Norepinephrine Onset Time and Mortality in Patients with Septic Shock Treated in the Emergency Department
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Definition of Terms
2.3. Sample Size Calculation
2.4. Error and Bias Control Strategies
- Information bias was controlled via systematic, orderly, and structured data collection by a dedicated research assistant using the prospective data collection instrument.
- Observer bias was controlled by direct data collection and variable values from medical records. The observer did not record any values, as this was a study in which no interventions were performed.
- Selection bias, despite the sample being from a single center, was controlled by the sepsis diagnostic criteria for study entry (sepsis-3). Furthermore, patient selection was controlled by the eligibility criteria used, and a representative sample of the population was ensured by calculating the sample size.
2.5. Data Analysis
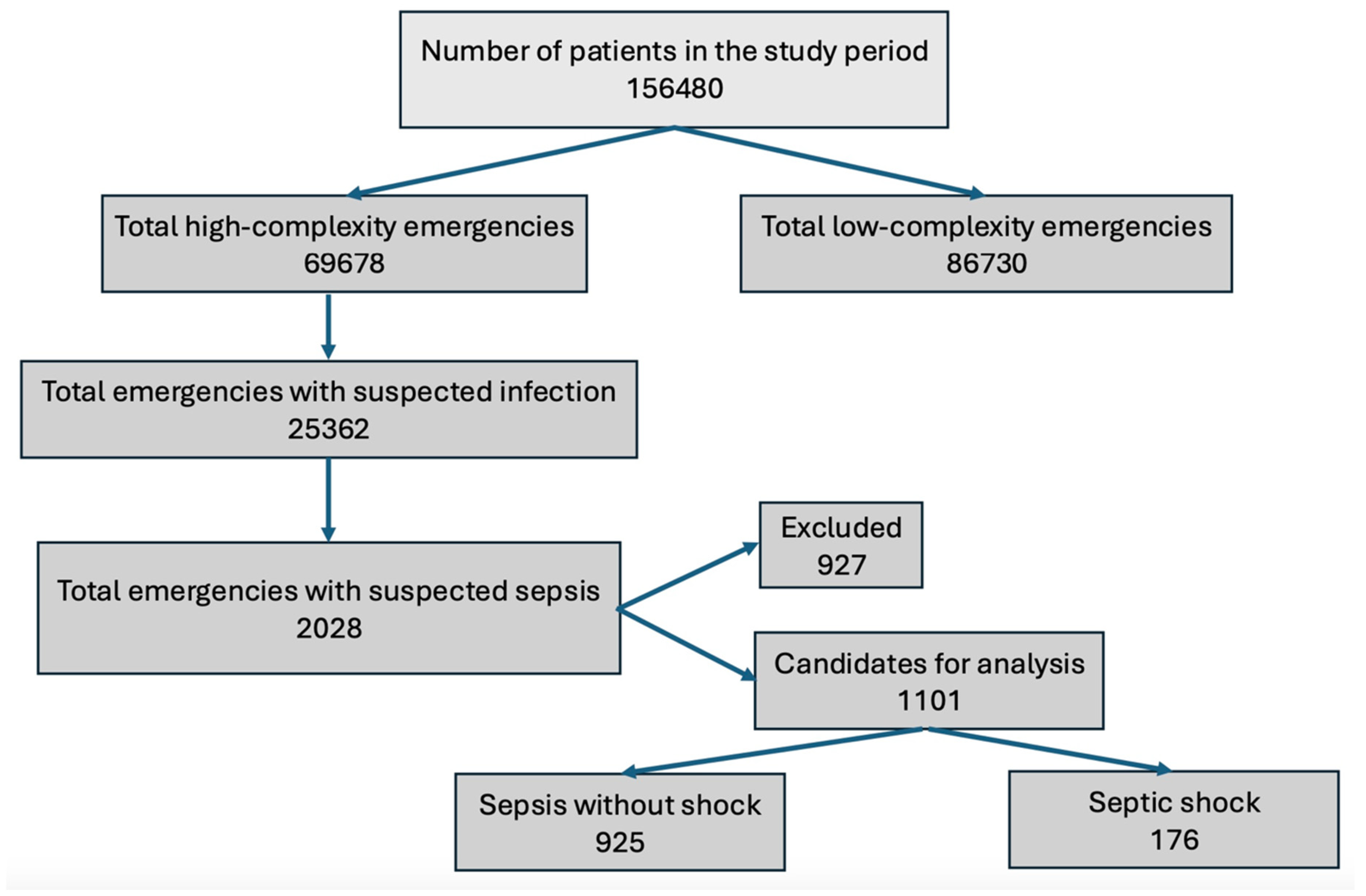
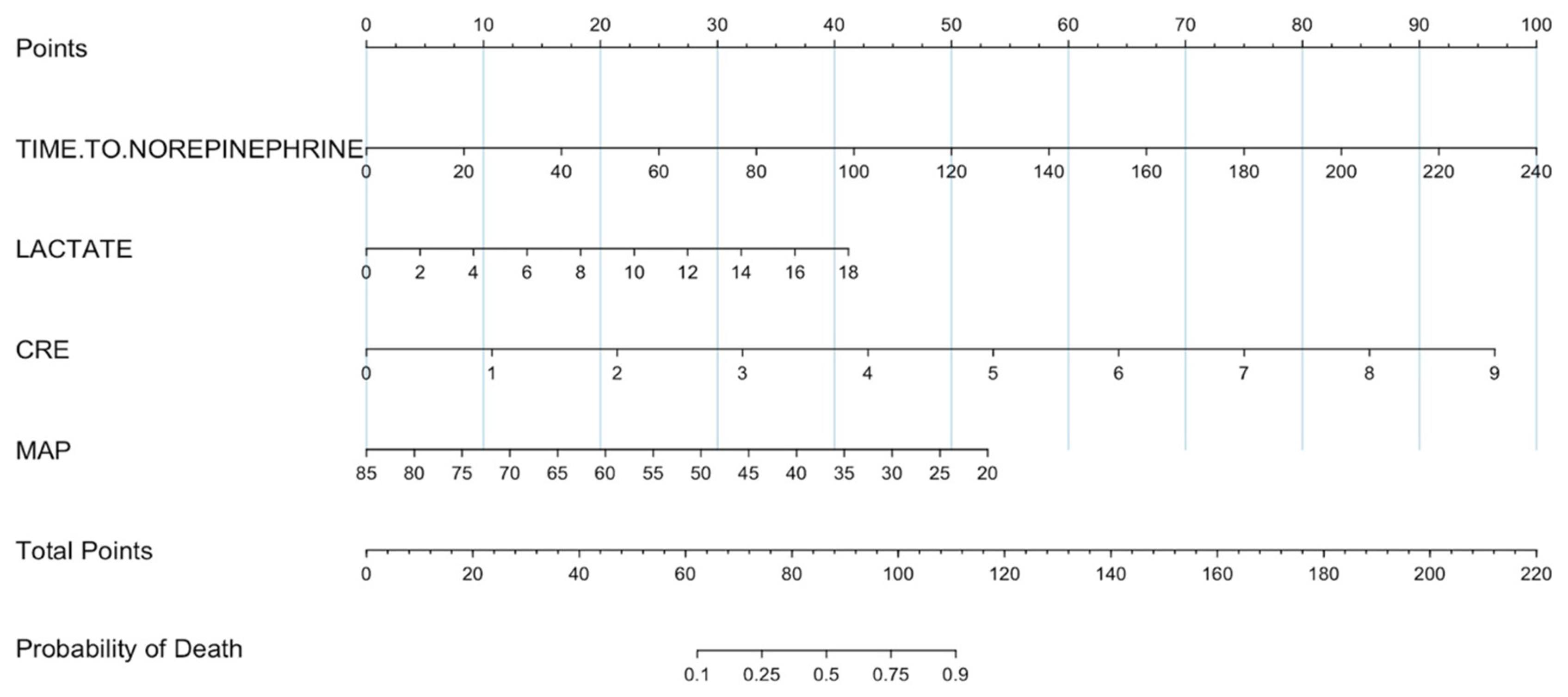
3. Results
4. Discussion
4.1. Limitation
4.2. Perspectives for the Future
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Receiver operating characteristic curve |
| PaFi | Arterial oxygen pressure divided by the fraction of inspired oxygen |
| SOFA | Sequential Organ Failure Assessment |
| ICU | Intensive care unit |
| HM | Hedonic motivation |
| Min | Minutes |
| MAP | Mean arterial pressure |
References
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Machado, F.R.; Cavalcanti, A.B.; Bozza, F.A.; Ferreira, E.M.; Angotti Carrara, F.S.; Sousa, J.L.; Caixeta, N.; Salomao, R.; Angus, D.C.; Pontes Azevedo, L.C.; et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): An observational study. Lancet Infect. Dis. 2017, 17, 1180–1189. [Google Scholar] [CrossRef]
- Rodríguez, F.; Barrera, L.; De La Rosa, G.; Dennis, R.; Dueñas, C.; Granados, M.; Londoño, D.; Molina, F.; Ortiz, G.; Jaimes, F. The epidemiology of sepsis in Colombia: A prospective multicenter cohort study in ten university hospitals. Crit. Care Med. 2011, 39, 1675–1682. [Google Scholar] [CrossRef]
- Devia Jaramillo, G.; Erazo Guerrero, L. Adjusting EWS scores for altitude above sea level: Is it necessary to predict sepsis mortality in the emergency room? Int. J. Emerg. Med. 2025, 18, 30. [Google Scholar] [CrossRef]
- Osborn, T.M. Severe Sepsis and Septic Shock Trials (ProCESS, ARISE, ProMISe): What is Optimal Resuscitation? Crit. Care Clin. 2017, 33, 323–344. [Google Scholar] [CrossRef]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef]
- Forero, R.; Hillman, K.M.; McCarthy, S.; Fatovich, D.M.; Joseph, A.P.; Richardson, D.B. Access block and ED overcrowding. Emerg. Med. Australas. 2010, 22, 119–135. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ko, R.-E.; Lim, S.Y.; Park, S.; Suh, G.Y.; Lee, Y.J. Sepsis Alert Systems, Mortality, and Adherence in Emergency Departments: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2422823. [Google Scholar] [CrossRef]
- Permpikul, C.; Tongyoo, S.; Viarasilpa, T.; Trainarongsakul, T.; Chakorn, T.; Udompanturak, S. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER). A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1097–1105. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhang, D. Timing of norepinephrine initiation in patients with septic shock: A systematic review and meta-analysis. Crit. Care 2020, 24, 488. [Google Scholar] [CrossRef]
- Ahn, C.; Yu, G.; Shin, T.G.; Cho, Y.; Park, S.; Suh, G.Y. Comparison of Early and Late Norepinephrine Administration in Patients with Septic Shock. Chest 2024, 166, 1417–1430. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; for the Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775. [Google Scholar] [CrossRef] [PubMed]
- Colon Hidalgo, D.; Patel, J.; Masic, D.; Park, D.; Rech, M.A. Delayed vasopressor initiation is associated with increased mortality in patients with septic shock. J. Crit. Care 2020, 55, 145–148. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Deutschman, C.S.; Hellman, J.; Myatra, S.N.; Ostermann, M.; Prescott, H.C.; Talmor, D.; Antonelli, M.; Pontes Azevedo, L.C.; Bauer, S.R.; et al. Surviving Sepsis Campaign Research Priorities 2023. Crit. Care Med. 2024, 52, 268–296. [Google Scholar] [CrossRef]
- Di Somma, S.; Paladino, L.; Vaughan, L.; Lalle, I.; Magrini, L.; Magnanti, M. Overcrowding in emergency department: An international issue. Intern. Emerg. Med. 2015, 10, 171–175. [Google Scholar] [CrossRef]
- LeDoux, D.; Astiz, M.E.; Carpati, C.M.; Rackow, E.C. Effects of perfusion pressure on tissue perfusion in septic shock. Crit. Care Med. 2000, 28, 2729–2732. [Google Scholar] [CrossRef]
- De Backer, D.; Creteur, J.; Preiser, J.-C.; Dubois, M.-J.; Vincent, J.-L. Microvascular Blood Flow Is Altered in Patients with Sepsis. Am. J. Respir. Crit. Care Med. 2002, 166, 98–104. [Google Scholar] [CrossRef]
- Sakr, Y.; Dubois, M.-J.; De Backer, D.; Creteur, J.; Vincent, J.-L. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004, 32, 1825–1831. [Google Scholar] [CrossRef]
- Annane, D.; Ouanes-Besbes, L.; De Backer, D.; Du, B.; Gordon, A.C.; Hernández, G.; Olsen, K.M.; Osborn, T.M.; Peake, S.; Russell, J.A.; et al. A global perspective on vasoactive agents in shock. Intensive Care Med. 2018, 44, 833–846. [Google Scholar] [CrossRef]
- Sandifer, J.P.; Jones, A.E. Is the Addition of Vasopressin to Norepinephrine Beneficial for the Treatment of Septic Shock? Ann. Emerg. Med. 2013, 62, 534–535. [Google Scholar] [CrossRef]
- Russell, J.A.; Walley, K.R.; Singer, J.; Gordon, A.C.; Hébert, P.C.; Cooper, D.J.; Holmes, C.L.; Mehta, S.; Granton, J.T.; Storms, M.M.; et al. Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock. N. Engl. J. Med. 2008, 358, 877–887. [Google Scholar] [CrossRef]
- Daley, M.J.; Lat, I.; Mieure, K.D.; Jennings, H.R.; Hall, J.B.; Kress, J.P. A Comparison of Initial Monotherapy with Norepinephrine Versus Vasopressin for Resuscitation in Septic Shock. Ann. Pharmacother. 2013, 47, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Braïk, R.; Monnet, X.; Gu, W.-J.; Ospina-Tascon, G.; Permpikul, C.; Djebbour, M.; Soumare, A.; Agaleridis, V.; Lai, C. Early norepinephrine for patients with septic shock: An updated systematic review and meta-analysis with trial sequential analysis. Crit. Care 2025, 29, 182. [Google Scholar] [CrossRef] [PubMed]
- Elbouhy, M.A.; Soliman, M.; Gaber, A.; Taema, K.M.; Abdel-Aziz, A. Early Use of Norepinephrine Improves Survival in Septic Shock: Earlier than Early. Arch. Med. Res. 2019, 50, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.J.; Lee, Y.S.; Kim, T.H.; Jang, J.H.; Lee, H.B.; Oh, D.K.; Park, M.H.; Lim, C.-M.; Cho, W.H.; on behalf of the Korean Sepsis Alliance (KSA) Investigators. Vasopressor Initiation Within 1 Hour of Fluid Loading Is Associated With Increased Mortality in Septic Shock Patients: Analysis of National Registry Data. Crit. Care Med. 2022, 50, e351–e360. [Google Scholar] [CrossRef]
- Devia Jaramillo, G.; Menendez Ramirez, S. USER Protocol as a Guide to Resuscitation of the Patient with Septic Shock in the Emergency Department. Open Access Emerg. Med. 2021, 13, 33–43. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Hernández, G.; Ospina-Tascón, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegría, L.; Teboul, J.-L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Williams, M.D.; Braun, L.A.; Cooper, L.M.; Johnston, J.; Weiss, R.V.; Qualy, R.L.; Linde-Zwirble, W. Hospitalized cancer patients with severe sepsis: Analysis of incidence, mortality, and associated costs of care. Crit. Care 2004, 8, R291. [Google Scholar] [CrossRef] [PubMed]
| Variable | Survivors (%) | No Survivors (%) | OR | p |
|---|---|---|---|---|
| Total (n = 176) | 120 (68.2) | 56 (31.8) | ||
| Sex (male) | 60 (50.0) | 21 (37.5) | 0.60 (0.31–1.14) | 0.165 |
| Age (median (IQ)) | 74 (63.5–81.5) | 74.5 (68–86.2) | 0.663 | |
| Sepsis origin (%) | 0.087 | |||
| Urinary | 47 (39.2) | 12 (21.4) | 0.76 (0.37–1.53) | 0.445 |
| Pulmonary | 20 (16.7) | 18 (32.1) | 2.73 (1.35–5.51) | 0.005 |
| Gastrointestinal | 21 (17.5) | 12 (21.4) | 3.04 (1.36–6.78) | 0.006 |
| Biliary tract | 10 (8.3) | 2 (3.6) | 2.93 (0.57–14.92) | 0.194 |
| Soft tissues | 6 (5) | 4 (7.1) | 11.73 (2.84–48.37) | <0.001 |
| Abdominal | 6 (5) | 6 (10.7) | 14.66 (4.10–52–42) | <0.001 |
| Bacteremia without focus | 6 (5) | 0 (0) | 2.08 (0.10–41.16) | 0.628 |
| Indeterminate | 2 (1.7) | 1 (1.8) | 29.33 (2.05–418.50) | 0.012 |
| Endocarditis | 1 (0.8) | 1 (1.8) | 88 (3.95–1958.41) | 0.004 |
| Malaria | 1 (0.8) | 0 (0) | 39.22 (1.09–1409.1) | 0.044 |
| Glasgow score (median (IQ)) | 15 (14–15) | 15 (14.75–15) | 0.891 | |
| Systolic blood pressure (mean (SD)) | 74.9 (10.8) | 72.6 (15.5) | 0.265 | |
| Diastolic blood pressure (median (IQ)) | 45 (40–49.2) | 42 (38–50) | 0.134 | |
| Mean arterial pressure (median (IQ)) | 56 (51–61) | 54.5 (46.7–60) | 0.078 | |
| PaFi (median (IQ)) | 253.5 (205.8–301.2) | 233 (122.2–292.2) | 0.058 | |
| Platelet count (median (IQ)) | 194.5 (138–278) | 216 (129–306.8) | 0.700 | |
| Bilirubin (median (IQ)) | 1.1 (0.7–1.9) | 1.2 (0.6–1.8) | 0.544 | |
| Creatinine (median (IQ)) | 1.2 (0.9–1.7) | 2.1 (1.4–2.9) | <0.001 | |
| Lactate levels (median (IQ)) | 2 (1.2–3.5) | 6 (2.5–8.2) | <0.001 | |
| SOFA (median (IQ)) | 5 (4–6) | 6 (5–8) | <0.001 | |
| Comorbidities | ||||
| Immune diseases | 13 (10.8) | 7 (12.5) | 1.18 (0.41–3.11) | 0.945 |
| Cardiovascular diseases | 60 (50) | 30 (53.6) | 1.15 (0.60–2.19) | 0.779 |
| Endocrinological diseases | 41 (34.2) | 24 (42.9) | 1.44 (0.74–2.77) | 0.344 |
| Liver diseases | 17 (14.2) | 8 (14.3) | 1.01 (0.38–2.48) | 1.000 |
| Chronic infections | 2 (1.7) | 2 (3.6) | 2.17 (0.22–21.34) | 0.805 |
| Neurological diseases | 8 (6.7) | 3 (5.4) | 0.81 (0.16–3.02) | 1.00 |
| Oncological diseases | 27 (22.5) | 23 (41.1) | 2.38 (1.19–4.76) | 0.018 |
| Kidney failure | 17 (14.2) | 8 (14.3) | 1.01 (0.38–2.48) | 1.00 |
| Lung diseases | 12 (10) | 9 (16.1) | 1.72 (0.65–4.39) | 0.364 |
| Presence of arrhythmias | 5 (4.2) | 6 (10.7) | 2.72 (0.76–10.1) | 0.181 |
| Time stay outside ICU (median (IQ)) | 12 (9–18) | 4.5 (3–7.7) | <0.001 | |
| Stay in ICU (median (IQ)) | 22 (13.7–24) | 17.5 (1–22.5) | <0.001 |
| Variable | Survivors | No Survivors | OR | p |
|---|---|---|---|---|
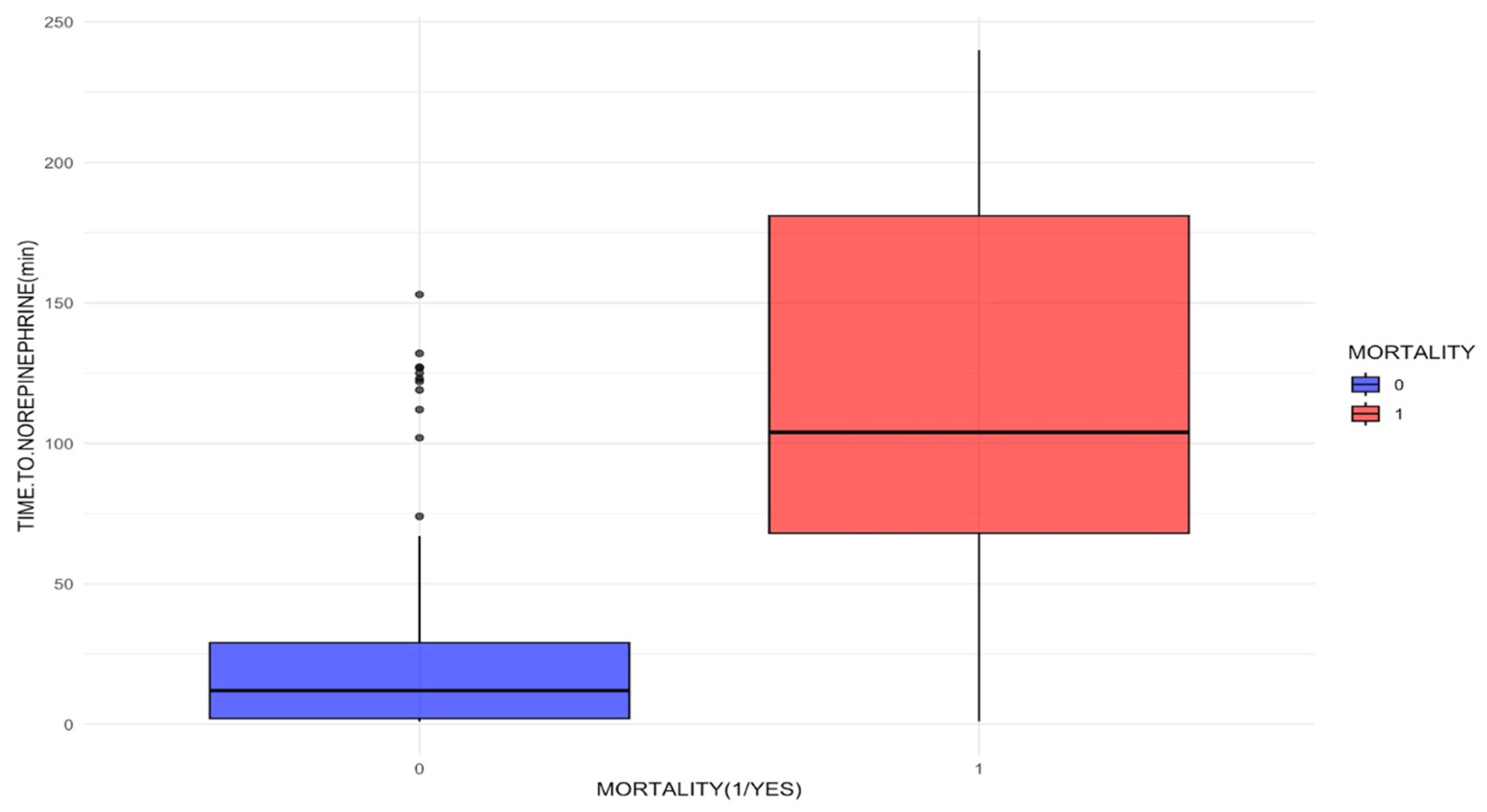
| Norepinephrine onset time (median (IQ)) min | 12 (2–29) | 104 (68–181) | <0.001 | |
| Time to norepinephrine (%) | ||||
| 60 min or less | 103 (85.8) | 11 (19.6) | 5.59 (2.67–11.6) | <0.001 |
| 61 to 179 min | 17 (14.2) | 20 (35.7) | 5.59 (2.67–11.6) | <0.001 |
| 180 min or more | 0 (0.0) | 25 (44.6) | 353 (20.8–5978.9) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devia Jaramillo, G.; Motta Hernández, J.W.; Donoso Zapata, W.G. Norepinephrine Onset Time and Mortality in Patients with Septic Shock Treated in the Emergency Department. J. Clin. Med. 2025, 14, 6025. https://doi.org/10.3390/jcm14176025
Devia Jaramillo G, Motta Hernández JW, Donoso Zapata WG. Norepinephrine Onset Time and Mortality in Patients with Septic Shock Treated in the Emergency Department. Journal of Clinical Medicine. 2025; 14(17):6025. https://doi.org/10.3390/jcm14176025
Chicago/Turabian StyleDevia Jaramillo, German, Jose Wdroo Motta Hernández, and William Gerardo Donoso Zapata. 2025. "Norepinephrine Onset Time and Mortality in Patients with Septic Shock Treated in the Emergency Department" Journal of Clinical Medicine 14, no. 17: 6025. https://doi.org/10.3390/jcm14176025
APA StyleDevia Jaramillo, G., Motta Hernández, J. W., & Donoso Zapata, W. G. (2025). Norepinephrine Onset Time and Mortality in Patients with Septic Shock Treated in the Emergency Department. Journal of Clinical Medicine, 14(17), 6025. https://doi.org/10.3390/jcm14176025





