Review of Neurostimulation Therapies for Obstructive Sleep Apnea: Hypoglossal Nerve Stimulation and Beyond
Abstract
Introduction
Funding
Conflicts of Interest
Abbreviations
| OSA | Obstructive Sleep Apnea |
| CPAP | Continuous Positive Airway Pressure |
| IOAs | Intraoral Appliances |
| UAS | Upper Airway Surgery |
| MMA | Maxillomandibular Advancement |
| HNS | Hypoglossal Nerve Stimulation |
| AHI | Apnea–Hypopnea Index |
| ODI | Oxygen Desaturation Index |
| STAR | Stimulation Treatment for Apnea Reduction |
| FOSQ | Functional Outcomes of Sleep Questionnaire |
| ESS | Epworth Sleepiness Scale |
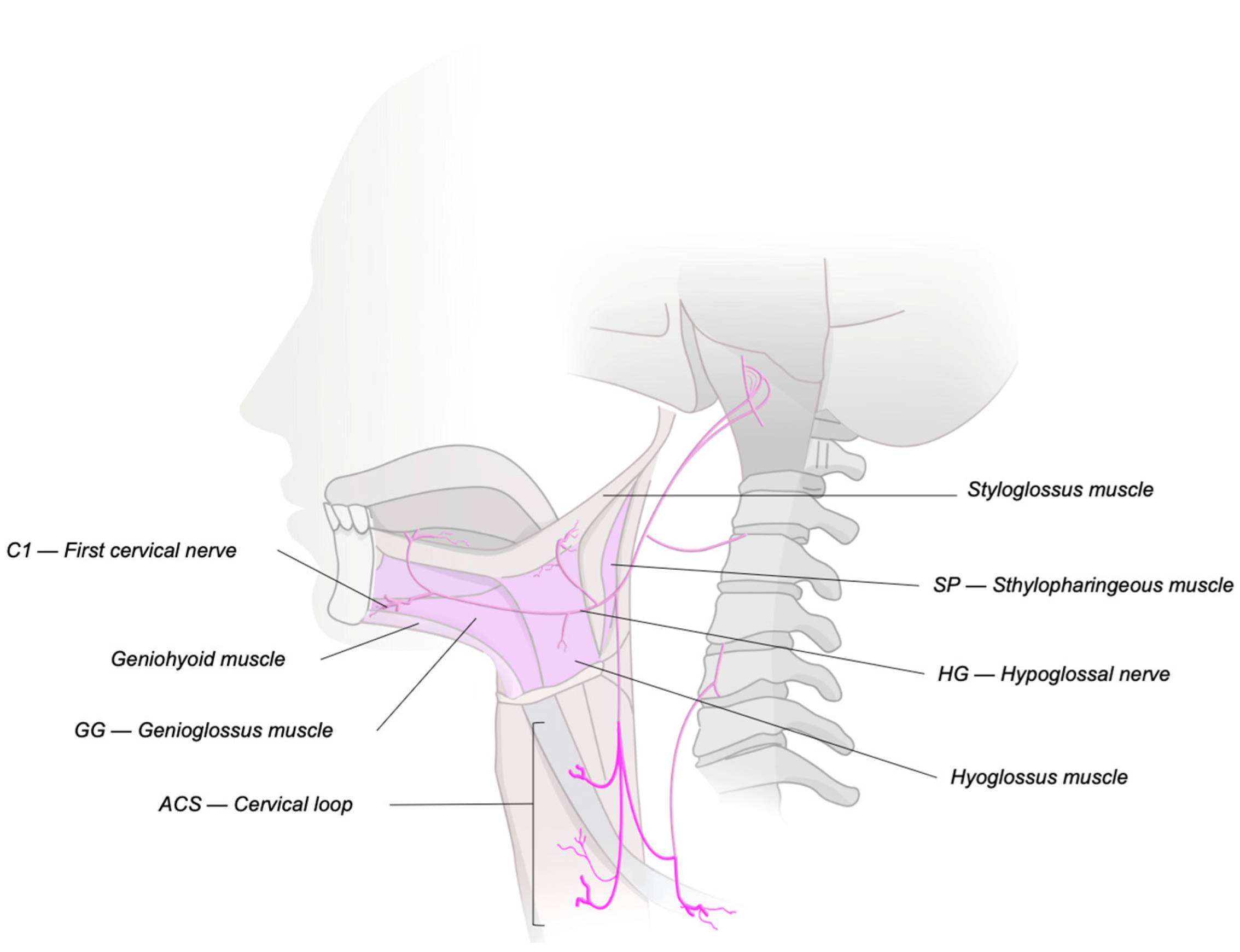
| C1 | First Cervical Spinal Nerve |
| DISE | Drug-Induced Sleep Endoscopy |
| CCC | Complete Concentric Collapse |
| ACS | Ansa Cervicalis Stimulation |
| RNS | Respiratory Neurostimulation |
| GNS | Glossopharyngeal Nerve Stimulation |
| PSG | Polysomnography |
| PAP | Positive Airway Pressure |
Appendix A
| Selective Stimulation | Targeted Stimulation | ||
|---|---|---|---|
| Laterality | Unilateral | Bilateral | Unilateral |
| Device | Inspire ® | Nyxoah Genio System TM | LivaNova aura6000 TM |
| Age | >18 years >13 years with Down Syndrome | >18 years | >22 years |
| AHI | 15–100 events/hour (adult) 15–50 events/hour (pediatric) | 15–65 events/hour | 20–65 events/hour |
| BMI | <40 kg/m2 | <35 kg/m2 | <35 kg/m2 |
| Central and Mixed Apneas | Central and/or mixed apnea index < 25 % of AHI | Central and/or mixed apnea index < 25 % of AHI | Central and/or mixed apnea index < 25 % of AHI |
| DISE | Required to exclude CCC | Not necessary; indicated for patients with CCC | Not necessary |
| Implantable Device | 3 pieces (IPG, stimulation electrode, sensor electrode) | 1 piece (stimulation electrode) | 2 pieces (IPG, stimulation electrode) |
| Implant Incisions | 2 | 1 | 2 |
| Stimulation Electrode | Medial and distal branches of the HN | Medial and distal branches of the HN | Main Branch of HN |
| Respiratory Cycle Dependence | Yes (intercostal sensor, pressure sensor) | Stimulation cycle dependent | Not dependent |
| Battery Life Expectancy | ~10.7 years | External Battery and IPG | ~10–15 years |
| Charging | Not required | Daily, external battery | 2 days for 10–15 min |
| Battery Replacement | IPG | Not required | IPG |
| Programming | Telemetry | External programming | Telemetry |
| MRI Compatibility | 1.5 T (FDA) | 3.0 T | Not compatible |
References
- Veugen, C.C.A.F.M.; Dieleman, E.; Hardeman, J.A.; Stokroos, R.J.; Copper, M.P. Upper Airway Stimulation in Patients with Obstructive Sleep Apnea: Long-Term Surgical Success, Respiratory Outcomes, and Patient Experience. Int. Arch. Otorhinolaryngol. 2023, 27, e43–e49. [Google Scholar] [CrossRef]
- Tong, J.Y.; Gocal, W.A.; Haft, S.J. Adverse Events Associated with Device Assisted Hyoid and Tongue Base Suspension for Obstructive Sleep Apnea. Am. J. Otolaryngol. 2024, 45, 104237. [Google Scholar] [CrossRef]
- Chang, C.P.; Poomkonsarn, S.; Giannakopoulos, H.; Ma, Y.; Riley, R.; Liu, S.Y. Comparative Efficacy of Obstructive Sleep Apnea Patients Undergoing Multilevel Surgery Followed by Upper Airway Stimulation versus Isolated Upper Airway Stimulation. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2023, 81, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Leiter, J.C. Upper Airway Shape: Is It Important in the Pathogenesis of Obstructive Sleep Apnea? Am. J. Respir. Crit. Care Med. 1996, 153, 894–898. [Google Scholar] [CrossRef]
- Fleury Curado, T.; Oliven, A.; Sennes, L.U.; Polotsky, V.Y.; Eisele, D.; Schwartz, A.R. Neurostimulation Treatment of OSA. Chest 2018, 154, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Schneider, H.; Marx, J.J.; Gladmon, E.; Schwartz, A.R.; Smith, P.L. Neuromechanical Control of Upper Airway Patency during Sleep. J. Appl. Physiol. 2007, 102, 547–556. [Google Scholar] [CrossRef]
- Jordan, A.S.; White, D.P.; Owens, R.L.; Eckert, D.J.; Rahangdale, S.; Yim-Yeh, S.; Malhotra, A. The Effect of Increased Genioglossus Activity and End-Expiratory Lung Volume on Pharyngeal Collapse. J. Appl. Physiol. 2010, 109, 469–475. [Google Scholar] [CrossRef]
- Strollo, P.J.; Gillespie, M.B.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Woodson, B.T.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: Durability of the Treatment Effect at 18 Months. Sleep 2015, 38, 1593–1598. [Google Scholar] [CrossRef]
- Serghani, M.; Heiser, C.; Schwartz, A.R.; Amatoury, J. Exploring Hypoglossal Nerve Stimulation Therapy for Obstructive Sleep Apnea: A Comprehensive Review of Clinical and Physiological Upper Airway Outcomes. Sleep Med. Rev. 2024, 76, 101947. [Google Scholar] [CrossRef]
- Alapati, R.; Wagoner, S.F.; Nieves, A.B.; Lawrence, A.; Rouse, D.; Larsen, C. Upper Airway Stimulation Device Failure: A 7-Year Single Center Experience. Am. J. Otolaryngol. 2024, 45, 104153. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Hida, W.; Inoue, H.; Takishima, T. A New Treatment for Obstructive Sleep Apnea Syndrome by Electrical Stimulation of Submental Region. Tohoku J. Exp. Med. 1988, 154, 91–92. [Google Scholar] [CrossRef]
- Decker, M.J.; Haaga, J.; Arnold, J.L.; Atzberger, D.; Strohl, K.P. Functional Electrical Stimulation and Respiration during Sleep. J. Appl. Physiol. 1993, 75, 1053–1061. [Google Scholar] [CrossRef]
- Strollo, P.J.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol.–Head Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, P.R.; Barnes, M.; MacKay, S.; Wheatley, J.R.; Hillman, D.R.; Nguyên, X.L.; Lewis, R.; Campbell, M.C.; Pételle, B.; Walsh, J.H.; et al. Bilateral Hypoglossal Nerve Stimulation for Treatment of Adult Obstructive Sleep Apnoea. Eur. Respir. J. 2019, 55, 1901320. [Google Scholar] [CrossRef] [PubMed]
- Certal, V.F.; Zaghi, S.; Riaz, M.; Vieira, A.S.; Pinheiro, C.T.; Kushida, C.; Capasso, R.; Camacho, M. Hypoglossal Nerve Stimulation in the Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Laryngoscope 2014, 125, 1254–1264. [Google Scholar] [CrossRef]
- Wray, C.M.; Thaler, E.R. Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea: A Review of the Literature. World J. Otorhinolaryngol.-Head Neck Surg. 2016, 2, 230–233. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Jacobowitz, O.; Eisele, D.W.; Mickelson, S.A.; Miller, M.B.; Oliven, A.; Certal, V.; Hopp, M.L.; Winslow, D.H.; Huntley, T.C.; et al. Targeted Hypoglossal Nerve Stimulation for Patients with Obstructive Sleep Apnea. JAMA Otolaryngol.—Head Neck Surg. 2023, 149, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Hida, W.; Shindoh, C.; Kikuchi, Y.; Chonan, T.; Taguchi, O.; Inoue, H.; Takishima, T. Effects of Electrical Stimulation of the Genioglossus on Upper Airway Resistance in Anesthetized Dogs. Am. Rev. Respir. Dis. 1989, 140, 1279–1284. [Google Scholar] [CrossRef]
- Baptista, P.M.; Costantino, A.; Moffa, A.; Rinaldi, V.; Casale, M. Hypoglossal Nerve Stimulation in the Treatment of Obstructive Sleep Apnea: Patient Selection and New Perspectives. Nat. Sci. Sleep 2020, 12, 151–159. [Google Scholar] [CrossRef]
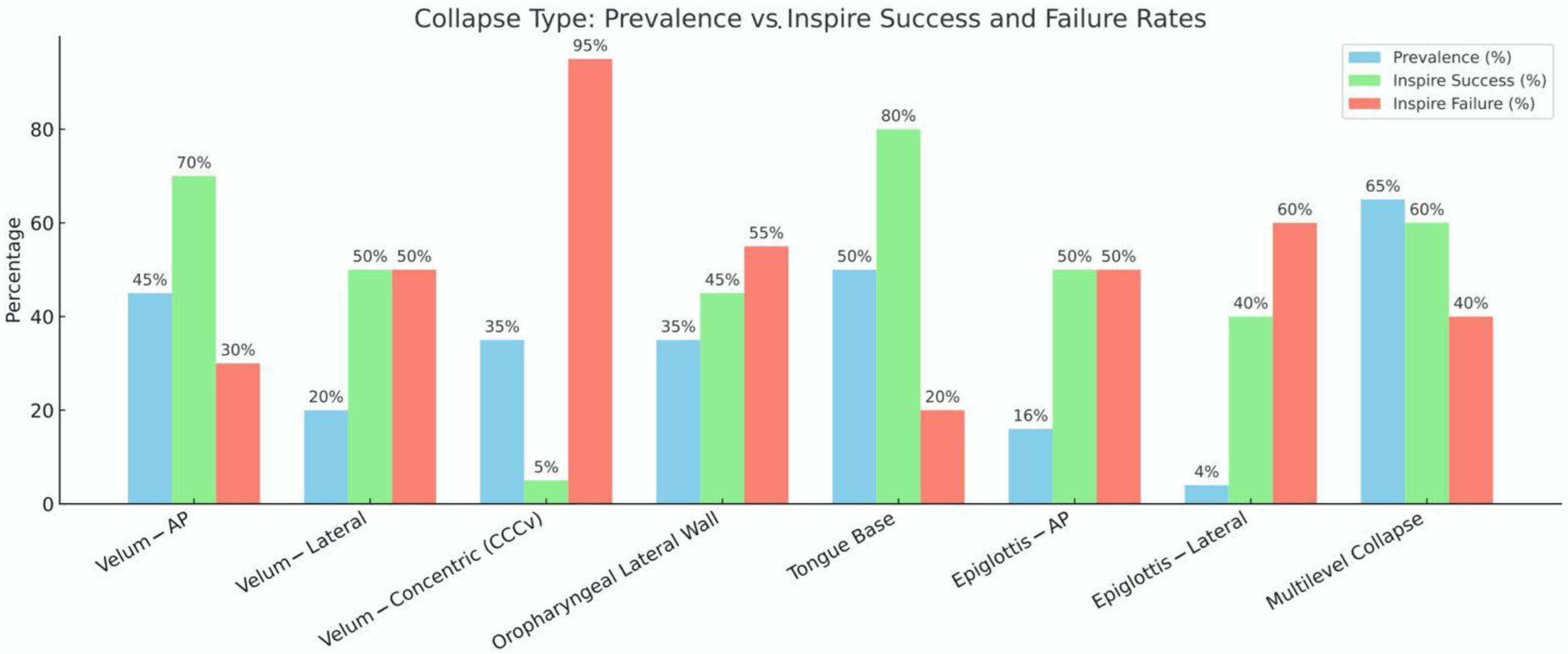
- Kezirian, E.J.; Hohenhorst, W.; de Vries, N. Drug-Induced Sleep Endoscopy: The VOTE Classification. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M.; Maurer, J.T.; Hohenhorst, W.; Hamans, E.; Lin, H.-S.; Vroegop, A.V.; Anders, C.; de Vries, N.; Van de Heyning, P.H. Evaluation of Drug-Induced Sleep Endoscopy as a Patient Selection Tool for Implanted Upper Airway Stimulation for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2013, 9, 433–438. [Google Scholar] [CrossRef]
- Ravesloot, M.J.L.; de Vries, N. One Hundred Consecutive Patients Undergoing Drug-Induced Sleep Endoscopy: Results and Evaluation. Laryngoscope 2011, 121, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
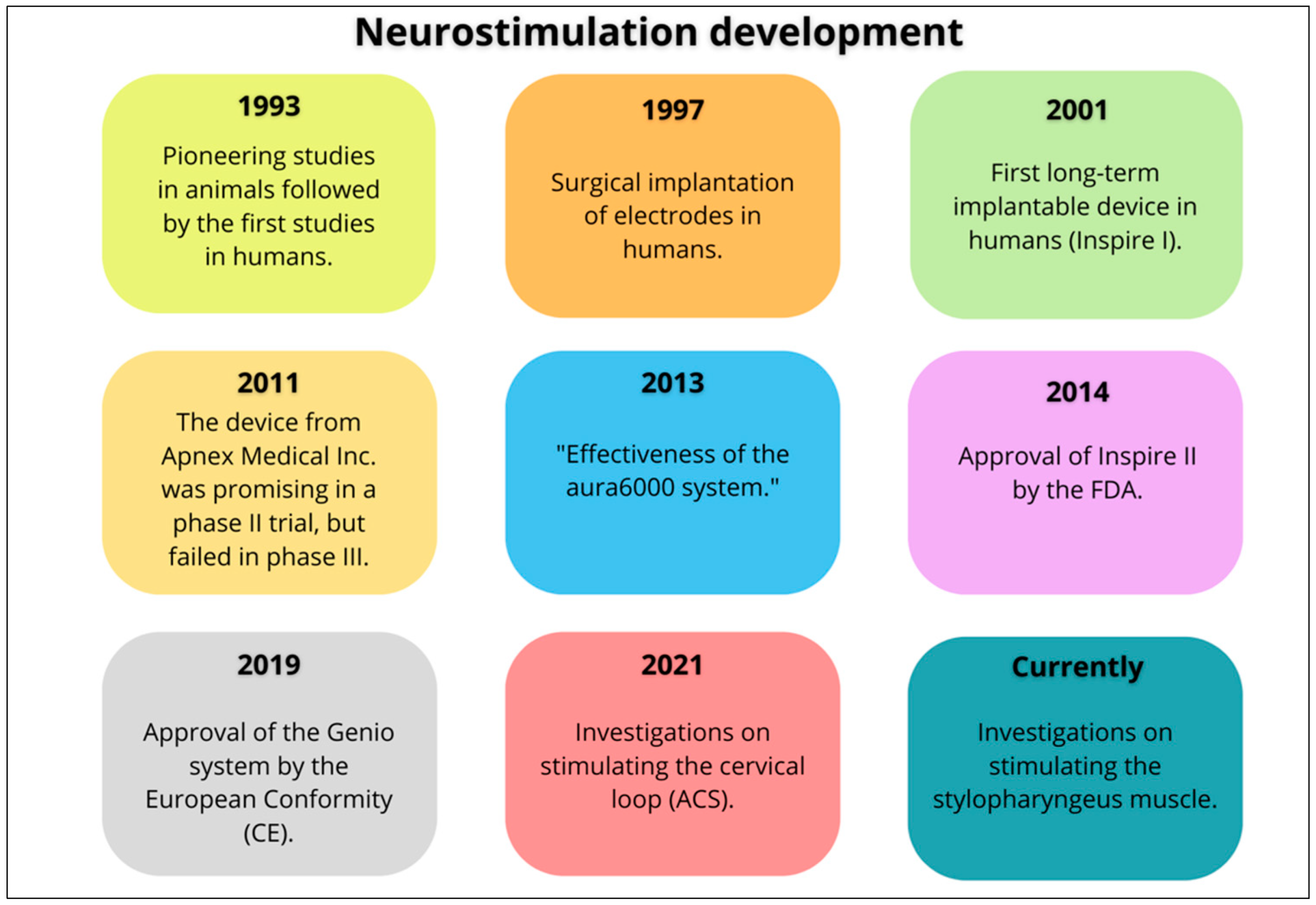
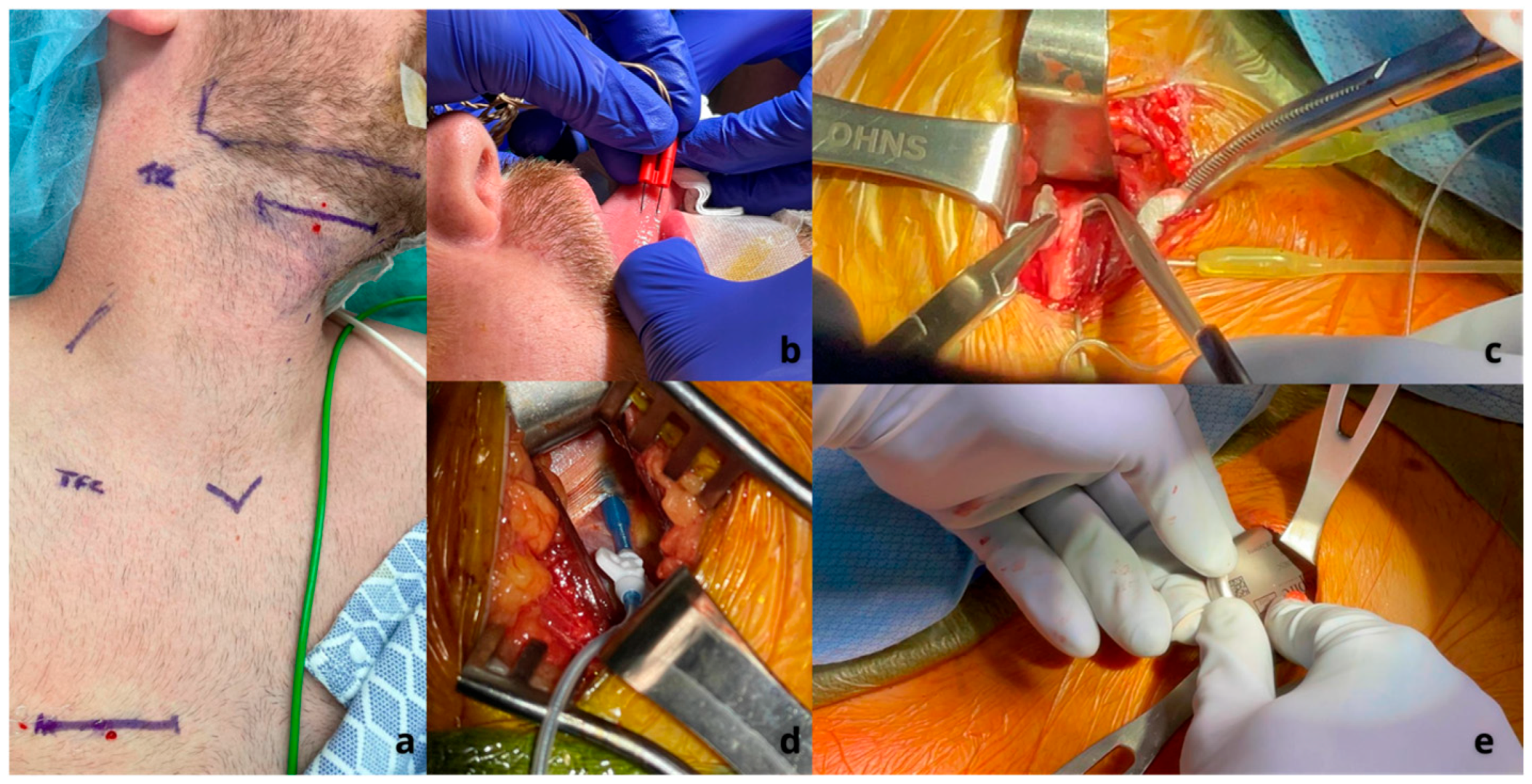
- Kent, D.T.; Zealear, D.; Schwartz, A.R. Ansa Cervicalis Stimulation. Chest 2021, 159, 1212–1221. [Google Scholar] [CrossRef]
- Guilleminault, C.; Hill, M.W.; Simmons, F.B.; Dement, W.C. Obstructive Sleep Apnea: Electromyographic and Fiberoptic Studies. Exp. Neurol. 1978, 62, 48–67. [Google Scholar] [CrossRef]
- Kent, D.T.; Ceremsak, J.J.; Li, Y.; Yalamanchi, P.; Mannion, K.; Zealear, D.; Shotwell, M.S.; Hall, M.E.; Lindsell, C.J.; Budnick, H.A.; et al. Role of Glossopharyngeal Nerve Stimulation in Stabilizing the Lateral Pharyngeal Wall and Ventilation in OSA: A Pilot Study. Chest 2025, 167, 1493–1496. [Google Scholar] [CrossRef]
- Alrubasy, W.A.; Abuawwad, M.T.; Taha, M.J.J.; Khurais, M.; Sayed, M.S.; Dahik, A.M.; Keshk, N.; Abdelhadi, S.; Serhan, H.A. Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea in Adults: An Updated Systematic Review and Meta-Analysis. Respir. Med. 2024, 234, 107826. [Google Scholar] [CrossRef]
- Wesson, T.; Rone, V.; Ramirez, M.; Manchanda, S.; Stahl, S.; Chernyak, Y.; Parker, N. Outcome Reporting in Prospective Studies Evaluating Neurostimulation for Obstructive Sleep Apnea. Laryngoscope 2024, 134, 4873–4881. [Google Scholar] [CrossRef] [PubMed]
- Pordzik, J.; Ludwig, K.; Seifen, C.; Ruckes, C.; Huppertz, T.; Bahr-Hamm, K.; Hackenberg, B.; Matthias, C.; Gouveris, H. Real-World Data on Polysomnography- and Patient-Reported Outcomes in Hypoglossal Nerve Stimulation and Auto-Titrating Positive Airway Pressure Therapy for Obstructive Sleep Apnea. Respir. Med. 2024, 232, 107750. [Google Scholar] [CrossRef]
- Crossley, J.R.; Wallerius, K.; Hoa, M.; Davidson, B.; Giurintano, J.P. Association between Conflict of Interest and Published Position on Hypoglossal Nerve Stimulation for Sleep Apnea. Otolaryngology 2021, 165, 375–380. [Google Scholar] [CrossRef]
- Wollny, M.; Heiser, C.; Sommer, U.; Schöbel, C.; Braun, M. Adverse Events with Hypoglossal Nerve Stimulation in the Treatment of Obstructive Sleep Apnea—A Systematic Review of Clinical Trials and Real-World Data. J. Clin. Med. 2024, 13, 4282. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cé, P.d.S.; Melo, M.E.S.; Machado, A.A.; Ridge, S.E.; Fleury Curado, T. Review of Neurostimulation Therapies for Obstructive Sleep Apnea: Hypoglossal Nerve Stimulation and Beyond. J. Clin. Med. 2025, 14, 5494. https://doi.org/10.3390/jcm14155494
Cé PdS, Melo MES, Machado AA, Ridge SE, Fleury Curado T. Review of Neurostimulation Therapies for Obstructive Sleep Apnea: Hypoglossal Nerve Stimulation and Beyond. Journal of Clinical Medicine. 2025; 14(15):5494. https://doi.org/10.3390/jcm14155494
Chicago/Turabian StyleCé, Patrícia dos Santos, Maria Eduarda Schiestl Melo, Alan Alves Machado, Sarah Eden Ridge, and Thomaz Fleury Curado. 2025. "Review of Neurostimulation Therapies for Obstructive Sleep Apnea: Hypoglossal Nerve Stimulation and Beyond" Journal of Clinical Medicine 14, no. 15: 5494. https://doi.org/10.3390/jcm14155494
APA StyleCé, P. d. S., Melo, M. E. S., Machado, A. A., Ridge, S. E., & Fleury Curado, T. (2025). Review of Neurostimulation Therapies for Obstructive Sleep Apnea: Hypoglossal Nerve Stimulation and Beyond. Journal of Clinical Medicine, 14(15), 5494. https://doi.org/10.3390/jcm14155494






