Levosimendan vs. Dobutamine in Patients with Septic Shock: A Systematic Review and Meta-Analysis with Trial Sequential Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Risk of Bias in Individual Studies
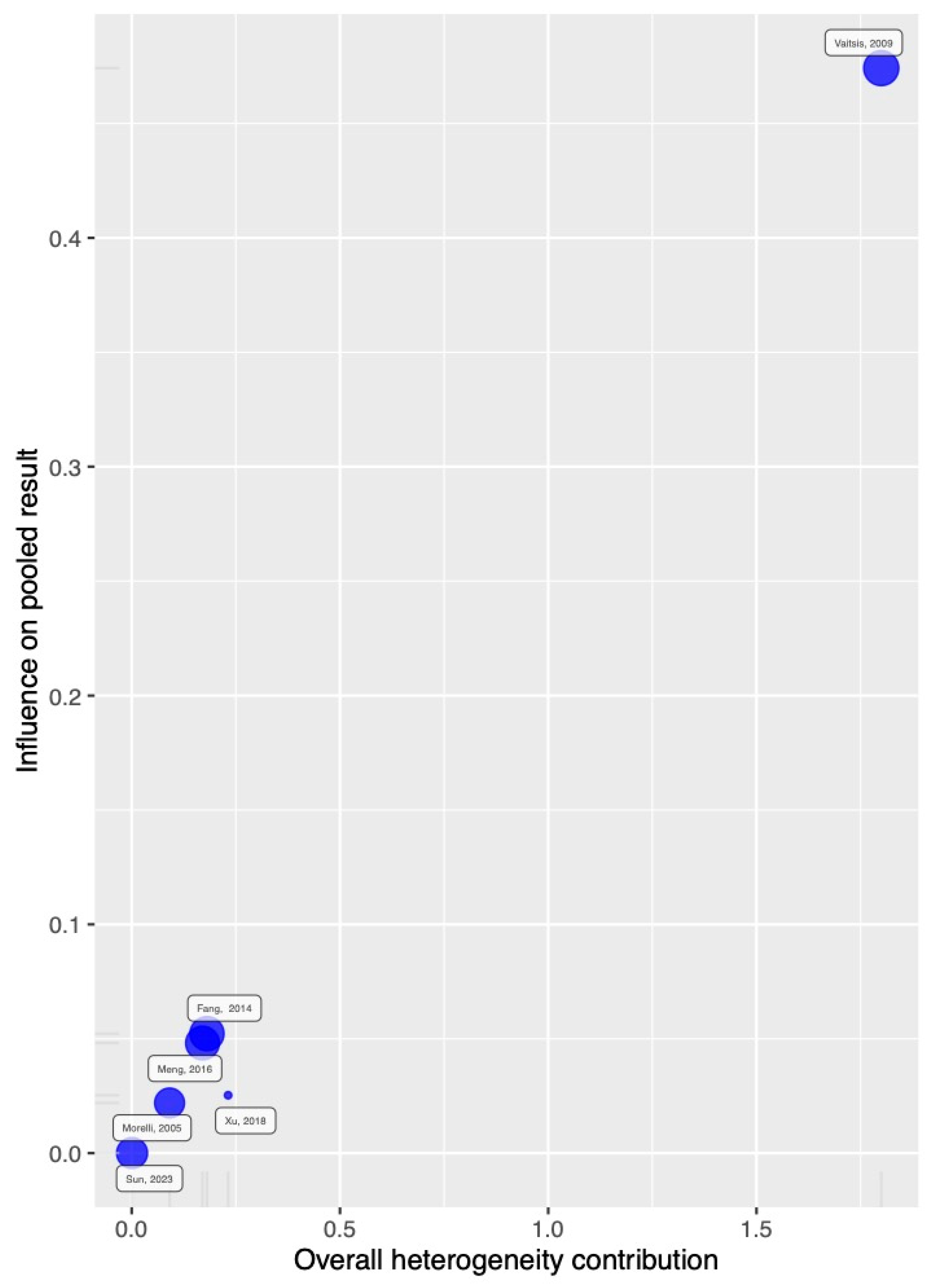
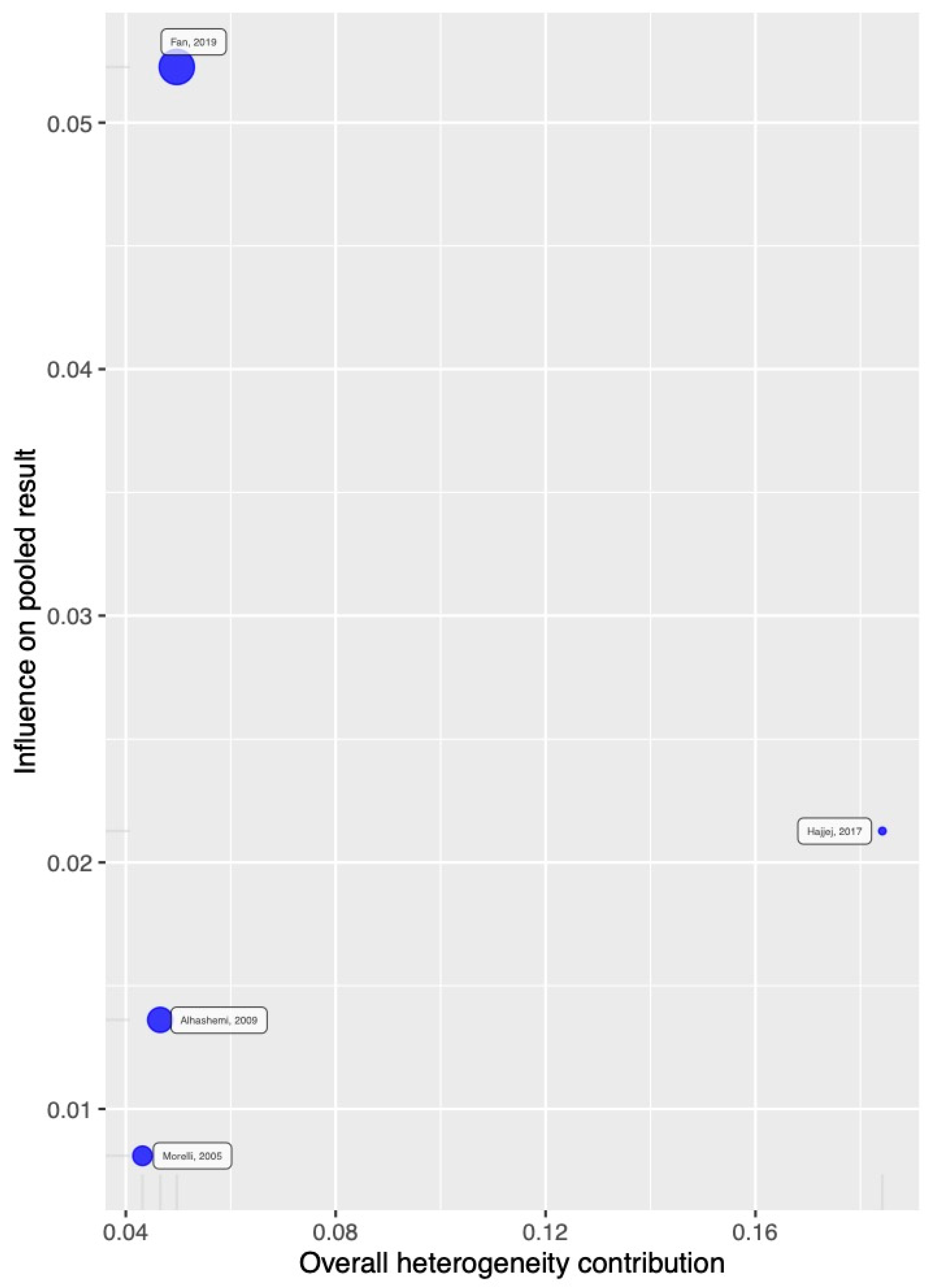
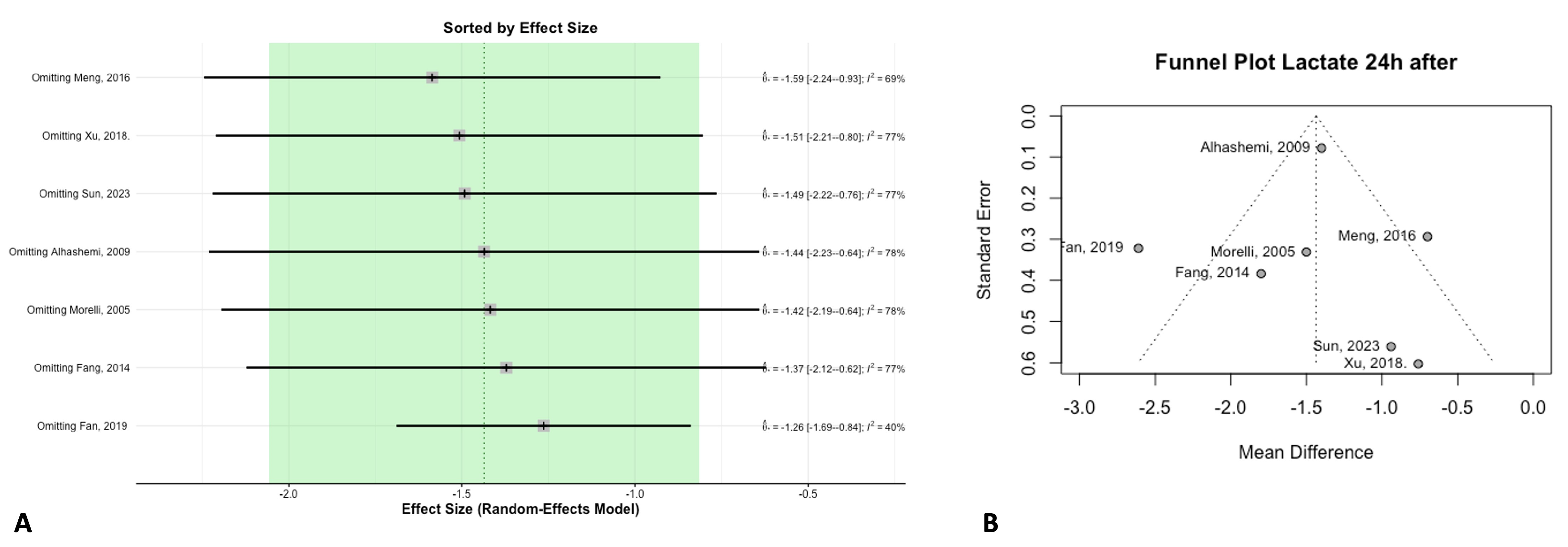
2.6. Publication Bias
2.7. Subgroup Analysis and TSA
3. Results
3.1. Study Selection and Characteristics of the Included Trial
3.2. ROB in the Studies
3.3. Mortality Analysis
Secondary Analysis with LeoPARDS
3.4. Biomarkers
3.5. Hemodynamic Parameters and ICU Stay
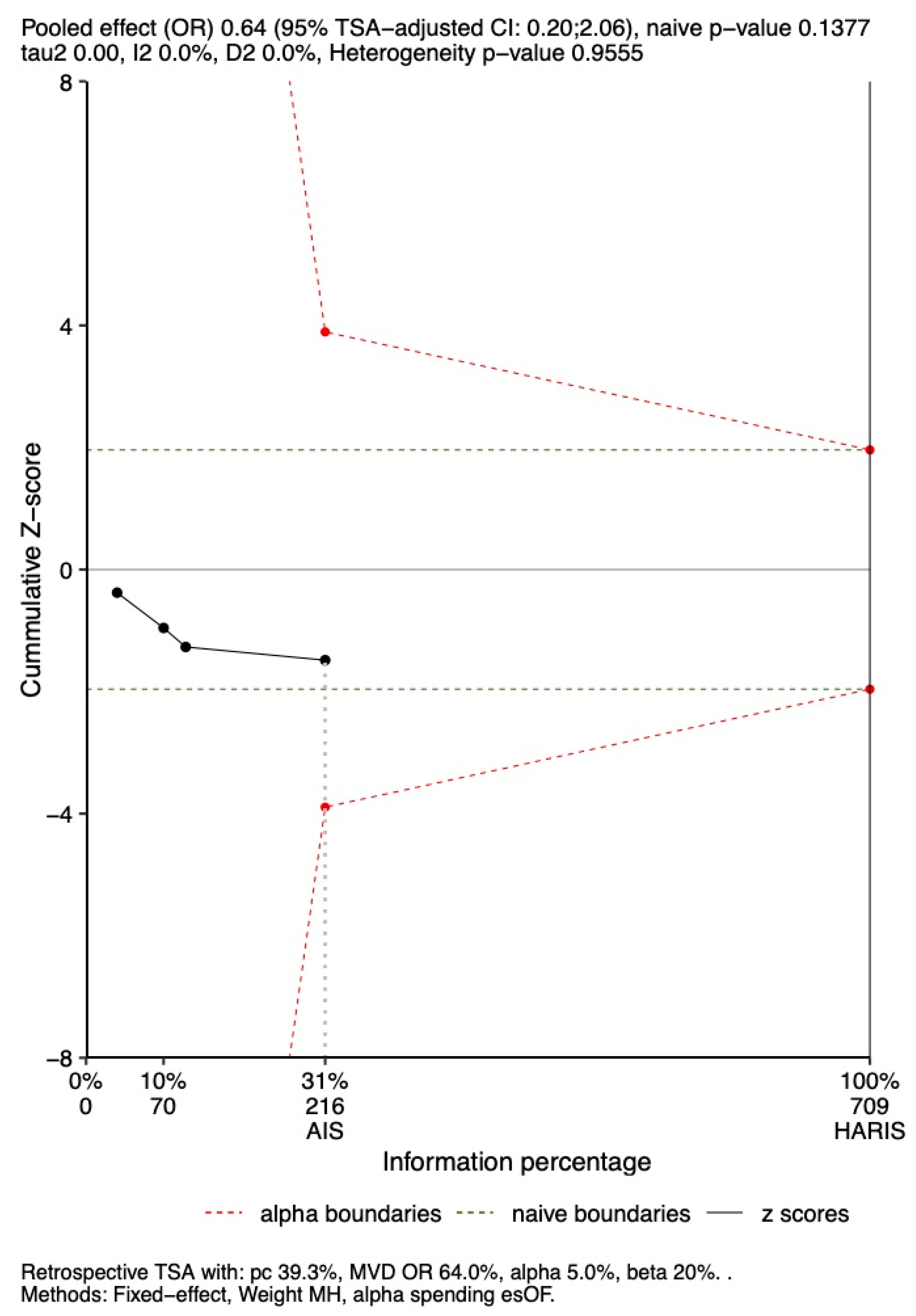
3.6. TSA
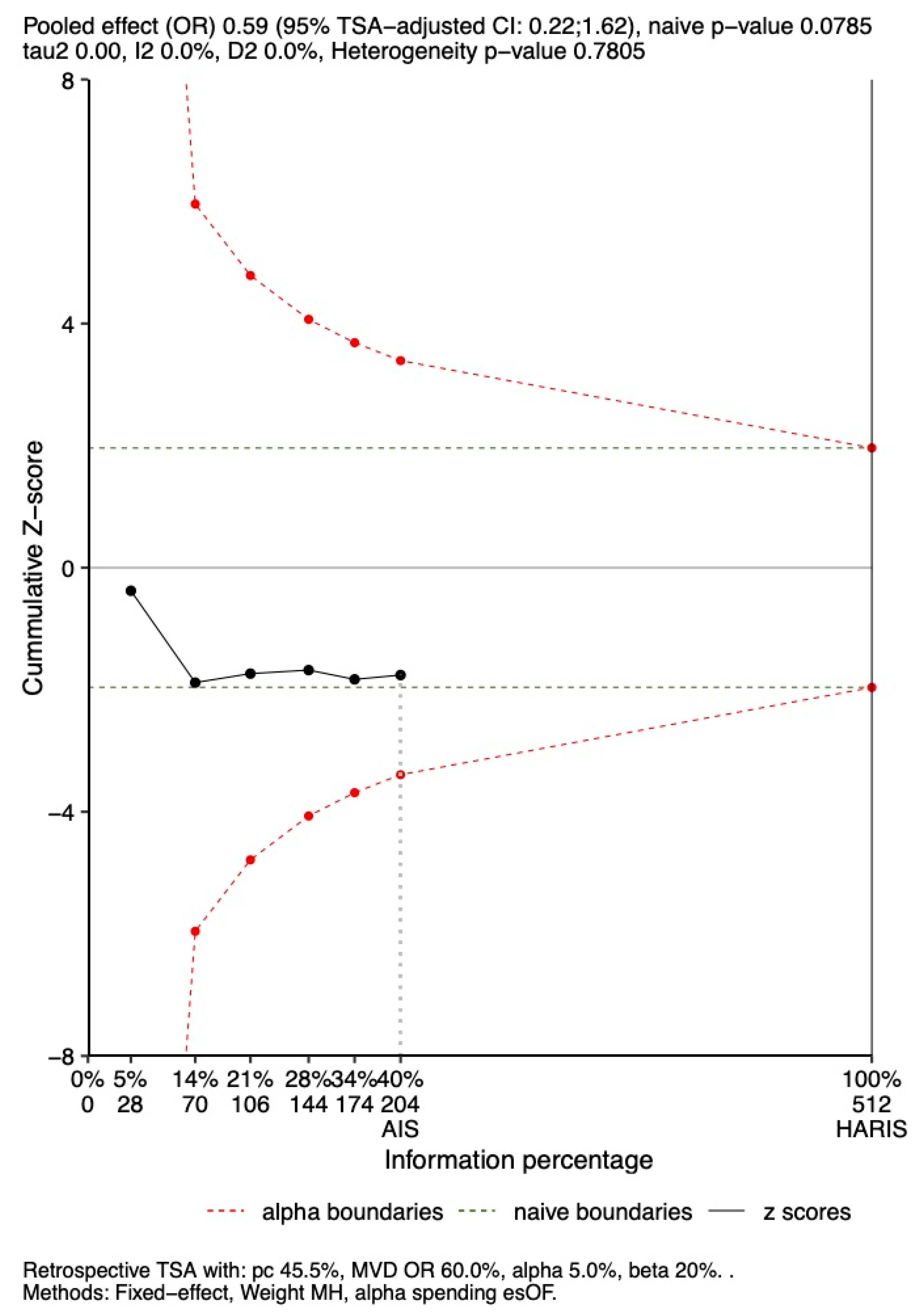
3.6.1. In-Hospital Mortality
3.6.2. 28-Day Mortality
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SICM | Septic-induced cardiomyopathy |
| TSA | Trial sequential analysis |
| OR | Odds ratio |
| CI | Confidence interval |
| SMD | Standardized mean difference |
| MAP | Mean arterial pressure |
| LVEF | Left ventricular ejection fraction |
| BNP | Brain natriuretic peptide |
| ICU | Intensive care unit |
| APACHE II | Acute Physiology and Chronic Health Evaluation |
| TnI | Troponin I |
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Dellinger, R.P.; Levy, M. The Surviving Sepsis Campaign: A History and a Perspective. Surg. Infect. 2010, 11, 275–281. [Google Scholar] [CrossRef]
- Srzić, I. Sepsis Definition: What’s New in the Treatment Guidelines. Acta Clin Croat [Internet]. 2022 [citado 1 de diciembre de 2024]. Available online: https://hrcak.srce.hr/clanak/407695 (accessed on 17 February 2025).
- Siegler, B.H.; Brenner, T.; Uhle, F.; Weiterer, S.; Weigand, M.A.; Hofer, S. Why a second look might be worth it: Immuno-modulatory therapies in the critically ill patient. J. Thorac. Dis. 2016, 8, E424–E430. [Google Scholar] [CrossRef] [PubMed]
- Balk, R.A. Systemic inflammatory response syndrome (SIRS): Where did it come from and is it still relevant today? Virulence 2014, 5, 20–26. [Google Scholar] [CrossRef]
- Parker, M.M. Profound but Reversible Myocardial Depression in Patients with Septic Shock. Ann. Intern. Med. 1984, 100, 483. [Google Scholar] [CrossRef]
- Lima, M.R.; Silva, D. Septic cardiomyopathy: A narrative review. Rev. Port. Cardiol. 2023, 42, 471–481. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Caille, V.; Charron, C.; Belliard, G.; Page, B.; Jardin, F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit. Care Med. 2008, 36, 1701–1706. [Google Scholar] [CrossRef]
- Zakynthinos, G.E.; Giamouzis, G.; Xanthopoulos, A.; Oikonomou, E.; Kalogeras, K.; Karavidas, N.; Dimeas, I.E.; Gialamas, I.; Gounaridi, M.I.; Siasos, G.; et al. Septic Cardiomyopathy: Difficult Definition, Challenging Diagnosis, Unclear Treatment. J. Clin. Med. 2025, 14, 986. [Google Scholar] [CrossRef]
- Boissier, F.; Aissaoui, N. Septic cardiomyopathy: Diagnosis and management. J. Intensive Med. 2022, 2, 8–16. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Soni, K.D.; Maitra, S.; Baidya, D.K. Levosimendan does not provide mortality benefit over dobutamine in adult patients with septic shock: A meta-analysis of randomized controlled trials. J. Clin. Anesth. 2017, 39, 67–72. [Google Scholar] [CrossRef]
- Herpain, A.; Bouchez, S.; Girardis, M.; Guarracino, F.; Knotzer, J.; Levy, B.; Liebregts, T.; Pollesello, P.; Ricksten, S.-E.; Riha, H.; et al. Use of Levosimendan in Intensive Care Unit Settings: An Opinion Paper. J. Cardiovasc. Pharmacol. 2019, 73, 3–14. [Google Scholar] [CrossRef]
- Gordon, A.C.; Perkins, G.D.; Singer, M.; McAuley, D.F.; Orme, R.M.L.; Santhakumaran, S.; Mason, A.J.; Cross, M.; Al-Beidh, F.; Best-Lane, J.; et al. Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis. N. Engl. J. Med. 2016, 375, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, N.; Cui, N.; Wang, S.H.; Ding, X.; Li, N.; Chen, N.; Yu, Z.B. Efficacy of Levosimendan in the Treatment of Patients With Severe Septic Cardiomyopathy. J. Cardiothorac. Vasc. Anesth. 2023, 37, 344–349. [Google Scholar] [CrossRef]
- Meng, J.B.; Hu, M.H.; Lai, Z.Z.; Ji, C.L.; Xu, X.J.; Zhang, G.; Tian, S. Levosimendan versus dobutamine in myocardial injury patients with septic shock: A randomized controlled trial. Med. Sci. Monit. 2016, 22, 1486–1496. [Google Scholar] [CrossRef]
- Morelli, A.; Donati, A.; Ertmer, C.; Rehberg, S.; Lange, M.; Orecchioni, A.; Cecchini, V.; Landoni, G.; Pelaia, P.; Pietropaoli, P.; et al. Levosimendan for resuscitating the microcirculation in patients with septic shock: A randomized controlled study. Crit. Care 2010, 14, R232. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; de Castro, S.; Teboul, J.L.; Singer, M.; Rocco, M.; Conti, G.; de Luca, L.; di Angelantonio, E.; Orecchioni, A.; Pandian, N.G.; et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 2005, 31, 638–644. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, H.; Yan, J.; Le, J.; Zhou, X.; Zhu, J. Levosimendan’s Influence on Myocardial Depression and Patients with Septic Shock. Acta Medica Mediterr. 2019, 35, 1103–1107. [Google Scholar]
- Memiş, D.; Inal, M.T.; Sut, N. The effects of levosimendan vs. dobutamine added to dopamine on liver functions assessed with noninvasive liver function monitoring in patients with septic shock. J. Crit. Care 2012, 27, e1–e318. [Google Scholar] [CrossRef]
- Vaitsis, J.; Michalopoulou, H.; Thomopoulos, C.; Massias, S.; Stamatis, P. Use of levosimendan in myocardial dysfunction due to sepsis. Crit. Care 2009, 13 (Suppl. 1), P165. [Google Scholar] [CrossRef]
- Alhashemi, J.A.; Alotaibi, Q.A.; Abdullah, G.M.; Shalabi, S.A. Levosimendan vs. dobutamine in septic shock. J. Crit. Care 2009, 24, e14–e15. [Google Scholar] [CrossRef] [PubMed]
- Hajjej, Z.; Meddeb, B.; Sellami, W.; Labbene, I.; Morelli, A.; Ferjani, M. Effects of Levosimendan on Cellular Metabolic Alterations in Patients with Septic Shock: A Randomized Controlled Pilot Study. Shock 2017, 48, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.X.; Li, L.; Gong, S.J.; Yu, Y.H.; Yan, J. The effects of levosimendan on the cardiac function and prognosis in elderly patients with septic shock and myocardial contractility impairment. Zhonghua Nei Ke Za Zhi 2018, 57, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Dong, S. Effects of levosimendan on hemodynamics and cardiac function in patients with septic shock. Chin. Crit. Care Med. 2014, 26, 692–696. [Google Scholar] [CrossRef]
- Coloretti, I.; Tosi, M.; Biagioni, E.; Busani, S.; Girardis, M. Management of Sepsis in the First 24 Hours: Bundles of Care and Individualized Approach. Semin. Respir. Crit. Care Med. 2024, 45, 503–509. [Google Scholar] [CrossRef]
- De Backer, D.; Cecconi, M.; Chew, M.S.; Hajjar, L.; Monnet, X.; Ospina-Tascón, G.A.; Ostermann, M.; Pinsky, M.R.; Vincent, J.-L. A plea for personalization of the hemodynamic management of septic shock. Crit. Care 2022, 26, 372. [Google Scholar] [CrossRef]
- Geri, G.; Vignon, P.; Aubry, A.; Fedou, A.-L.; Charron, C.; Silva, S.; Repessé, X.; Vieillard-Baron, A. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: A post hoc analysis. Intensive Care Med. 2019, 45, 657–667. [Google Scholar] [CrossRef]
- Guan, Q.; Zhang, C.; Li, B.; Huang, D.; Li, A.; Qin, J.; Zhang, X. Meta-Analysis of the Efficacy of Levosimendan in the Treatment of Severe Sepsis Complicated with Septic Cardiomyopathy. Heart Surg. Forum 2023, 26, E609–E620. [Google Scholar] [CrossRef]
- Hu, K.; Mathew, R. Inotrope and vasopressor use in cardiogenic shock: What, when and why? Curr. Opin. Crit. Care 2022, 28, 419–425. [Google Scholar] [CrossRef]
- Fernando, S.M.; Mathew, R.; Sadeghirad, B.; Brodie, D.; Belley-Côté, E.P.; Thiele, H.; Van Diepen, S.; Fan, E.; Di Santo, P.; Simard, T.; et al. Inotropes, vasopressors, and mechanical circulatory support for treatment of cardiogenic shock complicating myocardial infarction: A systematic review and network meta-analysis. Can. J. Anesth. Can. Anesth. 2022, 69, 1537–1553. [Google Scholar] [CrossRef]
- Xiong, F.; Hu, Z.; Liu, C.; Zhang, K.; Zhou, Q.; Chen, J.; Lu, H.; Yue, X.; Zhao, J.; Pan, P. The therapeutic effect of levosimendan in patients with prolonged ventilator weaning and cardiac dysfunction. J. Int. Med. Res. 2024, 52, 03000605241263166. [Google Scholar] [CrossRef]
- Sangalli, F.; Bellani, G.; Affronti, A.; Volpi, F.; Feri, M.; Marini, M.; Quacquarelli, A.; Vitale, D.; Guarracino, F. Levosimendan to facilitate weaning from cardiorespiratory support in critically ill patients: Current evidence and future directions. Minerva Anestesiol. 2020, 86, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Jacky, A.; Rudiger, A.; Krüger, B.; Wilhelm, M.J.; Paal, S.; Seifert, B.; Spahn, D.R.; Bettex, D. Comparison of Levosimendan and Milrinone for ECLS Weaning in Patients After Cardiac Surgery—A Retrospective Before-and-After Study. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2112–2119. [Google Scholar] [CrossRef]
- Sangalli, F.; Avalli, L.; Laratta, M.; Formica, F.; Maggioni, E.; Caruso, R.; Cristina Costa, M.; Guazzi, M.; Fumagalli, R. Effects of Levosimendan on Endothelial Function and Hemodynamics During Weaning From Veno-Arterial Extracorporeal Life Support. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Papp, Z.; Agostoni, P.; Alvarez, J.; Bettex, D.; Bouchez, S.; Brito, D.; Černý, V.; Comin-Colet, J.; Crespo-Leiro, M.G.; Delgado, J.F.; et al. Levosimendan Efficacy and Safety: 20 Years of SIMDAX in Clinical Use. J. Cardiovasc. Pharmacol. 2020, 76, 4–22. [Google Scholar] [CrossRef]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic Cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Pollesello, P.; Papp, Z.; Papp, J.G. Calcium sensitizers: What have we learned over the last 25years? Int. J. Cardiol. 2016, 203, 543–548. [Google Scholar] [CrossRef]
- Mebazaa, A.; Nieminen, M.S.; Packer, M.; Cohen-Solal, A.; Kleber, F.X.; Pocock, S.J.; Thakkar, R.; Padley, R.J.; Põder, P.; Kivikko, M.; et al. Levosimendan vs. Dobutamine for Patients With Acute Decompensated Heart Failure: The SURVIVE Randomized Trial. JAMA 2007, 297, 1883. [Google Scholar] [CrossRef]
- Packer, M.M. REVIVE II and SURVIVE: Use of Levosimendan for the Treatment of Acute Decompensated Heart Failure. Available online: https://www.medscape.org/viewarticle/523043 (accessed on 17 February 2025).
- Damiani, E.; Carsetti, A.; Casarotta, E.; Domizi, R.; Scorcella, C.; Donati, A.; Adrario, E. Microcirculation-guided resuscitation in sepsis: The next frontier? Front. Med. 2023, 10, 1212321. [Google Scholar] [CrossRef]
- González, R.; Urbano, J.; López-Herce, J. Resuscitating the macro- vs. microcirculation in septic shock. Curr. Opin. Pediatr. 2024, 36, 274–281. [Google Scholar] [CrossRef]
- Wang, H.; Ding, H.; Wang, Z.-Y.; Zhang, K. Research progress on microcirculatory disorders in septic shock: A narrative review. Medicine 2024, 103, e37273. [Google Scholar] [CrossRef] [PubMed]
- Mannino, F.; Urzì Brancati, V.; Lauro, R.; Pirrotta, I.; Rottura, M.; Irrera, N.; Cavallini, G.M.; Pallio, G.; Gitto, E.; Manti, S. Levosimendan and Dobutamin Attenuate LPS-Induced Inflammation in Microglia by Inhibiting the NF-κB Pathway and NLRP3 Inflammasome Activation via Nrf2/HO-1 Signalling. Biomedicines 2024, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Faivre, V.; Kaskos, H.; Callebert, J.; Losser, M.-R.; Milliez, P.; Bonnin, P.; Payen, D.; Mebazaa, A. Cardiac and Renal Effects of Levosimendan, Arginine Vasopressin, and Norepinephrine in Lipopolysaccharide-treated Rabbits. Anesthesiology 2005, 103, 514–521. [Google Scholar] [CrossRef]
- García-Bardon, A.; Kamuf, J.; Ziebart, A.; Liu, T.; Krebs, N.; Dünges, B.; Kelm, R.F.; Morsbach, S.; Mohr, K.; Heimann, A.; et al. Levosimendan increases brain tissue oxygen levels after cardiopulmonary resuscitation independent of cardiac function and cerebral perfusion. Sci. Rep. 2021, 11, 14220. [Google Scholar] [CrossRef]
- Coopersmith, C.M. Levosimendan and gut mucosal blood flow—Not all inotropes are created equal*. Crit. Care Med. 2005, 33, 246. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.; Klompas, M.; Rhee, C. What Is the Utility of Measuring Lactate Levels in Patients with Sepsis and Septic Shock? Semin. Respir. Crit. Care Med. 2021, 42, 650–661. [Google Scholar] [CrossRef]
- Hasslacher, J.; Bijuklic, K.; Bertocchi, C.; Kountchev, J.; Bellmann, R.; Dunzendorfer, S.; Joannidis, M. Levosimendan inhibits release of reactive oxygen species in polymorphonuclear leukocytes in vitro and in patients with acute heart failure and septic shock: A prospective observational study. Crit. Care 2011, 15, R166. [Google Scholar] [CrossRef]
- Antcliffe, D.B.; Santhakumaran, S.; Orme, R.M.L.; Ward, J.K.; Al-Beidh, F.; O’Dea, K.; Perkins, G.D.; Singer, M.; McAuley, D.F.; Mason, A.J.; et al. Levosimendan in septic shock in patients with biochemical evidence of cardiac dysfunction: A subgroup analysis of the LeoPARDS randomised trial. Intensive Care Med. 2019, 45, 1392–1400. [Google Scholar] [CrossRef]
- Claire, R.; Gluud, C.; Berlin, I.; Coleman, T.; Leonardi-Bee, J. Using Trial Sequential Analysis for estimating the sample sizes of further trials: Example using smoking cessation intervention. BMC Med. Res. Methodol. 2020, 20, 284. [Google Scholar] [CrossRef]
- Doorduin, J.; Sinderby, C.A.; Beck, J.; Stegeman, D.F.; Van Hees, H.W.H.; Van Der Hoeven, J.G.; Heunks, L.M.A. The Calcium Sensitizer Levosimendan Improves Human Diaphragm Function. Am. J. Respir. Crit. Care Med. 2012, 185, 90–95. [Google Scholar] [CrossRef]




| Study | Year | Levosimendan Group | Control Group | Levosimendan Dose (IV) | Dobutamine Dose (IV) | Inclusion Criteria | Outcomes |
|---|---|---|---|---|---|---|---|
| Sun, et al. [15] | 2023 | 15 | 15 | 0.2 μg/kg/min for 24 h | 5 μg/kg/min for 24 h. | Patients within the first 48 h from sepsis onset, with MAP maintained at 65 mmHg with norepinephrine, who presented LVEF ≤ 35% after fluid resuscitation and increase in myocardial injury markers after fluid resuscitation and vasoactive drug treatment. | Mortality 28-day. Hemodynamics. NE dose. Lactate, cTnI, NTproBNP, and PCR levels. Length of mechanical ventilation. ICU stay (days) ICU costs. |
| Meng, et al. [16] | 2016 | 19 | 19 | 0.2 μg/kg/min for 24 h. | 5 μg/kg/min for 24 h. | Patients with septic shock and established normovolemia using norepinephrine to maintain MAP of at least 65 mmHg, with LVEF ≤ 45% after fluid resuscitation and vasopressor therapy. | Hemodynamics. HFABP, TNI, BNP, and Lactate levels. Mortality 28 days. ICU stay. Hospital stay. Length of mechanical ventilation. NE dose (baseline and 24 h). |
| Morelli, et al. [17] | 2010 | 20 | 30 | 0.2 μg/kg/min for 24 h. | 5 μg/kg/min for 24 h. | Patients within the first 24 h from the onset of septic shock after having established normovolemia and an MAP of at least 65 mmHg using norepinephrine. | Hemodynamics. Microcirculatory flow variables. Lactate. NE dose (baseline and 24 h). Mortality (ICU). ICU stay. |
| Morelli, et al. [18] | 2005 | 15 | 13 | 0.2 μg/kg/min for 24 h. | 5 μg/kg/min for 24 h. | Patients within the first 24 h from the onset of septic shock after having established normovolemia, norepinephrine, and dobutamine given to maintain MAP of at least 65 mmHg while maintaining Hb > 7 g/dL. | Hemodynamics. Gastric perfusion. Lactate and TnI levels. Mortality (in-hospital, ICU, 30-day). |
| Fan, et al. [19] | 2019 | 63 | 63 | Bolus 6–12 μg/kg, continued at 0.1 μg/kg/min for 24 h. | 5 μg/kg/min for 3 days. | Patients with septic shock managed under early goal-directed therapy, needing norepinephrine for blood pressure maintenance. | Cardiac function parameters. Lactate, BNP, PCT, and cTnI concentrations. APACHE II score. NE total dose. O2 inhalation time of mechanical ventilation. In-hospital mortality. ICU stay. |
| Memiş, et al. [20] | 2012 | 15 | 15 | 0.1 μg/kg/min for 24 h. | 10 μg/kg/min for 24 h. | Critically ill patients who met at least two of the criteria of septic shock, as defined by CHEST, with MAP of ≤65 mmHg despite dopamine infusion. | Liver function. Hemodynamic variables. ICU stay. |
| Vaitsis, et al. [21] | 2009 | 23 | 19 | 0.1 μg/kg/min for 24 h | 5–10 μg/kg/min for 24 h. | Patients with sepsis and severe cardiac dysfunction (CI ≤ 2.2, LVEF ≤ 35%). | Mortality at 7 and 30 days. |
| Alhashemi, et al. [22] | 2009 | 21 | 21 | 0.05–0.2 μg/kg/min for 24 h. | 5–20 μg/kg/min for 7 days maximum. | Patients with severe sepsis or septic shock managed with norepinephrine infusion titrated to an MAP of at least 65 mmHg. | ICU mortality, first-day serum lactate. |
| Hajjej, et al. [23] | 2017 | 10 | 10 | 0.2 μg/kg/min for 24 h. | 5 μg/kg/min for 72 h. | Patients with septic shock requiring norepinephrine to maintain an MAP of at least 65 mmHg despite appropriate volume resuscitation. | Systemic hemodynamics. Global oxygen transport. Acid-base homeostasis. Muscle microdialysis variables. |
| Xu, et al. [24] | 2018 | 15 | 15 | 0.2 μg/kg/min for 24 h. | 5 μg/kg/min for 24 h. | Elderly patients with sepsis with LVEF ≤ 50% after fluid resuscitation. | Cardiac function parameters. Lactate levels. Length of mechanical ventilation. ICU stay. Mortality 28-day. |
| Fang, et al. [25] | 2014 | 18 | 18 | Dobutamine + 0.2 μg/kg/min for 24 h. | 5 μg/kg/min for 48 h. | Patients with septic shock with LVEF ≤ 45% after fluid resuscitation. | Hemodynamics and cardiac function. Lactate levels. 24-h urinary output. NE total dose. Mortality (ICU and 28-day). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, E.E.; Jaramillo, G.A.D.; Cuellar, L.M.R.; Herran, S.E.P.; Lima, D.R.R.; Herpain, A. Levosimendan vs. Dobutamine in Patients with Septic Shock: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. J. Clin. Med. 2025, 14, 5496. https://doi.org/10.3390/jcm14155496
Rodríguez EE, Jaramillo GAD, Cuellar LMR, Herran SEP, Lima DRR, Herpain A. Levosimendan vs. Dobutamine in Patients with Septic Shock: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Journal of Clinical Medicine. 2025; 14(15):5496. https://doi.org/10.3390/jcm14155496
Chicago/Turabian StyleRodríguez, Edith Elianna, German Alberto Devia Jaramillo, Lissa María Rivera Cuellar, Santiago Eduardo Pérez Herran, David René Rodríguez Lima, and Antoine Herpain. 2025. "Levosimendan vs. Dobutamine in Patients with Septic Shock: A Systematic Review and Meta-Analysis with Trial Sequential Analysis" Journal of Clinical Medicine 14, no. 15: 5496. https://doi.org/10.3390/jcm14155496
APA StyleRodríguez, E. E., Jaramillo, G. A. D., Cuellar, L. M. R., Herran, S. E. P., Lima, D. R. R., & Herpain, A. (2025). Levosimendan vs. Dobutamine in Patients with Septic Shock: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Journal of Clinical Medicine, 14(15), 5496. https://doi.org/10.3390/jcm14155496







