pediatr. Res. 2023, 93, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kikuya, M.; Asayama, K.; Ohkubo, T. Blood pressure variability and arterial stiffness parameters derived from ambulatory blood pressure monitoring. Kardiol. Pol. 2019, 77, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.G.; Dolan, E.; Gao, P.J.; Guo, H.F.; Nawrot, T.; Stanton, A.V.; Zhu, D.L.; O’Brien, E.; Staessen, J.A. Ambulatory arterial stiffness index derived from 24-h ambulatory blood pressure monitoring. Hypertension 2006, 47, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Maloberti, A.; Facchetti, R.; Cuspidi, C.; Bombelli, M.; Laurent, S.; Redon, J.; Mancia, G. Within-visit BP variability, cardiovascular risk factors, and BP control in central and eastern Europe: Findings from the BP-CARE study. J. Hypertens. 2015, 33, 2250–2256. [Google Scholar] [CrossRef]
- Mancia, G.; Schumacher, H.; Böhm, M.; Grassi, G.; Teo, K.K.; Mahfoud, F.; Parati, G.; Redon, J.; Yusuf, S. Impact of seasonal blood pressure changes on visit-to-visit blood pressure variability and related cardiovascular outcomes. J. Hypertens. 2024, 42, 1269–1281. [Google Scholar] [CrossRef]
- Parati, G.; Ochoa, J.E.; Bilo, G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr. Hypertens. Rep. 2012, 14, 421–431. [Google Scholar] [CrossRef]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef]
- Mancia, G.; Ferrari, A.; Gregorini, L.; Parati, G.; Pomidossi, G.; Bertinieri, G.; Grassi, G.; di Rienzo, M.; Pedotti, A.; Zanchetti, A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ. Res. 1983, 53, 96–104. [Google Scholar] [CrossRef]
- He, B.; Ji, D.; Zhang, B. Hypertension and its correlation with autonomic nervous system dysfunction, heart rate variability and chronic inflammation. Blood Press. 2024, 33, 2405156. [Google Scholar] [CrossRef]
- Hanevold, C.D.; Seo, J.D.; Daniels, S.R.; Falkner, B.E.; Ferguson, M.A.; Flynn, J.T.; Ingelfinger, J.R.; Khoury, P.R.; Lande, M.B.; Meyers, K.E.; et al. Ambulatory blood pressure variability in prediction of target organ injury: The SHIP AHOY study. Pediatr. Nephrol. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- El Mokadem, M.; Boshra, H.; Abd El Hady, Y.; Kasla, A.; Gouda, A. Correlation between blood pressure variability and subclinical target organ damage in patients with essential hypertension. J. Hum. Hypertens. 2020, 34, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, L.; Lin, Y.; Zheng, T.; Li, X.H.; Liu, Y.Y.; Liu, J.J.; Liu, D. Relationship between blood pressure variability and target organ damage in children with essential hypertension. Chin. J. Pediatr. 2019, 57, 93–97. [Google Scholar]
- Sharma, A.P.; Mohammed, J.; Thomas, B.; Lansdell, N.; Norozi, K.; Filler, G. Nighttime blood pressure, systolic blood pressure variability, and left ventricular mass index in children with hypertension. Pediatr. Nephrol. 2013, 28, 1275–1282. [Google Scholar] [CrossRef]
- Stabouli, S.; Papakatsika, S.; Kotronis, G.; Papadopoulou-Legbelou, K.; Rizos, Z.; Kotsis, V. Arterial stiffness and SBP variability in children and adolescents. J. Hypertens. 2015, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar]
- Kim, K.I.; Lee, J.H.; Chang, H.J.; Cho, Y.S.; Youn, T.J.; Chung, W.Y.; Chae, I.H.; Choi, D.J.; Park, K.U.; Kim, C.H. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ. J. 2008, 72, 293–298. [Google Scholar] [CrossRef]
- White, F.N.; Grollman, A. Autoimmune factors associated with infarction of the kidney. Nephron 1964, 1, 93–102. [Google Scholar] [CrossRef]
- Guzik, T.J.; Nosalski, R.; Maffia, P.; Drummond, G.R. Immune and inflammatory mechanisms in hypertension. Nat. Rev. Cardiol. 2024, 21, 396–416. [Google Scholar] [CrossRef]
- Imiela, A.M.; Mikołajczyk, T.P.; Siedliński, M.; Dobrowolski, P.; Konior-Rozlachowska, A.; Wróbel, A.; Biernat-Kałuża, E.; Januszewicz, M.; Guzik, B.; Guzik, T.J.; et al. Th17/Treg imbalance in patients with primary hyperaldosteronism and resistant hypertension. Pol. Arch. Intern. Med. 2022, 132, 16171. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, E.; Koo, S.; Kim, C.W.; Kim, I. Transfer of Th17 from Adult Spontaneous Hypertensive Rats Accelerates Development of Hypertension in Juvenile Spontaneous Hypertensive Rats. BioMed Res. Int. 2021, 2021, 6633825. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, T.P.; Śliwa, T.; Guzik, T.J. Immunometabolic switch in hypertension: How dendritic cell mineralocorticoid receptors drive Th17 polarization and blood pressure control. Eur. Heart J. 2025, 46, 1352–1354. [Google Scholar] [CrossRef]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef]
- Calcaterra, V.; Croce, S.; Vinci, F.; De Silvestri, A.; Cordaro, E.; Regalbuto, C.; Zuccotti, G.V.; Mameli, C.; Albertini, R.; Avanzini, M.A. Th17 and Treg Balance in Children with Obesity and Metabolically Altered Status. Front. Pediatr. 2020, 8, 591012. [Google Scholar] [CrossRef]
- Litwin, M.; Sladowska, J.; Antoniewicz, J.; Niemirska, A.; Wierzbicka, A.; Daszkowska, J.; Wawer, Z.T.; Janas, R.; Grenda, R. Metabolic abnormalities, insulin resistance, and metabolic syndrome in children with primary hypertension. Am. J. Hypertens. 2007, 20, 875–882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abramson, J.L.; Lewis, C.; Murrah, N.V.; Anderson, G.T.; Vaccarino, V. Relation of C-reactive protein and tumor necrosis factor-alpha to ambulatory blood pressure variability in healthy adults. Am. J. Cardiol. 2006, 98, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, H.; Yu, J. Correlation between hs-CRP, VCAM-1, LEP levels and blood pressure variability in OSAHS patients. Cell. Mol. Biol. 2023, 69, 63–66. [Google Scholar] [CrossRef]
- Anyfanti, P.; Triantafyllou, A.; Lazaridis, A.; Malliora, A.; Margouta, A.; Chionidou, A.; Nikolaidou, B.; Kotsis, V.; Gkaliagkousi, E. Short-Term Variability of Both Brachial and Aortic Blood Pressure is Increased in Patients with Immune-mediated Chronic Inflammation. High Blood Press. Cardiovasc. Prev. 2024, 31, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Stojan, G.; Magder, L.S.; Petri, M. Blood Pressure Variability and Age-related Blood Pressure Patterns in Systemic Lupus Erythematosus. J. Rheumatol. 2020, 47, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Reese, T.; Dickson, A.L.; Shuey, M.M.; Gandelman, J.S.; Barnado, A.; Barker, K.A.; Neal, J.E.; Khan, O.A.; Dupont, W.D.; Stein, C.M.; et al. Increased blood pressure visit-to-visit variability in patients with systemic lupus erythematosus: Association with inflammation and comorbidity burden. Lupus 2019, 28, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef]
- Wang, L.; Gao, J.; Liu, B.; Fu, Y.; Yao, Z.; Guo, S.; Song, Z.; Zhang, Z.; He, J.; Wang, C.; et al. The association between lymphocyte-to-monocyte ratio and all-cause mortality in obese hypertensive patients with diabetes and without diabetes: Results from the cohort study of NHANES 2001-2018. Front. Endocrinol. 2024, 15, 1387272. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Sato, H.; Shimajiri, S.; Umehara, T.; Noguchi, H.; Niino, D.; Nakayama, T. Association of troponin I and macrophages in cardiac tamponade with Stanford type A aortic dissection. Heliyon 2023, 9, e20791. [Google Scholar] [CrossRef]
- Dukić, V.; Muršić, D.; Popović Grle, S.; Jakopović, M.; Ružić, A.; Vukić Dugac, A. Monocyte-related hematological indices in acute exacerbations of chronic obstructive pulmonary disease—A new biomarker? Monaldi. Arch. Chest. Dis. 2023, 94, 2706. [Google Scholar]
- Mika, T.; Ladigan, S.; Schork, K.; Turewicz, M.; Eisenacher, M.; Schmiegel, W.; Schroers, R.; Baraniskin, A. Monocytes-neutrophils-ratio as predictive marker for failure of first induction therapy in AML. Blood Cells Mol. Dis. 2019, 77, 103–108. [Google Scholar] [CrossRef]
- Gembillo, G.; Siligato, R.; Cernaro, V.; Satta, E.; Conti, G.; Salvo, A.; Romeo, A.; Calabrese, V.; Sposito, G.; Ferlazzo, G.; et al. Monocyte to HDL ratio: A novel marker of resistant hypertension in CKD patients. Int. Urol. Nephrol. 2022, 54, 395–403. [Google Scholar] [CrossRef]
- Gan, L.; Ye, D.; Feng, Y.; Pan, H.; Lu, X.; Wan, J.; Ye, J. Immune cells and hypertension. Immunol. Res. 2024, 72, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Q.; Gao, L.; Lv, Y.; Wu, Z. Macrophage Polarization in Left Ventricular Structural Remodeling Induced by Hypertension. Rev. Cardiovasc. Med. 2024, 25, 121. [Google Scholar] [CrossRef]
- Kim, K.W.; Ivanov, S.; Williams, J.W. Monocyte Recruitment, Specification, and Function in Atherosclerosis. Cells 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic-Jankowska, K.; Pietrzak, R.; Szyszka, M.; Bujanowicz, A.; Stelmaszczyk-Emmel, A.; Werner, B.; Skrzypczyk, P. Monocyte-to-Neutrophil Ratio as an Immunological Marker of Left Ventricular Hypertrophy in Children with Primary Hypertension. J. Clin. Med. 2025, 14, 3896. [Google Scholar] [CrossRef]
- Sasaki, S.; Yoneda, Y.; Fujita, H.; Uchida, A.; Takenaka, K.; Takesako, T.; Itoh, H.; Nakata, T.; Takeda, K.; Nakagawa, M. Association of blood pressure variability with induction of atherosclerosis in cholesterol-fed rats. Am. J. Hypertens. 1994, 7, 453–459. [Google Scholar] [CrossRef]
- Kai, H.; Kudo, H.; Takayama, N.; Yasuoka, S.; Kajimoto, H.; Imaizumi, T. Large blood pressure variability and hypertensive cardiac remodeling--role of cardiac inflammation. Circ. J. 2009, 73, 2198–2203. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Xie, H.H.; Shu, H.; Yuan, W.J.; Su, D.F. Inflammation is involved in the organ damage induced by sinoaortic denervation in rats. J. Hypertens. 2003, 21, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Hjortkjær, H.; Persson, F.; Theilade, S.; Winther, S.A.; Tofte, N.; Ahluwalia, T.S.; Rossing, P. Non-dipping and higher nocturnal blood pressure are associated with risk of mortality and development of kidney disease in type 1 diabetes. J. Diabetes Complicat. 2022, 36, 108270. [Google Scholar] [CrossRef]
- Seeman, T.; Hradský, O.; Gilík, J. Nocturnal blood pressure non-dipping is not associated with increased left ventricular mass index in hypertensive children without end-stage renal failure. Eur. J. Pediatr. 2016, 175, 1091–1097. [Google Scholar] [CrossRef]
- Szyszka, M.; Skrzypczyk, P.; Ofiara, A.; Wabik, A.M.; Pietrzak, R.; Werner, B.; Pańczyk-Tomaszewska, M. Circadian Blood Pressure Profile in Pediatric Patients with Primary Hypertension. J. Clin. Med. 2022, 11, 5325. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Jiang, W. Neutrophil and monocyte ratios to high-density lipoprotein cholesterol as biomarkers in non-dipping hypertension. Clin. Exp. Hypertens. 2023, 45, 2210785. [Google Scholar] [CrossRef]
- Güntürk, E.E.; Güntürk, İ.; Topuz, A.N.; Akkaya, H.; Topuz, M. Serum interleukin-18 levels are associated with non-dipping pattern in newly diagnosed hypertensive patients. Blood Press. Monit. 2021, 26, 87–92. [Google Scholar] [CrossRef]
- Gerdes, N.; Sukhova, G.K.; Libby, P.; Reynolds, R.S.; Young, J.L.; Schönbeck, U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for atherogenesis. J. Exp. Med. 2002, 195, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Niemirska, A. Intima-media thickness measurements in children with cardiovascular risk factors. Pediatr. Nephrol. 2009, 24, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Jaworski, M.; Niemirska, A.; Litwin, M.; Szalecki, M.; Karczmarewicz, E.; Michalkiewicz, J. Vitamin D status, body composition and hypertensive target organ damage in primary hypertension. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Obrycki, Ł.; Sarnecki, J.; Pac, M.; Dereziński, T.; Lewandowska, W.; Feber, J.; Litwin, M. Accelerated vascular age in adolescents with primary hypertension. J. Hypertens. 2023, 41, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Litwin, M.; Niemirska, A.; Jaworski, M.; Sladowska, J.; Kryskiewicz, E.; Karczmarewicz, E.; Neuhoff-Murawska, J.; Wierzbicka, A.; Lorenc, R.S. Accelarated skeletal maturation in children with primary hypertension. Hypertension 2009, 54, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Boos, C.J.; Hein, A.; Khattab, A. Ambulatory arterial stiffness index, mortality, and adverse cardiovascular outcomes; Systematic review and meta-analysis. J. Clin. Hypertens. 2024, 26, 89–101. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Kollias, A.; Giovas, P.P.; Papagiannis, J.; Roussias, L.G. Ambulatory arterial stiffness index, pulse pressure and pulse wave velocity in children and adolescents. Hypertens. Res. 2010, 33, 1272–1277. [Google Scholar] [CrossRef]
- Paszynska, E.; Dmitrzak-Weglarz, M.; Ostalska-Nowicka, D.; Nowicki, M.; Gawriolek, M.; Zachwieja, J. Association of Oral Status and Early Primary Hypertension Biomarkers among Children and Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 7981. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Primary Hypertension | Control Group | p |
|---|---|---|---|
| Number of patients (n) | 56 | 30 | - |
| Age [years] | 15.1 ± 2.3 (13.9–16.8) | 14.9 ± 1.4 (13.8–15.9) | 0.159 |
| Sex [M/F] | 40/16 | 19/11 | 0.472 |
| Duration of PH [months] | 12.57 ± 0.86 (3.0–17.0) | - | - |
| Duration of pregnancy [weeks] | 39.3 ± 2.0 (40.0–40.0) | 39.5 ± 1.5 (40.0–40.0) | 0.564 |
| Birth weight [g] | 3201.1 ± 634.9 (2920–3600) | 3435.4 ± 416.3 (3050–3790) | 0.133 |
| BMI Z-score | 1.47 ± 0.86 (0.92–2.16) | 0.13 ± 0.72 (−0.30–0.75) | <0.001 |
| Serum creatinine [mg/dL] | 0.8 ± 0.2 (0.6–0.9) | 0.7 ± 0.1 (0.6–0.8) | 0.129 |
| Serum urea [mg/dL] | 26.4 ± 5.7 (22.5–30) | 25.1 ± 5.5 (22–28) | 0.328 |
| Serum uric acid [mg/dL] | 6.1 ± 1.5 (5.2–6.9) | 5.1 ± 1.1 (4–5.7) | 0.002 |
| Serum total cholesterol [mg/dL] | 163.9 ± 26.0 (145.5–179) | 157.2 ± 30.7 (133–182) | 0.233 |
| Serum LDL-cholesterol [mg/dL] | 90.4 ± 24.3 (69.5–105.7) | 86.2 ± 27.2 (64.6–109) | 0.447 |
| Serum HDL-cholesterol [mg/dL] | 50.7 ± 12.9 (43–58.5) | 57.8 ± 11.1 (50–65) | 0.013 |
| Serum triglyceride [mg/dL] | 113.7 ± 56.9 (65.5–144) | 65.7 ± 25.9 (50–78) | <0.001 |
| Urinary ACR [mg/g] | 17.5 ± 47.2 (5.0–11.4) | 9.8 ± 8.7 (4–11.8) | 0.566 |
| hsCRP [mg/L] | 4.9 ± 5.2 (1.5–7.3) | 2.8 ± 5.4 (0.5–1.9) | <0.001 |
| IL-18 [pg/mL] | 81.8 ± 76.7 (21.6–130.4) | 92.5 ± 58.1 (43.7–117.5) | 0.201 |
| Neutrophil count [×103/µL] | 3.89 ± 1.44 (2.88–4.80) | 2.63 ± 0.96 (1.94–3.16) | <0.001 |
| Monocyte count [×103/µL] | 0.56 ± 0.17 (0.45–0.65) | 0.46 ± 0.14 (0.34–0.53) | 0.003 |
| Lymphocyte count [×103/µL] | 2.44 ± 0.88 (1.90–2.71) | 2.09 ± 0.49 (1.70–2.38) | 0.060 |
| Platelet count [×103/µL] | 263.7 ± 55.3 (227.0–297.0) | 239.2 ± 52.6 (215–258) | 0.037 |
| Mean platelet volume (MPV) [fL] | 10.44 ± 1.34 (9.70–11.35) | 10.92 ± 0.73 (10.20–11.50) | 0.069 |
| Neutrophil-to-lymphocyte ratio (NLR) | 1.73 ± 0.88 (1.22–2.0) | 1.27 ± 0.41 (0.92–1.55) | 0.005 |
| Platelet-to-lymphocyte ratio (PLR) | 117.15 ± 34.25 (88.75–137.87) | 118.59 ± 32.35 (98.04–145.68) | 0.851 |
| Monocyte-to-lymphocyte ratio (MLR) | 0.25 ± 0.08 (0.19–0.30) | 0.22 ± 0.06 (0.18–0.26) | 0.173 |
| Monocyte-to-neutrophil ratio (MNR) | 0.16 ± 0.06 (0.11–0.19) | 0.19 ± 0.05 (0.14–0.22) | 0.010 |
| Platelet-to-MPV ratio (PMPVR) (1012/fL) | 25.81 ± 6.76 (20.85–30.09) | 22.02 ± 5.11 (19.8–23.45) | 0.010 |
| Parameter | Primary Hypertension | Control Group | p |
|---|---|---|---|
| Office SBP [mm Hg] | 141.7 ± 10.2 (135–148) | 113.5 ± 7.6 (109–119) | <0.001 |
| Office SBP Z-score | 2.26 ± 0.90 (1.81–2.94) | −0.15 ± 0.81 (−0.64–0.44) | <0.001 |
| Office DBP [mm Hg] | 83.1 ± 10.2 (76–91) | 65.1 ± 5.8 (60–69) | <0.001 |
| Office DBP Z-score | 2.39 ± 1.39 (1.36–3.47) | −0.01 ± 0.81 (−0.66–0.55) | <0.001 |
| 24 h ABPM SBP [mm Hg] | 134.6 ± 5.3 (131–138.5) | 113.4 ± 5.9 (110–118) | <0.001 |
| 24 h ABPM SBP Z-score | 2.34 ± 0.86 (1.73–2.76) | −0.31 ± 0.68 (−0.85–0.25) | <0.001 |
| 24 h ABPM DBP [mm Hg] | 72.2 ± 7.1 (69–77) | 62.7 ± 3.8 (60–66) | <0.001 |
| 24 h ABPM DBP Z-score | 0.77 ± 1.27 (0.20–1.54) | −0.91 ± 0.74 (−1.37–−0.42) | <0.001 |
| 24 h ABPM MAP [mm Hg] | 92.2 ± 6.3 (87–96) | 75.9 ± 4.7 (73–78) | <0.001 |
| 24 h ABPM MAP Z-score | 1.52 ± 1.26 (0.71–2.04) | −1.05 ± 0.68 (−1.44–−0.78) | <0.001 |
| PP 24 h | 62.4 ± 7.2 (58.5–68) | 50.9 ± 5.4 (47–55) | <0.001 |
| HR [bpm] | 77.0 ± 12.0 (69–87) | 74.6 ± 9.4 (75–84) | 0.338 |
| 24 h HR Z-score | −0.22 ± 1.39 (−1.14–0.68) | −0.66 ± 1.35 (−0.92–0.23) | 0.176 |
| SBPL/24 h (%) | 57.8 ± 19.4 (41–74) | 7.8 ± 6.2 (3–10) | <0.001 |
| DBPL/24 h (%) | 25.7 ± 19.4 (13–37) | 3.9 ± 3.6 (1–6) | <0.001 |
| 24 h ABPM SBP SD | 13.7 ± 2.5 (11.9–15.4) | 12.6 ± 1.8 (11–14) | 0.036 |
| 24 h ABPM DBP SD | 11.3 ± 2.5 (9.5–12.8) | 10.7 ± 2.1 (9.3–11.3) | 0.225 |
| 24 h ABPM MAP SD | 11.4 ± 2.6 (9.7–12.7) | 10.0 ± 1.9 (8.7–10.9) | 0.009 |
| 24 h ABPM HR SD | 12.5 ± 3.4 (9.8–14.5) | 12.0 ± 3.5 (9.1–14.6) | 0.602 |
| SBP DIP [%] | 11.8 ± 5.5 (8.9–15.2) | 12.9 ± 4.3 (10.5–16) | 0.337 |
| DBP DIP [%] | 16.4 ± 8.6 (11–21.6) | 19.6 ± 7 (15.7–24.4) | 0.083 |
| Morning BP surge [mm Hg] | 10.9 ± 12 (4–18) | 15.2 ± 8.1 (9–23) | 0.066 |
| 24 h ABPM PP/SBP | 0.46 ± 0.05 (0.43–0.48) | 0.45 ± 0.03 (0.43–0.47) | 0.128 |
| 24 h rate–pressure index [bpm x mm Hg] | 10,352 ± 1584 (9205–11,228) | 8466 ± 1203 (7810–9266) | <0.001 |
| 24 h weighted SBP variation | 11.4 ± 1.8 (10.2–12.5) | 10.3 ± 1.8 (9.1–11.8) | 0.009 |
| 24 h weighted DBP variation | 9.6 ± 1.7 (8.3–10.8) | 8.7 ± 1.8 (7.6–10.3) | 0.033 |
| 24 h weighted MAP variation | 9.2 ± 1.6 (7.9–9.9) | 8.0 ± 1.7 (6.8–9.8) | 0.002 |
| 24 h coefficient of SBP variation [%] | 10.2 ± 1.9 (8.9–11.5) | 11.1 ± 1.6 (9.9–12.7) | 0.012 |
| 24 h coefficient of DBP variation [%] | 15.8 ± 3.5 (13.3–18.4) | 17.1 ± 3.6 (14.8–18.1) | 0.237 |
| 24 h coefficient of MAP variation [%] | 12.4 ± 2.8 (10.3–14.1) | 13.2 ± 2.4 (11.7–13.9) | 0.134 |
| AASI | 0.39 ± 0.14 (0.32–0.50) | 0.38 ± 0.11 (0.30–0.46) | 0.709 |
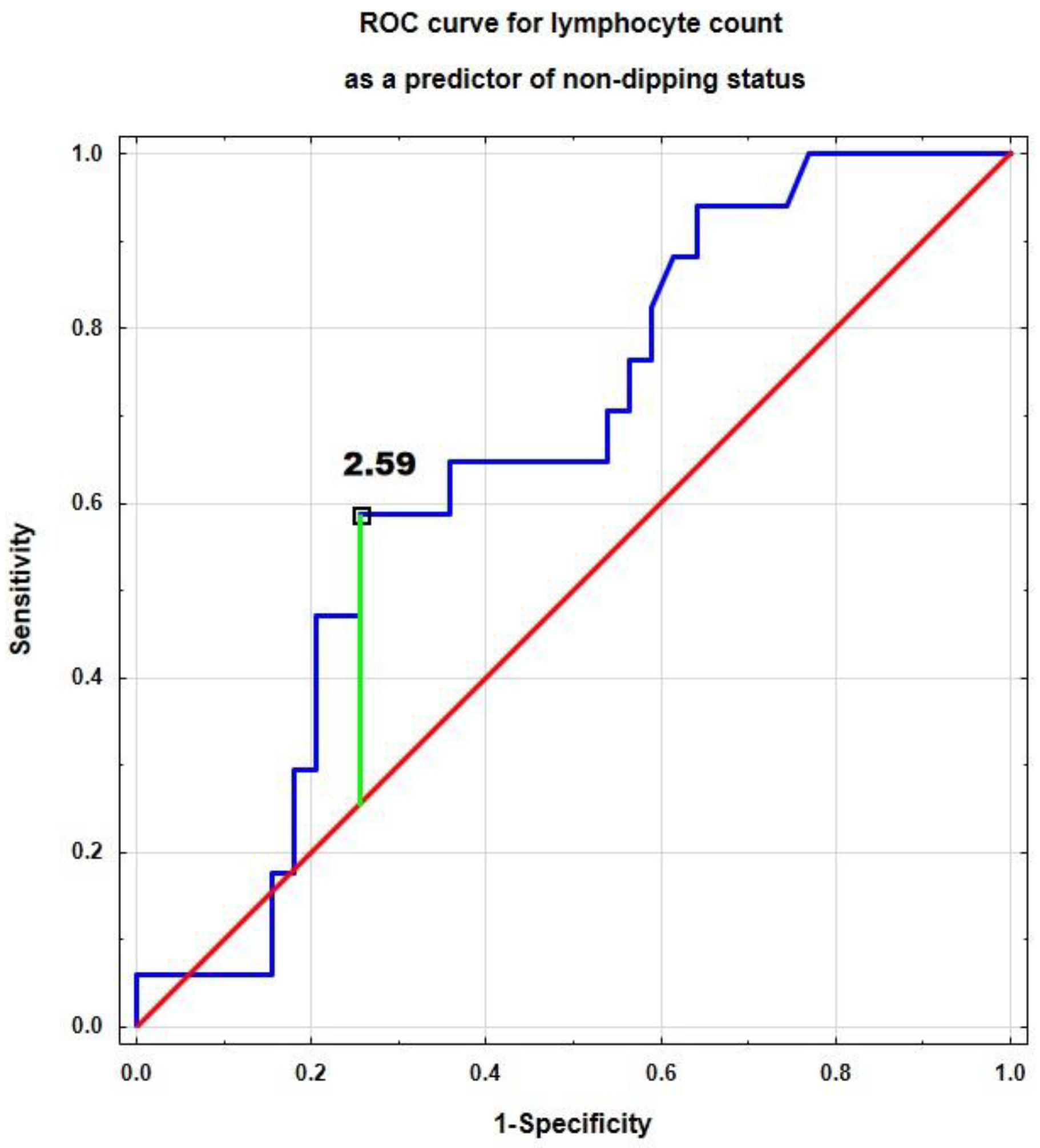
| Parameter | Area Under the Curve (95% CI) | p | Cut-Off Value | Sensitivity | Specificity | ACC |
|---|---|---|---|---|---|---|
| hsCRP [mg/L] | 0.509 (0.344–0.675) | 0.915 | 2.54 | 0.846 | 0.294 | 0.735 |
| IL-18 [pg/mL] | 0.520 (0.354–0.686) | 0.810 | 7.7 | 0.294 | 0.821 | 0.661 |
| Neutrophil count [×103/µL] | 0.574 (0.422–0.726) | 0.341 | 3.48 | 0.765 | 0.487 | 0.571 |
| Monocyte count [×103/µL] | 0.579 (0.416–0.742) | 0.341 | 0.51 | 0.765 | 0.462 | 0.554 |
| Lymphocyte count [×103/µL] | 0.656 (0.510–0.803) | 0.037 | 2.59 | 0.588 | 0.744 | 0.696 |
| Platelet count [×103/µL] | 0.659 (0.490–0.828) | 0.065 | 320 | 0.353 | 0.974 | 0.786 |
| Mean platelet volume (MPV) [fL] | 0.534 (0.370–0.698) | 0.685 | 10.6 | 0.590 | 0.647 | 0.607 |
| Neutrophil-to-lymphocyte ratio (NLR) | 0.502 (0.347–0.657) | 0.977 | 2.54 | 0.179 | 1.000 | 0.429 |
| Platelet-to-lymphocyte ratio (PLR) | 0.525 (0.361–0.689) | 0.766 | 122 | 0.538 | 0.647 | 0.571 |
| Monocyte-to-lymphocyte ratio (MLR) | 0.563 (0.401–0.725) | 0.443 | 0.26 | 0.462 | 0.765 | 0.554 |
| Monocyte-to-neutrophil ratio (MNR) | 0.545 (0.382–0.708) | 0.586 | 0.217 | 0.179 | 1.000 | 0.429 |
| Platelet-to-MPV ratio (PMPVR) (1012/fL) | 0.637 (0.470–0.803) | 0.107 | 24.8 | 0.706 | 0.564 | 0.607 |
| Blood Pressure Variability Index (Dependent Variable) | Predictor (Independent Variable) | Standardized Beta (95% CI) | p |
|---|---|---|---|
| 24 h ABPM MAP SD | MNR | 0.290 (0.029–0.551) | 0.030 |
| 24 h rate–pressure index [bpm·mm Hg] | Age [years] | −0.336 (−0.572–−0.101) | 0.006 |
| Monocytes [×103/µL] | 0.281 (0.041–0.521) | 0.023 | |
| MNR | −0.348 (−0.587–−0.108) | 0.005 | |
| 24 h weighted DBP variation | Age [years] | 0.298 (0.001–0.596) | 0.049 |
| hsCRP [mg/L] | 0.310 (0.055–0.564) | 0.018 | |
| MNR | 0.286 (0.032–0.540) | 0.028 | |
| Sex (M/F) | −0.364 (−0.663–−0.065) | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic-Jankowska, K.; Szyszka, M.; Bujanowicz, A.; Stelmaszczyk-Emmel, A.; Skrzypczyk, P. Blood Pressure Variability and Low-Grade Inflammation in Pediatric Patients with Primary Hypertension. J. Clin. Med. 2025, 14, 5737. https://doi.org/10.3390/jcm14165737
Dziedzic-Jankowska K, Szyszka M, Bujanowicz A, Stelmaszczyk-Emmel A, Skrzypczyk P. Blood Pressure Variability and Low-Grade Inflammation in Pediatric Patients with Primary Hypertension. Journal of Clinical Medicine. 2025; 14(16):5737. https://doi.org/10.3390/jcm14165737
Chicago/Turabian StyleDziedzic-Jankowska, Katarzyna, Michał Szyszka, Adam Bujanowicz, Anna Stelmaszczyk-Emmel, and Piotr Skrzypczyk. 2025. "Blood Pressure Variability and Low-Grade Inflammation in Pediatric Patients with Primary Hypertension" Journal of Clinical Medicine 14, no. 16: 5737. https://doi.org/10.3390/jcm14165737
APA StyleDziedzic-Jankowska, K., Szyszka, M., Bujanowicz, A., Stelmaszczyk-Emmel, A., & Skrzypczyk, P. (2025). Blood Pressure Variability and Low-Grade Inflammation in Pediatric Patients with Primary Hypertension. Journal of Clinical Medicine, 14(16), 5737. https://doi.org/10.3390/jcm14165737







