Finerenone: A Third-Generation MRA and Its Impact on Cardiovascular Health—Insights from Randomized Controlled Trials
Abstract
1. Introduction
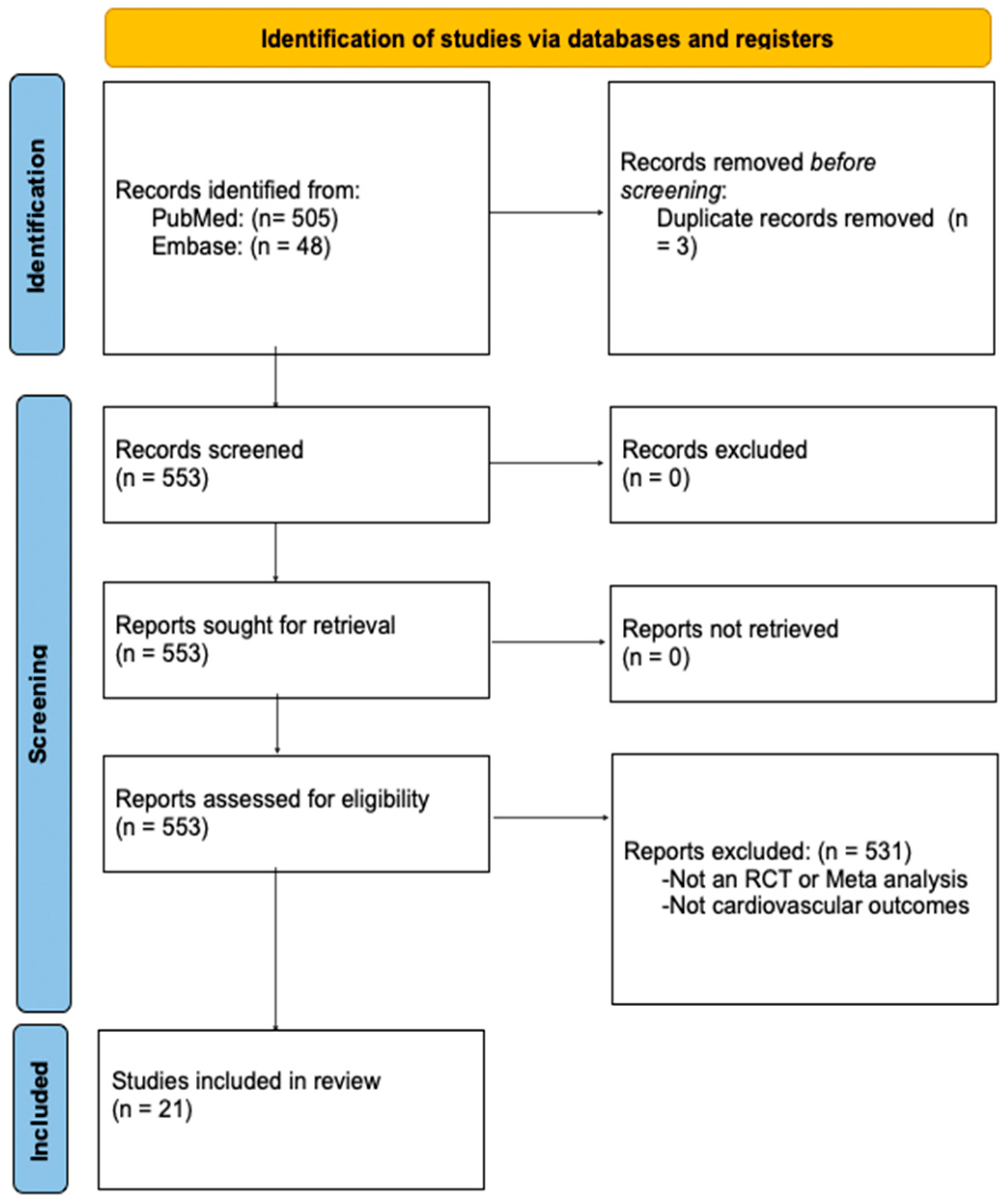
2. Methods
3. Results
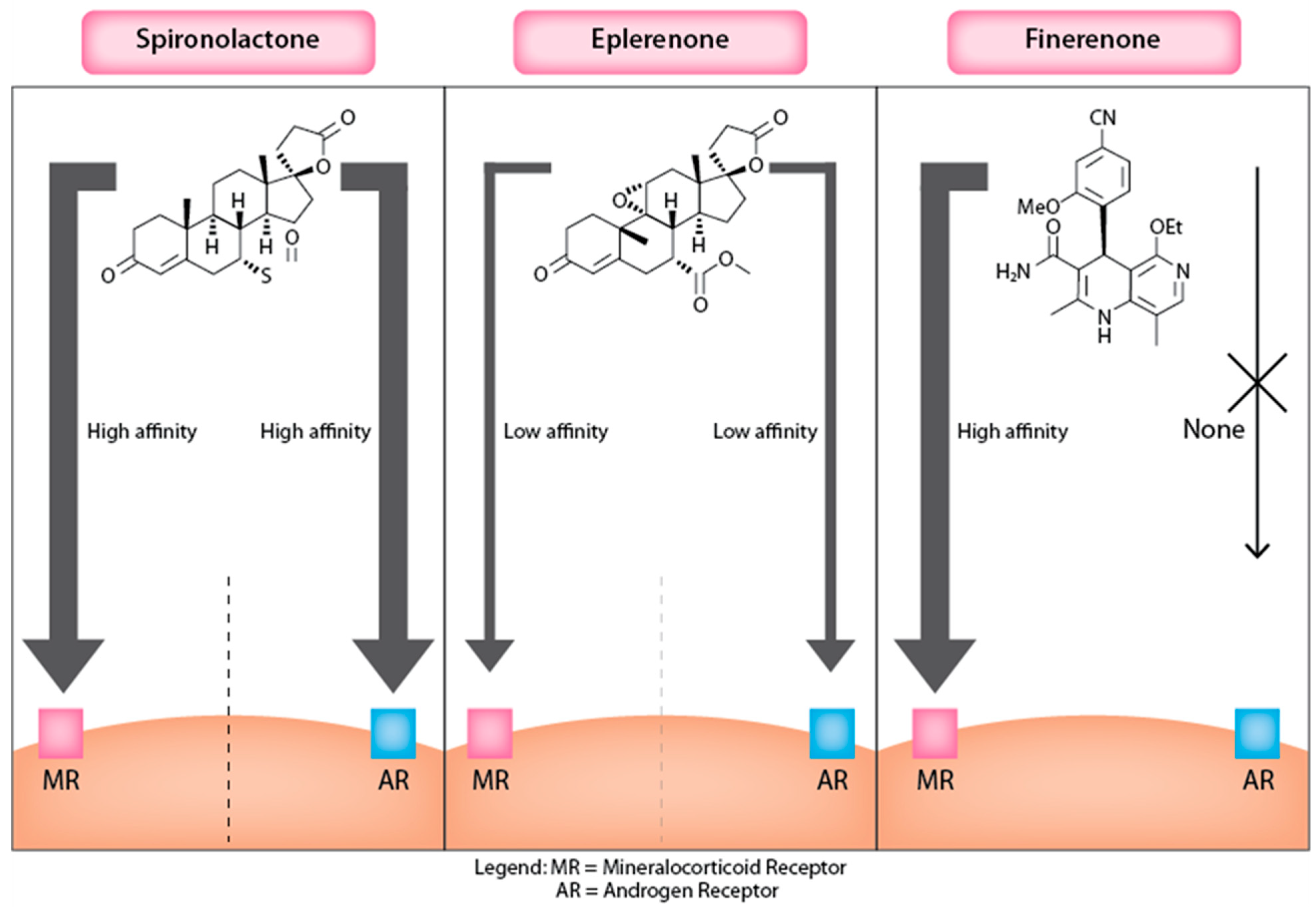
3.1. Mineralocorticoid Receptor Antagonists
3.1.1. Spironolactone
3.1.2. Eplerenone
3.1.3. Finerenone
3.2. Chronic Kidney Disease and Type 2 Diabetes Mellitus
3.3. Blood Pressure
3.4. Heart Failure
3.5. Atrial Fibrillation
3.6. Ethnicity and Sex Differences
3.7. Head-to-Head Studies with SGLT-2s, GLP-1 RAs, and MRAs
3.8. Future Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [PubMed]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar] [CrossRef]
- Young, D.B.; Smith, M.J., Jr.; Jackson, T.E.; Scott, R.E. Multiplicative interaction between angiotensin II and K concentration in stim-ulation of aldosterone. Am. J. Physiol. 1984, 247, E328. [Google Scholar] [PubMed]
- Ghose, R.P.; Hall, P.M.; Bravo, E.L. Medical management of aldosterone-producing adenomas. Ann. Intern. Med. 1999, 131, 105–108. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Yoshimura, M.; Yasue, H.; Sakamoto, T.; Ogawa, H.; Kugiyama, K.; Harada, E.; Nakayama, M.; Nakamura, S.; Ito, T.; et al. Aldosterone production is activated in failing ventricle in humans. Circulation 2001, 103, 72–77. [Google Scholar] [CrossRef]
- Lijnen, P.; Petrov, V. Induction of cardiac fibrosis by aldosterone. J. Mol. Cell. Cardiol. 2000, 32, 865–879. [Google Scholar] [CrossRef]
- Cooper, H.A.; Dries, D.L.; Davis, C.E.; Shen, Y.L.; Domanski, M.J. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation 1999, 100, 1311–1315. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Milliez, P.; Girerd, X.; Plouin, P.F.; Blacher, J.; Safar, M.E.; Mourad, J.-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243. [Google Scholar] [CrossRef] [PubMed]
- Jorde, U.P.; Vittorio, T.; Katz, S.D.; Colombo, P.C.; Latif, F.; Le Jemtel, T.H. Elevated plasma aldosterone levels despite complete inhibition of the vascular angioten-sin-converting enzyme in chronic heart failure. Circulation 2002, 106, 1055. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Young, W.F.; MacDonald, T.M. A review of the medical treatment of primary aldosteronism. J. Hypertens. 2001, 19, 353–361. [Google Scholar] [CrossRef]
- Layton, A.M.; Eady, E.A.; Whitehouse, H.; Del Rosso, J.Q.; Fedorowicz, Z.; van Zuuren, E.J. Oral Spironolactone for Acne Vulgaris in Adult Females: A Hybrid Systematic Review. Am. J. Clin. Dermatol. 2017, 18, 169–191. [Google Scholar] [CrossRef]
- Struthers, A.; Krum, H.; Williams, G.H. A Comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin. Cardiol. 2008, 31, 153–158. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Pre-vention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar]
- Parthasarathy, H.K.; Ménard, J.; White, W.B.; Young, W.F.; Williams, G.H.; Williams, B.; Ruilope, L.M.; McInnes, G.T.; Connell, J.M.; MacDonald, T.M. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J. Hypertens. 2011, 29, 980–990. [Google Scholar] [CrossRef]
- Fagart, J.; Hillisch, A.; Huyet, J.; Bärfacker, L.; Fay, M.; Pleiss, U.; Pook, E.; Schäfer, S.; Rafestin-Oblin, M.-E.; Kolkhof, P. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J. Biol. Chem. 2010, 285, 29932–29940. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Effect of Finerenone on Albuminuria in Patients with Diabetic Nephropathy: A Randomized Clinical Trial. JAMA 2015, 314, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Rossing, P.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; McGill, J.B.; Rosas, S.E.; Joseph, A.; Gebel, M.; et al. Finerenone in Patients with Chronic Kidney Disease and Type 2 Diabetes by Sodium–Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care 2022, 45, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Ruilope, L.M.; Ruiz-Hurtado, G.; Haller, H.; Schmieder, R.E.; Anker, S.D.; Filippatos, G.; Pitt, B.; Rossing, P.; Lambelet, M.; et al. Effect of finerenone on ambulatory blood pressure in chronic kidney disease in type 2 diabetes. J. Hypertens. 2022, 41, 295–302. [Google Scholar] [CrossRef]
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.-Y.; Zannad, F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013, 34, 2453–2463. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Böhm, M.; Gheorghiade, M.; Køber, L.; Krum, H.; Maggioni, A.P.; Ponikowski, P.; Voors, A.A.; Zannad, F.; et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J. 2016, 37, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients with Chronic Kidney Disease and Type 2 Diabetes: Analyses From the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with Finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484, Correction in Eur. Heart J. 2022, 43, 1989. [Google Scholar] [CrossRef]
- Agarwal, R.; Pitt, B.; Rossing, P.; Anker, S.D.; Filippatos, G.; Ruilope, L.M.; Kovesdy, C.P.; Tuttle, K.; Vaduganathan, M.; Wanner, C.; et al. Modifiability of Composite Cardiovascular Risk Associated with Chronic Kidney Disease in Type 2 Diabetes with Finerenone. JAMA Cardiol. 2023, 8, 732–741. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.; Pitt, B.; Senni, M.; et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2024, 391, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Filippatos, G.; Claggett, B.L.; Desai, A.S.; Jhund, P.S.; Henderson, A.; Brinker, M.; Kolkhof, P.; Schloemer, P.; Lay-Flurrie, J.; et al. Finerenone in Heart Failure and Chronic Kidney Disease with Type 2 Diabetes: The FINE-HEART pooled analysis of cardi-ovascular, kidney, and mortality outcomes. Nat. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Bakris, G.L.; Pitt, B.; Agarwal, R.; Rossing, P.; Ruilope, L.M.; Butler, J.; Lam, C.S.; Kolkhof, P.; Roberts, L.; et al. Finerenone Reduces New-Onset Atrial Fibrillation in Patients with Chronic Kidney Disease and Type 2 Diabetes. J. Am. Coll. Cardiol. 2021, 78, 142–152. [Google Scholar] [CrossRef]
- Koya, D.; Anker, S.D.; Ruilope, L.M.; Rossing, P.; Liu, Z.; Lee, B.W.; Lee, C.-T.; Scott, C.; Kolkhof, P.; Lawatscheck, R.; et al. Cardiorenal Outcomes with Finerenone in Asian Patients with Chronic Kidney Disease and Type 2 Diabetes: A FIDELIO-DKD post hoc Analysis. Am. J. Nephrol. 2023, 54, 370–378. [Google Scholar] [CrossRef]
- Bansal, S.; Canziani, M.E.F.; Birne, R.; Anker, S.D.; Bakris, G.L.; Filippatos, G.; Rossing, P.; Ruilope, L.M.; Farjat, A.E.; Kolkhof, P.; et al. Finerenone cardiovascular and kidney outcomes by age and sex: FIDELITY post hoc analysis of two phase 3, multicentre, double-blind trials. BMJ Open 2024, 14, e076444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, L.; Wang, J.; Wang, T.; Chien, C.; Huang, W.; Fu, X.; Xiao, Y.; Fu, Q.; Wang, S.; et al. Network meta-analysis on the effects of finerenone versus SGLT2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Cardiovasc. Diabetol. 2022, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Randomized Trial to Determine the Efficacy and Safety of Finerenone on Morbidity and Mortality Among Heart Failure Patients with Left Ventricular Ejection Fraction Greater than or Equal to 40% Hospitalized Due to an Episode of Acute Decompensated Heart Failure (REDEFINE-HF). ClinicalTrials.gov Identifier: NCT06008197. EudraCT: 2023-508581-15-00. Available online: https://clinicaltrials.gov/ct2/show/NCT06008197 (accessed on 1 August 2024).
- Colorado Prevention Center. A Study to Evaluate Finerenone on Clinical Efficacy and Safety in Patients with Heart Failure Who Are Intolerant or Not Eligible for Treatment with Steroidal Mineralocorticoid Receptor Antagonists (FINALITY-HF). ClinicalTri-als.gov Identifier: NCT06033950. Updated 17 April 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT06033950 (accessed on 1 August 2024).
- Green, J.B.; Green, J.B.; Mottl, A.K.; Mottl, A.K.; Bakris, G.; Bakris, G.; Heerspink, H.J.L.; Heerspink, H.J.L.; Mann, J.F.E.; Mann, J.F.E.; et al. Design of the Combination effect of Finerenone and Empagliflozin in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE). Nephrol. Dial. Transplant. 2023, 38, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Colorado Prevention Center. A Study to Determine the Efficacy and Safety of Finerenone and SGLT2i in Combination in Hos-pitalized Patients with Heart Failure (CONFIRMATION-HF) (CONFIRMATION). ClinicalTrials.gov Identifier: NCT06024746. Updated 22 July 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT06024746 (accessed on 1 August 2024).
- Tanaka, A.; Shibata, H.; Imai, T.; Yoshida, H.; Miyazono, M.; Takahashi, N.; Fukuda, D.; Okada, Y.; Teragawa, H.; Suwa, S.; et al. Rationale and design of an investigator-initiated, multicenter, prospective, placebo-controlled, double-blind, randomized trial to evaluate the effects of finerenone on vascular stiffness and cardiorenal biomarkers in type 2 diabetes and chronic kidney disease (FIVE-STAR). Cardiovasc. Diabetol. 2023, 22, 194. [Google Scholar] [CrossRef]
- Schaefer, F.; Montini, G.; Kang, H.G.; Walle, J.V.; Zaritsky, J.; Schreuder, M.F.; Litwin, M.; Scalise, A.; Scott, H.; Potts, J.; et al. Investigating the use of finerenone in children with chronic kidney disease and proteinuria: Design of the FIONA and open-label extension studies. Trials 2024, 25, 203. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Birkenfeld, A.L.; Cherney, D.Z.; Colhoun, H.M.; Ji, L.; Mathieu, C.; Groop, P.-H.; Pratley, R.E.; Rosas, S.E.; Rossing, P.; et al. Rationale and design of a randomised phase III registration trial investigating finerenone in participants with type 1 diabetes and chronic kidney disease: The FINE-ONE trial. Diabetes Res. Clin. Pract. 2023, 204, 110908. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Agarwal, R.; Bakris, G.L.; Cherney, D.Z.I.; Lam, C.S.P.; Neuen, B.L.; Sarafidis, P.A.; Tuttle, K.R.; Wanner, C.; Brinker, M.D.; et al. Design and baseline characteristics of the Finerenone, in addition to standard of care, on the progression of kidney disease in patients with Non-Diabetic Chronic Kidney Disease (FIND-CKD) randomized trial. Nephrol. Dial. Transplant. 2024, gfae132. [Google Scholar] [CrossRef] [PubMed]

| Study (Year) | Design | Intervention | Control | Main Outcomes | Effect Size |
|---|---|---|---|---|---|
| ARTS-DN (2015) [21] | RCT | Finerenone 1.25–20 mg once daily | Placebo | UACR over 90 days | There were reductions in UACR across all doses of Finerenone |
| FIDELIO-DKD (2020) [22] | RCT | Finerenone 10 or 20 mg once daily | Placebo | Kidney composite | Finerenone reduced the risk of CKD progression by 18% |
| FIGARO-DKD (2021) [23] | RCT | Finerenone 10 or 20 mg once daily | Placebo | Cardiovascular composite | Finerenone reduced the risk of composite CV events by 13% |
| The FIDELITY analysis (2022) [24] | Pooled analysis of FIDELIO-DKD and FIGARO-DKD | Finerenone 10 or 20 mg once daily | Finerenone + SGLT2i | Cardiovascular composite and kidney composite | Finerenone without an SGLT2i was equally as effective in reducing cardiorenal outcomes as Finerenone with SGLT2i |
| Study (Year) | Design | Population | Intervention vs. Control | Primary Outcome | Result |
|---|---|---|---|---|---|
| ARTS part A and B (2013) [26] | RCT | HFrEF and CKD | Finerenone 2.5–10 mg once daily vs. placebo (part A) and spironolactone (part B) | Change in serum potassium | Finerenone 10 mg once daily and 5 mg twice daily led to higher mean increases in serum potassium than the placebo but lower levels than spironolactone. |
| ARTS-HF (2016) [27] | RCT | HFrEF and T2D and/or CKD | Finerenone 2.5–15 mg once daily vs. eplerenone 25–50 mg once daily | >30% decline in NT-proBNP | There was no difference in NT-proBNP decline in Finerenone versus eplerenone. |
| Filippatos et al., 2022 [28] | Post hoc | Finerenone 10 or 20 mg once daily vs. placebo | First HHF, the combined risk of cardiovascular death or first HHF, and the risk of new-onset heart failure. | Finerenone had a 29% reduction in first HHF, 18% in cardiovascular death or first HHF, and 32% in new-onset HF. | |
| Agarwal et al. (2022) [29] | Post hoc | CKD and T2D | Finerenone 10 or 20 mg once daily vs. placebo | Cardiovascular and renal outcomes | Finerenone reduced the risk of cardiovascular and kidney outcomes vs. placebo across the spectrum of CKD in patients with type 2 diabetes. |
| Agarwal et al. (2023) [30] | Post hoc | CKD and T2D | Finerenone 10 or 20 mg once daily vs. placebo | Incidence rates of cardiovascular events | Finerenone was associated with a reduction in composite cardiovascular risk irrespective of eGFR and UACR. |
| FINEARTS-HF (2024) [31] | RCT | HFmrEF and HFpEF | Finerenone 20 or 40 mg once daily vs. placebo | Composite of total worsening heart failure events and death from cardiovascular causes | Finerenone resulted in a lower rate of total worsening heart failure events and death from cardiovascular causes than the placebo. |
| FINE-HEART (2024) [32] | Pooled analysis of FIGARO-DKD, FIDELIO-DKD, and FINEARTS-HF trials | - | Finerenone 20 or 40 mg once daily vs. placebo | Cardiorenal outcomes | Failed to demonstrate significant reductions in cardiovascular death; however, Finerenone was associated with significantly lower deaths of any cause, cardiovascular events, and kidney outcomes |
| Study (Year) | Design | Population | Intervention vs. Control | Primary Outcome | Effect Size |
|---|---|---|---|---|---|
| Zhang et al. (2022) [36] | Network meta-analysis | - | Finerenone vs. SGLT2i and GLP-1 agonist | Cardiorenal outcomes | SGLT2i significantly decreased the risk of renal events and HHF in comparison. All three were comparable in MACE, ACD, and CVD. |
| ARTS part A and B (2013) [26] | RCT | HFrEF and CKD | Finerenone 2.5–10 mg once daily vs. placebo (part A) and spironolactone (part B) | Change in potassium | Finerenone 10 mg once daily and 5 mg twice daily led to higher mean increases in serum potassium than the placebo but lower levels than spironolactone |
| ARTS-HF (2016) [27] | RCT | HFrEF and T2D and/or CKD | Finerenone 2.5–15 mg once daily vs. eplerenone 25–50 mg once daily | >30% decline in NT-proBNP | There was no difference in the NT-proBNP decline in Finerenone versus eplerenone |
| Study | Design | Anticipated Completion Date | Population | Intervention vs. Control | Primary Outcome |
|---|---|---|---|---|---|
| REDEFINE-HF [37] | RCT | April 2026 | Adults with heart failure with an EF > 40% | Finerenone vs. placebo | Composite total of HF events and CV death |
| FINALITY-HF [38] | RCT | August 2026 | HFrEF and intolerant of non steroidal MRAs | Finerenone vs. placebo | Composite total of HF events and CV death |
| CONFIDENCE [39] | RCT | January 2025 | Patients with CKD and T2DM on ACEis or ARBs | Finerenone + empagliflozin vs. placebo + Finerenone or empagliflozin | Urinary albumin to-creatinine ratio (UACR) |
| CONFIRMATION-HF [40] | RCT | November 2025 | Adults with heart failure | Finerenone + empagliflozin vs. standard of care | Clinical benefit |
| FIVE-STAR [41] | RCT | July 2026 | CKD and T2DM | Finerenone vs. placebo | Change in CAVI (Cardio–ankle vascular index) at 24 weeks after initiation of the protocol treatment compared with baseline |
| FIONA [42] | RCT | March 2027 | Children (6 months to 18 years old) | Finerenone + ACEi/ARB vs. placebo | Urinary protein-to-creatinine ratio (UPCR) reduction of at least 30% from baseline to day 180 |
| FIONA-OLE [42] | RCT | 18-month extension of FIONA | Children (6 months to 18 years old) | Finerenone + ACEi/ARB vs. placebo | Long-term safety |
| FINE-ONE [43] | RCT | October 2025 | Adults with T1DM | Finerenone vs. placebo | Change in UACR |
| FIND-CKD [44] | RCT | February 2026 | CKD without diabetes | Finerenone vs. placebo | Change in eGFR from baseline to 32 months |
| Steroidal MRA | Non-Steroidal MRA | ||
|---|---|---|---|
| Characteristics | Spironolactone | Eplerenone | Finerenone |
| Antagonism over MR | High | Low | High |
| Antagonism over AR | High | Low | Low |
| Effect on proteinuria and kidney damage | Moderate | Low | High |
| BP reduction | High | Low | High |
| Effect on HFpEF | Moderate | Moderate | High |
| Effect on HFmrEF | Moderate | Moderate | High |
| Effect on HFrEF | High | High | High |
| Hyperkalemia | High | Moderate | Low |
| Gynecomastia | High | Moderate | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabina, M.; Trube, J.; Shah, S.; Lurie, A.; Grimm, M.; Bizanti, A. Finerenone: A Third-Generation MRA and Its Impact on Cardiovascular Health—Insights from Randomized Controlled Trials. J. Clin. Med. 2024, 13, 6398. https://doi.org/10.3390/jcm13216398
Sabina M, Trube J, Shah S, Lurie A, Grimm M, Bizanti A. Finerenone: A Third-Generation MRA and Its Impact on Cardiovascular Health—Insights from Randomized Controlled Trials. Journal of Clinical Medicine. 2024; 13(21):6398. https://doi.org/10.3390/jcm13216398
Chicago/Turabian StyleSabina, Michael, Jennifer Trube, Shrinand Shah, Andrew Lurie, Mason Grimm, and Anas Bizanti. 2024. "Finerenone: A Third-Generation MRA and Its Impact on Cardiovascular Health—Insights from Randomized Controlled Trials" Journal of Clinical Medicine 13, no. 21: 6398. https://doi.org/10.3390/jcm13216398
APA StyleSabina, M., Trube, J., Shah, S., Lurie, A., Grimm, M., & Bizanti, A. (2024). Finerenone: A Third-Generation MRA and Its Impact on Cardiovascular Health—Insights from Randomized Controlled Trials. Journal of Clinical Medicine, 13(21), 6398. https://doi.org/10.3390/jcm13216398





