Diagnostic Accuracy of Bisphosphonate Scintigraphy in Glu54GlnATTR Cardiomyopathy
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Serum Biomarkers
2.4. Electrocardiography (ECG)
2.5. Echocardiography
2.6. Bisphosphonates Scintigraphy (BS)
2.6.1. Patient Preparation and Data Acquisition
2.6.2. Processing and Interpretation
2.7. Histologic Testing
2.8. Genetic Testing
2.9. Statistical Analysis
3. Results
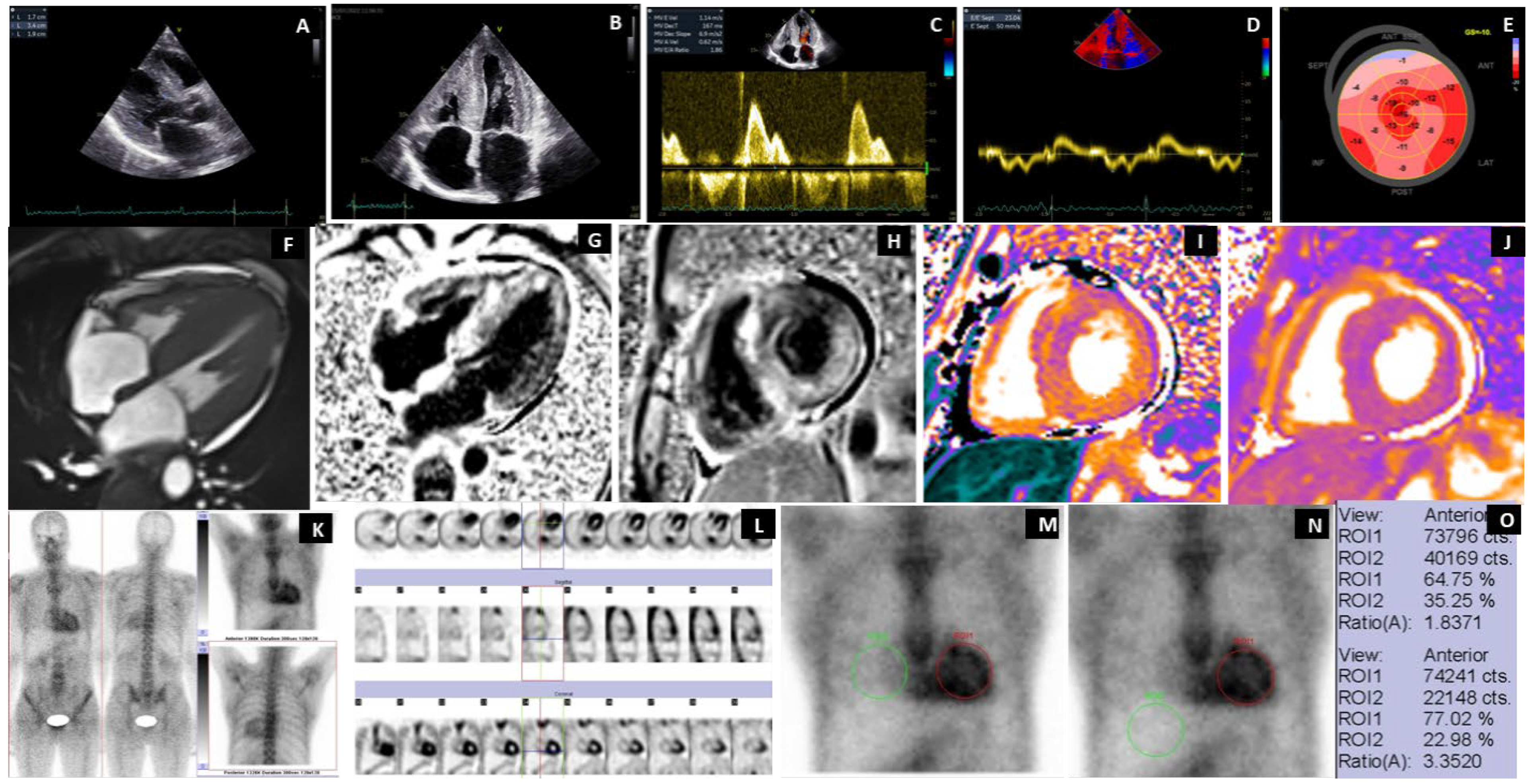
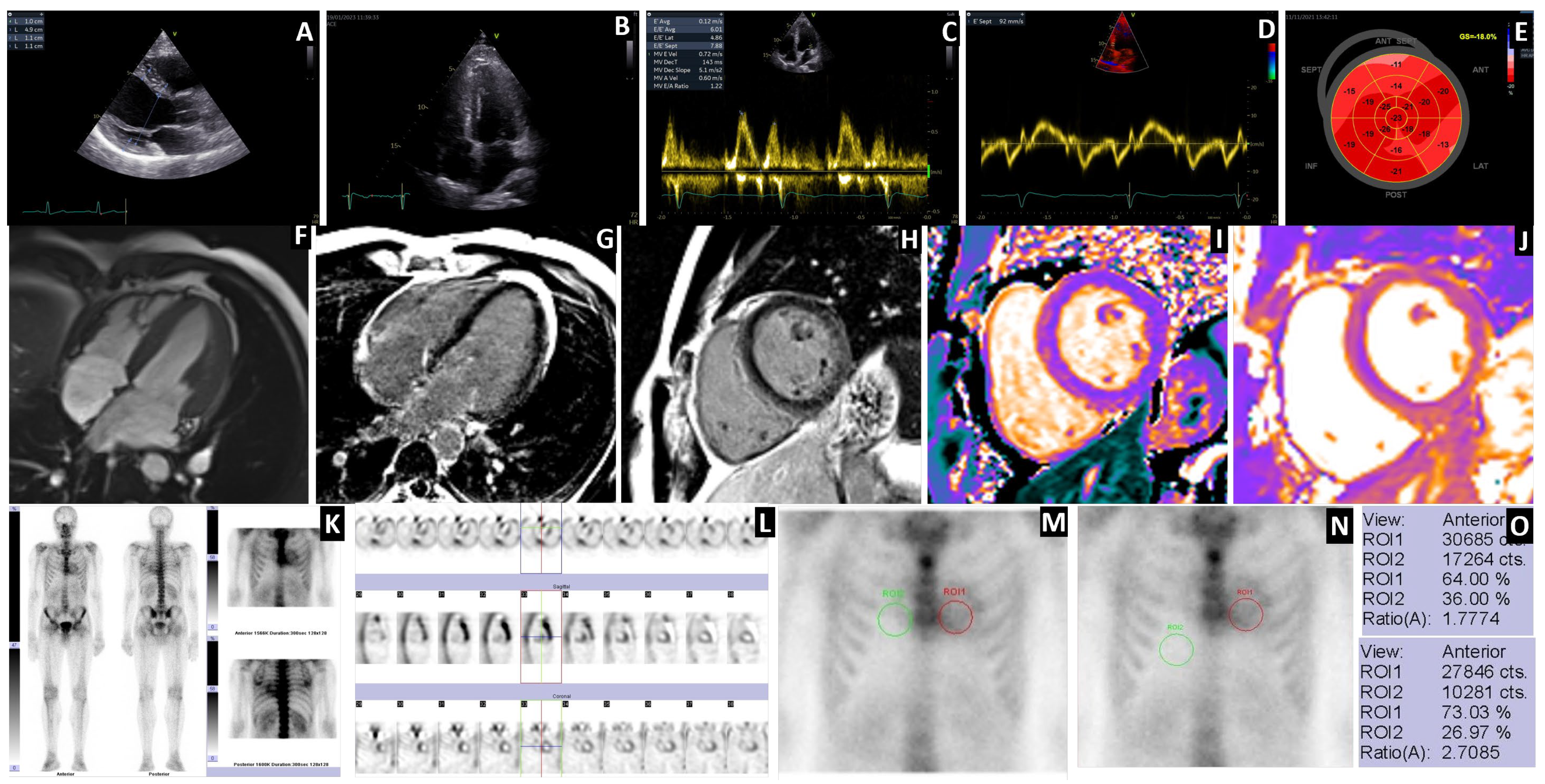
3.1. Scintigraphy Findings
3.2. Diagnostic Sensitivity
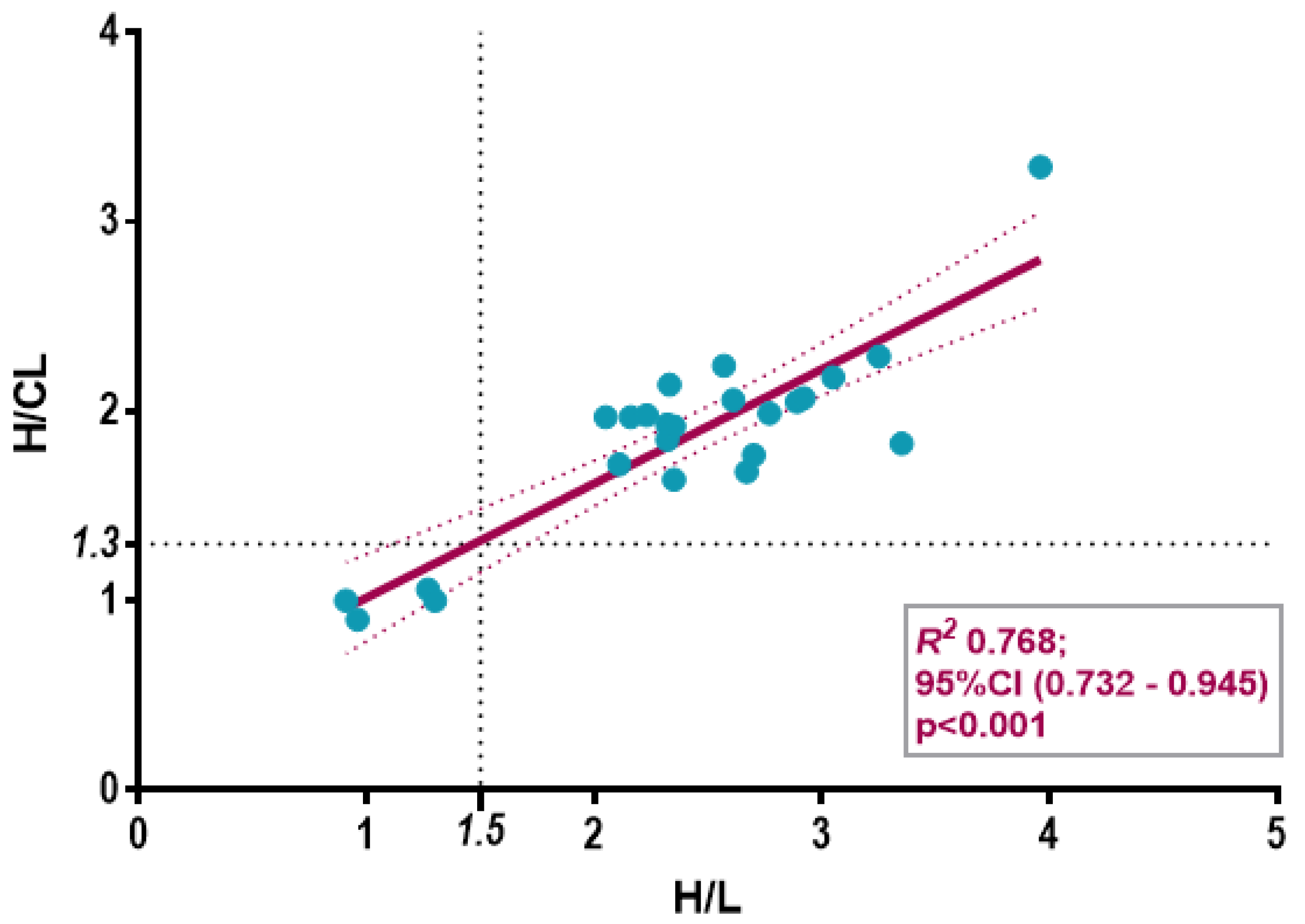
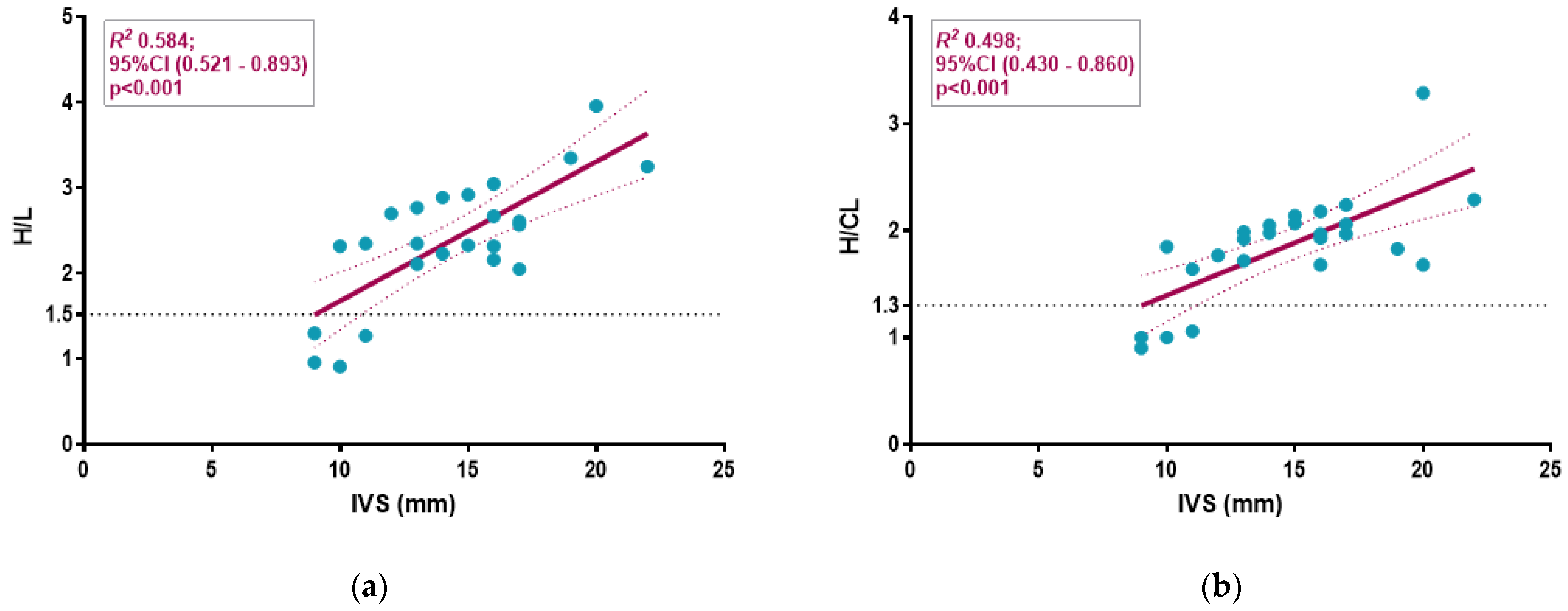
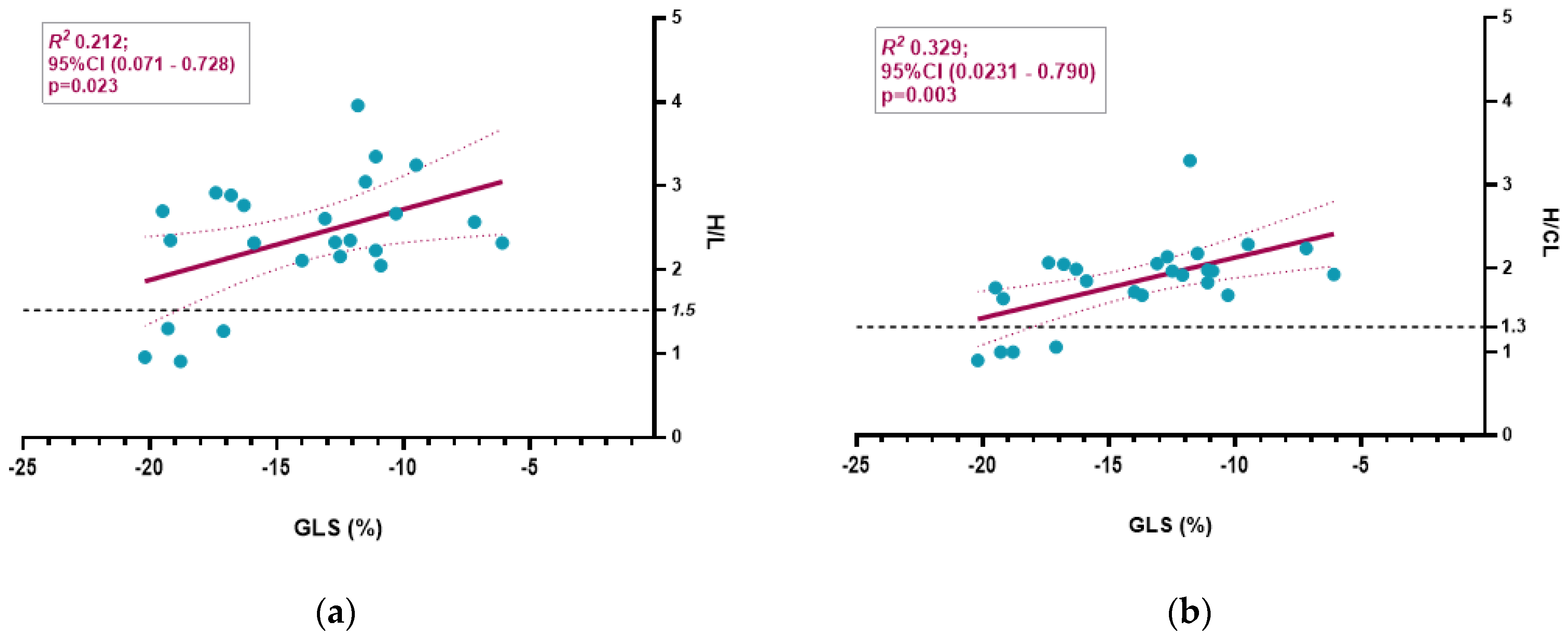
3.3. Semiquantitative Cardiac BS Assessment
3.4. Extracardiac WB Uptake and SPECT
3.5. Case Highlights
4. Discussion
4.1. Prevalence of ATTRGlu54Gln Amyloidosis in Romania
4.2. Diagnosis of ATTR Amyloidosis
4.3. Binding of Bisphosphonates to TTR Amyloid Fibrillar Proteins
4.4. Diagnostic Accuracy in Detecting Glu54Gln ATTR-CA
4.5. Conceptual Differences Between H/CL and H/L Ratios
4.6. Hepatic Uptake of Bisphosphonates
4.7. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 99mTc | metastable technetium |
| AL | amyloid light chain |
| ATTR | amyloid transthyretin |
| ATTR-CA | amyloid transthyretin-cardiomyopathy |
| BS | bisphosphonate scintigraphy |
| cMRI | cardiac magnetic resonance imaging |
| DPD | 3,3-diphosphono-1,2-propanodicarboxylic acid |
| ECG | electrocardiography |
| G | cardiac uptake Perugini score |
| Glu54Gln | glutamic acid 54 glutamine |
| GLS | global longitudinal strain |
| H/CL | heart to contralateral uptake |
| HDP | oxidronate |
| HMDP | oxidronate |
| H/L | heart to liver uptake |
| LV | left ventricle |
| LEHR | low energy and high resolution |
| LVEF | left ventricular ejection fraction |
| MDP | methylene diphosphonate |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
| PET | positron emission tomography |
| PIP | pseudo-infarction pattern |
| ROI | region of interest |
| RBBB | right bundle branch block |
| RV | right ventricle |
| SPECT | single-photon emission computed tomography |
| TnI | high-sensitivity troponin I |
| TTE | transthoracic echocardiography |
| TTR | transthyretin |
| WB | whole body |
References
- Buxbaum, J.N.; Eisenberg, D.S.; Fändrich, M.; McPhail, E.D.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Westermark, P. Amyloid nomenclature 2024: Update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2024, 31, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rowczenio, D.M.; Noor, I.; Gillmore, J.D.; Lachmann, H.J.; Whelan, C.; Hawkins, P.N.; Obici, L.; Westermark, P.; Grateau, G.; Wechalekar, A.D. Online Registry for Mutations in Hereditary Amyloidosis Including Nomenclature Recommendations. Hum. Mutat. 2014, 35, E2403–E2412. [Google Scholar] [CrossRef] [PubMed]
- Hazenberg, B.P.C.; Bijzet, J. XIIIth International Symposium on Amyloidosis 2012 “From Misfolded Proteins to Well-Designed Treatment”. 2012. Available online: https://www.researchgate.net/publication/260086782_XIIIth_International_Symposium_on_Amyloidosis_2012_From_misfolded_proteins_to_well-designed_treatment (accessed on 19 October 2024).
- Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2—Evidence base and standardized methods of imaging. J. Nucl. Cardiol. 2019, 26, 2065–2123. [Google Scholar] [CrossRef]
- Musumeci, M.B.; Cappelli, F.; Russo, D.; Tini, G.; Canepa, M.; Milandri, A.; Bonfiglioli, R.; Di Bella, G.; My, F.; Luigetti, M.; et al. Low Sensitivity of Bone Scintigraphy in Detecting Phe64Leu Mutation-Related Transthyretin Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 1314–1321. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 2 of 2—Diagnostic criteria and appropriate utilization. J. Nucl. Cardiol. 2019, 27, 659–673. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. Addendum to ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2—Evidence base and standardized methods of imaging. J. Nucl. Cardiol. 2021, 28, 1769–1774. [Google Scholar] [CrossRef]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.T.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef]
- Jercan, A.; Ene, A.; Jurcut, R.; Draghici, M.; Badelita, S.; Dragomir, M.; Dobrea, C.; Popescu, M.; Jardan, D.; Stoica, E.; et al. Clinical characteristics in patients with hereditary amyloidosis with Glu54Gln transthyretin identified in the Romanian population. Orphanet J. Rare Dis. 2020, 15, 34. [Google Scholar] [CrossRef]
- Porcari, A.; Baggio, C.; Fabris, E.; Merlo, M.; Bussani, R.; Perkan, A.; Sinagra, G. Endomyocardial biopsy in the clinical context: Current indications and challenging scenarios. Heart Fail. Rev. 2022, 28, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Ihse, E.; Rapezzi, C.; Merlini, G.; Benson, M.D.; Ando, Y.; Suhr, O.B.; Ikeda, S.-I.; Lavatelli, F.; Obici, L.; Quarta, C.C.; et al. Amyloid fibrils containing fragmented ATTR may be the standard fibril composition in ATTR amyloidosis. Amyloid 2013, 20, 142–150. [Google Scholar] [CrossRef]
- Pilebro, B.; Suhr, O.B.; Näslund, U.; Westermark, P.; Lindqvist, P.; Sundström, T. 99mTc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Upsala J. Med. Sci. 2016, 121, 17–24. [Google Scholar] [CrossRef]
- Landreh, M.; Rising, A.; Presto, J.; Jörnvall, H.; Johansson, J. Specific Chaperones and Regulatory Domains in Control of Amyloid Formation. J. Biol. Chem. 2015, 290, 26430–26436. [Google Scholar] [CrossRef]
- Tana, M.; Tana, C.; Palmiero, G.; Mantini, C.; Coppola, M.G.; Limongelli, G.; Schiavone, C.; Porreca, E. Imaging findings of right cardiac amyloidosis: Impact on prognosis and clinical course. J. Ultrasound 2023, 26, 605–614. [Google Scholar] [CrossRef]
- Porcari, A.; Fontana, M.; Canepa, M.; Biagini, E.; Cappelli, F.; Gagliardi, C.; Longhi, S.; Pagura, L.; Tini, G.; Dore, F.; et al. Clinical and Prognostic Implications of Right Ventricular Uptake on Bone Scintigraphy in Transthyretin Amyloid Cardiomyopathy. Circulation 2024, 149, 1157–1168. [Google Scholar] [CrossRef]
- Lee, S.P.; Lee, E.S.; Choi, H.; Im, H.J.; Koh, Y.; Lee, M.H.; Kwon, J.-H.; Paeng, J.C.; Kim, H.-K.; Cheon, G.J.; et al. 11C-Pittsburgh B PET Imaging in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2015, 8, 50–59. [Google Scholar] [CrossRef]
- Park, M.A.; Padera, R.F.; Belanger, A.; Dubey, S.; Hwang, D.H.; Veeranna, V.; Falk, R.H.; Di Carli, M.F.; Dorbala, S. 18F-Florbetapir Binds Specifically to Myocardial Light Chain and Transthyretin Amyloid Deposits: Autoradiography Study. Circ. Cardiovasc. Imaging 2015, 8, e002954. [Google Scholar] [CrossRef]
- Clerc, O.F.; Cuddy, S.A.M.; Robertson, M.; Vijayakumar, S.; Neri, J.C.; Chemburkar, V.; Kijewski, M.F.; Di Carli, M.F.; Bianchi, G.; Falk, R.H.; et al. Cardiac Amyloid Quantification Using 124I-Evuzamitide (124I-P5+14) Versus 18F-Florbetapir: A Pilot PET/CT Study. JACC Cardiovasc. Imaging 2023, 16, 1419–1432. [Google Scholar] [CrossRef]
- Galat, A.; Van der Gucht, A.; Guellich, A.; Bodez, D.; Cottereau, A.-S.; Guendouz, S.; Hittinger, L.; Dubois-Randé, J.-L.; Plante-Bordeneuve, V.; Itti, E.; et al. Early Phase 99Tc-HMDP Scintigraphy for the Diagnosis and Typing of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2017, 10, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Digamber, N.; Sharma, R. Technetium-99m-Methylene Diphosphonate Uptake in Hepatic Necrosis Secondary to Respiratory Failure. World J. Nucl. Med. 2013, 12, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Zuckier, L.S.; Freeman, L.M. Nonosseous, Nonurologic Uptake on Bone Scintigraphy: Atlas and Analysis. Semin. Nucl. Med. 2010, 40, 242–256. [Google Scholar] [CrossRef]
- Munoz, S.J.; Nagelberg, S.B.; Green, S.J.; Angstadt, J.D.; Yang, S.L.; Jarrell, B.E.; Maddrey, W.C. Ectopic Soft Tissue Calcium Deposition Following Liver Transplantation. Hepatology 1988, 8, 476–483. [Google Scholar] [CrossRef]
- Wale, D.J.; Wong, K.K.; Savas, H.; Kandathil, A.; Piert, M.; Brown, R.K.J. Extraosseous Findings on Bone Scintigraphy Using Fusion SPECT/CT and Correlative Imaging. Am. J. Roentgenol. 2015, 205, 160–172. [Google Scholar] [CrossRef]
- Malka, N.; Abulizi, M.; Kharoubi, M.; Oghina, S.; Galat, A.; Le Bras, F.; Moktefi, A.; Guendouz, S.; Molinier-Frenkel, V.; Fanen, P.; et al. Extracardiac Soft Tissue Uptake, Evidenced on Early 99mTc-HMDP SPECT/CT, Helps Typing Cardiac Amyloidosis and Demonstrates High Prognostic Value. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2396–2406. [Google Scholar] [CrossRef]
| Clinical Characteristics | ATTRGlu54Gln Symptomatic Patients’ Data (n = 22) |
|---|---|
| Age at diagnosis (y); median [IQR] | 45 [43–48] |
| Female, n (%) | 15 (68) |
| Family history of ATTR amyloidosis, n (%) | 21 (95.4) |
| BMI (kg/m2) [IQR] | 23.4 [19.4–25.9] |
| Proband status n (%) | 22 (100%) |
| Birthplace: Suceava county, Romania n (%) | 21 (95.45) |
| NT-proBNP (pg/mL) [IQR] | 1457 [94.8–3707.5] |
| TnI (ng/mL) [IQR] | 0.017 [0.01–0.02] |
| Cardiac involvement n (%) | 22 (100%) |
| Phenotype n (%) | |
| Cardiac incipient | 1 (5%) |
| Mixed phenotype (cardiac + neurologic) | 21 (95%) |
| NYHA functional class n (%) | |
| No HF symptoms reported | 6 (27.4%) |
| I | 4 (18.1%) |
| II | 9 (40.9%) |
| III | 3 (13.6%) |
| IV | 0 |
| Syncope, n (%) | 3 (13.6%) |
| ICD, n (%) | 2 (9%) |
| Pacemaker, n (%) | 2 (9%) |
| Autonomic involvement, n (%) | 20 (91%) |
| Peripheral neuropathy, n (%) | 21 (95%) |
| Bilateral carpal tunnel syndrome, n (%) | 19 (86%) |
| G0 Score at BS (Carriers 4pts.) | G2 Score at BS (4 pts.) | G3 Score at BS (18 pts.) | p-Value | |
|---|---|---|---|---|
| Age at Diagnosis (y) | 33.5 [30–34] | 42.5 [35–45] | 45 [43–48] # | <0.001 |
| Male | 3 (75) | 1 (25) | 6 (33.3) | NS |
| NYHA II/III | 0 | 1 (25) | 11 (61.1) | 0.05 |
| ICD | 0 | 0 | 2 (11.1) | NS |
| Pacemaker | 0 | 0 | 2 (11.1) | NS |
| NT-proBNP (pg/mL) | 17.25 [12.2–22.15] | 512.0 [76.5–2229] | 1632.5 [884–4218.5] | NS |
| TnI (ng/mL) | 0.005 [0.001–0.02] | 0.01 [0.0045–0.0165] | 0.02 [0.01–0.0255] | NS |
| Normal ECG | 4 (100) | 2 (50) | 3 (16.6) | NS |
| Low QRS Voltage | - | 0 | 11 (61.1) | 0.002 |
| Voltage/mass ratio | 0.33 [0.24–0.39] | 0.13 [0.06–0.22] | 0.10 [0.06–0.185] | NS |
| Pseudo-infarction pattern | - | 1 (25) | 11 (61.1) | 0.05 |
| RBBB | - | 0 | 6 (33.3) | NS |
| AVB | NS | |||
| 1st AVB | - | 2 (28.5) | 4 (22.2) | |
| 2nd AVB | - | 2 | 1 (5.5) | |
| 3rd AVB | - | 0 | 2 (11.1) | |
| AF | - | 0 | 2 (11.1) | NS |
| NSVT | - | 1 (25) | 5 (27.7) | NS |
| IVS (mm) | 9.5 [9–10.5] | 13.5 [11.5–17.5] | 16 [13.5–17] # | 0.003 |
| LVPW (mm) | 9.5 [9–11] | 12.5 [11–15] | 14 [12–16] # | 0.02 |
| LVED Diameter (mm) | 45.5 [44–47] | 44.5 [40.5–50] | 42 [38–48.5] | NS |
| LVMi (g/m2) | 73.0 [63.25–81.32] | 139.7 [116.3–173.0] | 146.3 [116.7–166.1] # | 0.01 |
| LVED Volume (mL) | 96 [79–118] | 74.5 [62–91] | 77 [67.5–94.5] | NS |
| LVEF (%) | 58.5 [58–60] | 62 [59.5–65] | 55 [46.5–58.5] | 0.049 |
| LV GLS | −19.0 [−19.7–−17.9] | −15.9 [−19.5–−11.5] | −12.5 [−15.2–−10.6] # | 0.002 |
| LA Diameter (mm) | 33.5 [32.5–36.5] | 35 [34–39] | 41 [38–43] | NS |
| RV FW (mm) | 4.0 [4–4.5] | 5.5 [4.5–6] | 7 [6–8] # | 0.004 |
| High LV filling pressure | - | 2 (50) | 11 (61) | NS |
| Pericardial fluid (Y/n) | - | 0 | 4 (22.2) | NS |
| H/CL | 1 [0.95–1.03] | 1.77 [1.72–1.81] # | 1.99 [1.92–2.14] # | <0.001 |
| H/L | 1.12 [0.94–1.29] | 2.42 [2.37–2.56] # | 2.59 [2.32–3.25] # | <0.001 |
| Autonomous involvement | - | 3(75) | 17 (94) | <0.001 |
| SMP | - | 3 (75) | 18 (100) | 0.05 |
| Carpal tunnel syndrome | 1 (25) | 3 (75) | 16 (89) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stan, C.; Neculae, G.; Adam, R.-D.; Jercan, A.; Badelita, S.-N.; Draghici, M.-R.; Dobrea, C.; Onciul, S.; Capşa, R.; Chirion, C.; et al. Diagnostic Accuracy of Bisphosphonate Scintigraphy in Glu54GlnATTR Cardiomyopathy. J. Clin. Med. 2025, 14, 3734. https://doi.org/10.3390/jcm14113734
Stan C, Neculae G, Adam R-D, Jercan A, Badelita S-N, Draghici M-R, Dobrea C, Onciul S, Capşa R, Chirion C, et al. Diagnostic Accuracy of Bisphosphonate Scintigraphy in Glu54GlnATTR Cardiomyopathy. Journal of Clinical Medicine. 2025; 14(11):3734. https://doi.org/10.3390/jcm14113734
Chicago/Turabian StyleStan, Claudiu, Gabriela Neculae, Robert-Daniel Adam, Andreea Jercan, Sorina-Nicoleta Badelita, Mirela-Ramona Draghici, Camelia Dobrea, Sebastian Onciul, Razvan Capşa, Cristina Chirion, and et al. 2025. "Diagnostic Accuracy of Bisphosphonate Scintigraphy in Glu54GlnATTR Cardiomyopathy" Journal of Clinical Medicine 14, no. 11: 3734. https://doi.org/10.3390/jcm14113734
APA StyleStan, C., Neculae, G., Adam, R.-D., Jercan, A., Badelita, S.-N., Draghici, M.-R., Dobrea, C., Onciul, S., Capşa, R., Chirion, C., Stanescu, D., Stefanescu, C., Grierosu, I.-C., Ionescu, T.-M., Statescu, A.-M., Gutu, M., Argiro, A., Cappelli, F., Coriu, D., & Jurcuţ, R. (2025). Diagnostic Accuracy of Bisphosphonate Scintigraphy in Glu54GlnATTR Cardiomyopathy. Journal of Clinical Medicine, 14(11), 3734. https://doi.org/10.3390/jcm14113734






