Current Therapeutic Strategies for Patients with Hypopharyngeal Carcinoma: Oncologic and Functional Outcomes
Abstract
1. Introduction
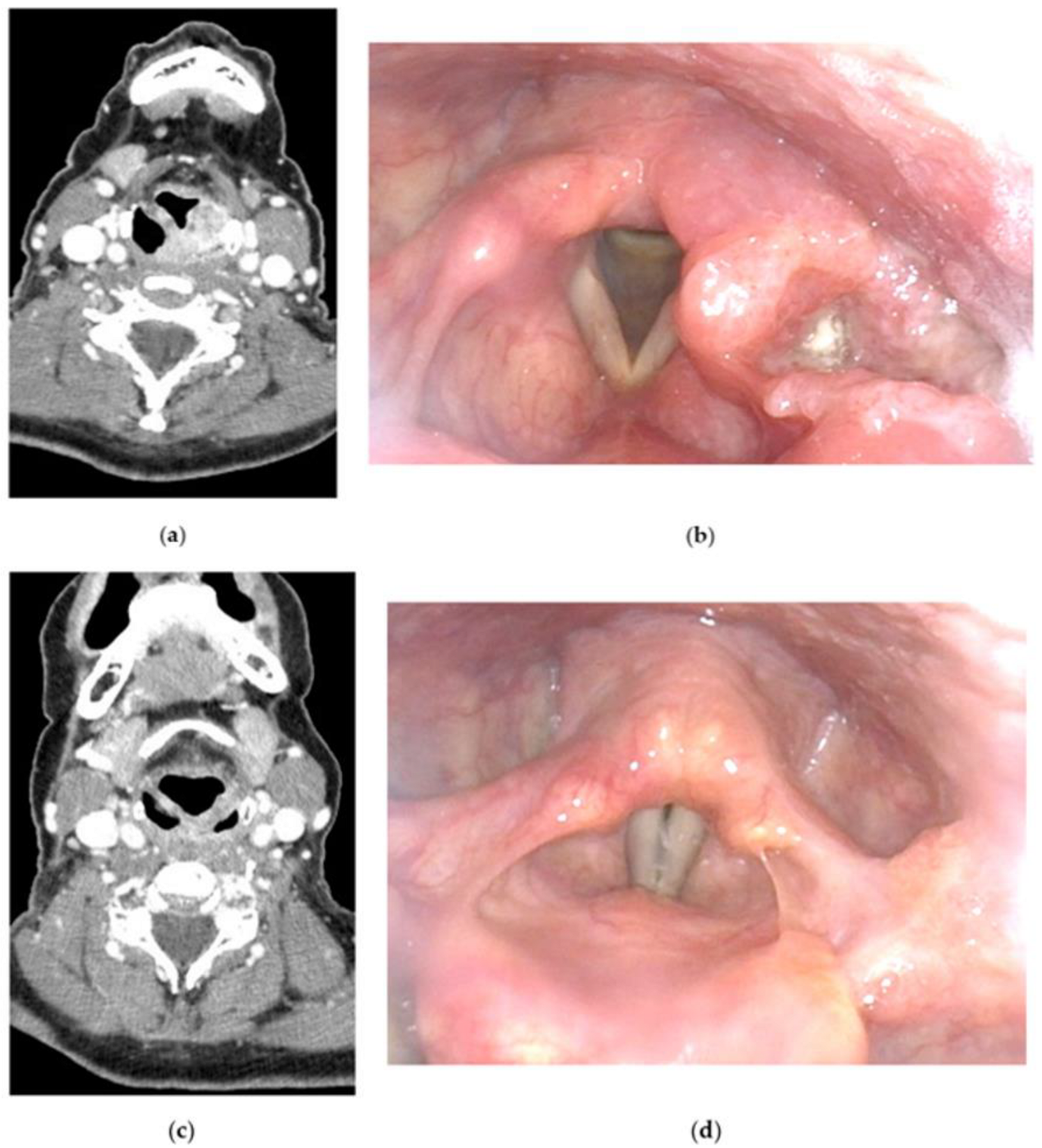
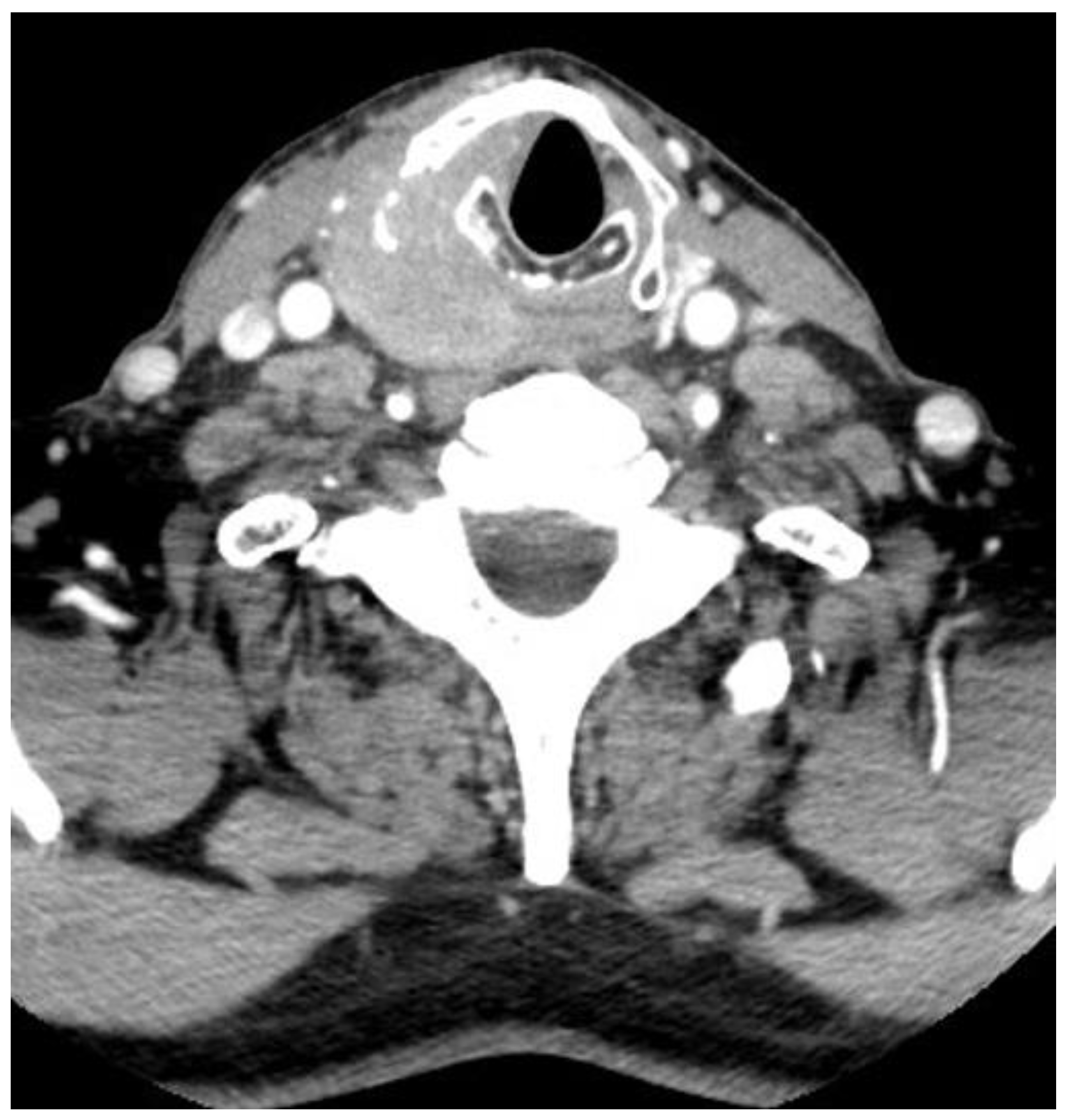
2. General Considerations
3. Management of Patients with Early-Stage Disease
3.1. Primary Surgical Treatment
3.1.1. Open Surgical Approaches
3.1.2. Transoral Surgical Approaches
3.2. Nonsurgical Treatment
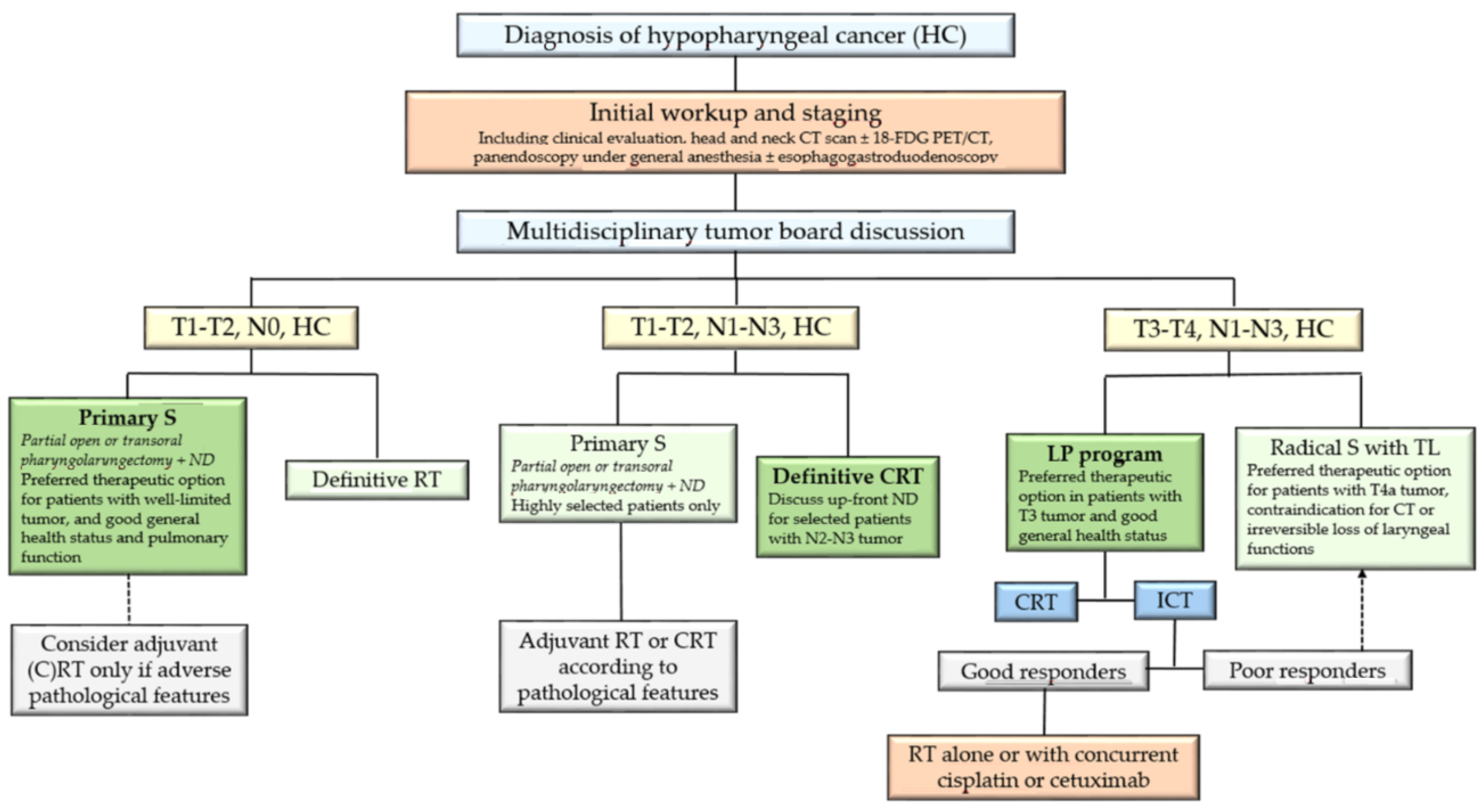
3.3. Choice of the Therapeutic Strategy
4. Management of Patients with T1-2, N1-3, M0 Tumors
5. Management of Patients with T3-4, N1-3, M0 Tumors
5.1. Radical Surgery
5.1.1. Oncologic Surgery
5.1.2. Reconstructive Surgery
5.1.3. Rehabilitation Measures
5.2. Larynx Preservation (LP) Approaches
5.3. Choice of the Therapeutic Strategy
6. Management of Patients with Recurrent and/or Metastatic Disease
7. Summary of Oncologic and Functional Outcomes
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 Cancer Groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Habib, A. Management of advanced hypopharyngeal carcinoma: Systematic review of survival following surgical and non-surgical treatments. J. Laryngol. Otol. 2018, 132, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Singh, C.A.; Thakar, A.; Kakkar, A.; Sikka, K.; Kumar, R.; Sharma, S.C. Prevalence of synchronous ESCN in head and neck cancer: A single-institution perspective. Laryngoscope 2021, 131, E807–E814. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.M.; Wang, H.H.; Le, C.T.; Tai, C.M.; Tseng, C.H.; Chen, C.C.; Tsai, Y.N.; Chen, T.H.; Hsu, M.H.; Wang, C.C.; et al. A nationwide population-based study to access the risk of metachronous esophageal cancers in head and neck cancer survivors. Sci. Rep. 2020, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Ishihara, R.; Morishima, T.; Maekawa, A.; Nakagawa, K.; Arao, M.; Ohmori, M.; Iwagami, H.; Matsuno, K.; Inoue, S.; et al. Impact of age at diagnosis of head and neck cancer on incidence of metachronous cancer. BMC Cancer 2019, 19, 3. [Google Scholar] [CrossRef]
- Haerle, S.K.; Schmid, D.T.; Ahmad, N.; Hany, T.F.; Stoeckli, S.J. The value of (18)F-FDG PET/CT for the detection of distant metastases in high-risk patients with head and neck squamous cell carcinoma. Oral Oncol. 2011, 47, 653–659. [Google Scholar] [CrossRef]
- Takes, R.P.; Rinaldo, A.; Silver, C.E.; Haigentz, M., Jr.; Woolgar, J.A.; Triantafyllou, A.; Mondin, V.; Paccagnella, D.; de Bree, R.; Shaha, A.R.; et al. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol. 2012, 48, 775–779. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: A narrative review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Nakayama, M.; Gosho, M.; Adachi, M.; Ii, R.; Matsumoto, S.; Miyamoto, H.; Hirose, Y.; Nishimura, B.; Tanaka, S.; Wada, T.; et al. The geriatric nutritional risk index as a prognostic factor in patients with advanced head and neck cancer. Laryngoscope 2021, 131, E151–E156. [Google Scholar] [CrossRef]
- Yanni, A.; Dequanter, D.; Lechien, J.R.; Loeb, I.; Rodriguez, A.; Javadian, R.; Van Gossum, M. Malnutrition in head and neck cancer patients: Impacts and indications of a prophylactic percutaneous endoscopic gastrostomy. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, S27–S33. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Benezery, K.; Chamorey, E.; Ettaiche, M.; Vandersteen, C.; Dassonville, O.; Poissonnet, G.; Riss, J.C.; Hannoun-Lévi, J.M.; Chand, M.E.; et al. Nutritional status and feeding-tube placement in patients with locally advanced hypopharyngeal cancer included in an induction chemotherapy-based larynx preservation program. Eur. Arch. Otorhinolaryngol. 2016, 273, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Sewnaik, A.; Baatenburg de Jong, R.J. Sequelae and complications of treatment for hypopharyngeal cancer: Minimising the risks. Adv. Otorhinolaryngol. 2019, 83, 109–117. [Google Scholar]
- Kwon, D.I.; Miles, B.A.; Education Committee of the American Head and Neck Society (AHNS). Hypopharyngeal carcinoma: Do you know your guidelines? Head Neck 2019, 41, 569–576. [Google Scholar] [CrossRef]
- Argiris, A.; Lefebvre, J.L. Laryngeal preservation strategies in locally advanced laryngeal and hypopharyngeal cancers. Front. Oncol. 2019, 9, 419. [Google Scholar] [CrossRef]
- Takes, R.P.; Strojan, P.; Silver, C.E.; Bradley, P.J.; Haigentz, M., Jr.; Wolf, G.T.; Shaha, A.R.; Hartl, D.M.; Olofsson, J.; Langendijk, J.A.; et al. Current trends in initial management of hypopharyngeal cancer: The declining use of open surgery. Head Neck 2012, 34, 270–281. [Google Scholar] [CrossRef]
- Cristalli, G.; Ferri, E.; Di Maio, P.; Spriano, G.; Mercante, G.; Ferreli, F.; Pellini, R.; Nata, F.B. Lateral conservative approach for recurrent/persistent hypopharyngeal carcinoma: A case series. Eur. Arch. Otorhinolaryngol. 2020, 277, 2375–2380. [Google Scholar] [CrossRef]
- Makeieff, M.; Mercante, G.; Jouzdani, E.; Garrel, R.; Crampette, L.; Guerrier, B. Supraglottic hemipharyngolaryngectomy for the treatment of T1 and T2 carcinomas of laryngeal margin and piriform sinus. Head Neck 2004, 26, 701–705. [Google Scholar] [CrossRef]
- Joo, Y.H.; Cho, K.J.; Park, J.O.; Nam, I.C.; Kim, C.S.; Kim, S.Y.; Kim, M.S. Swallowing function in patients with vertical hemipharyngolaryngectomy for hypopharyngeal squamous cell carcinoma. Head Neck 2016, 38, 191–195. [Google Scholar] [CrossRef]
- De Virgilio, A.; Iocca, O.; Malvezzi, L.; Di Maio, P.; Pellini, R.; Ferreli, F.; Cugini, G.; Colombo, G.; Spriano, G. The emerging role of robotic surgery among minimally invasive surgical approaches in the treatment of hypopharyngeal carcinoma: Systematic review and meta-analysis. J. Clin. Med. 2019, 8, 256. [Google Scholar] [CrossRef]
- Mazerolle, P.; Philouze, P.; Garrel, R.; Aubry, K.; Morinière, S.; El Bedoui, S.; Ton Van, J.; Ferron, C.; Malard, O.; Jegoux, F.; et al. Oncological and functional outcomes of trans-oral robotic surgery for pyriform sinus carcinoma: A French GETTEC group study. Oral Oncol. 2018, 86, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Razafindranaly, V.; Lallemant, B.; Aubry, K.; Moriniere, S.; Vergez, S.; Mones, E.D.; Malard, O.; Ceruse, P. Clinical outcomes with transoral robotic surgery for supraglottic squamous cell carcinoma: Experience of a French evaluation cooperative subgroup of GETTEC. Head Neck 2016, 38 (Suppl. S1), E1097–E1101. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Shioyama, Y.; Kawashima, M.; Saito, Y.; Nakamura, N.; Nakata, K.; Hareyama, M.; Takada, T.; Karasawa, K.; Watanabe, T.; et al. Multi-institutional analysis of early squamous cell carcinoma of the hypopharynx treated with radical radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Nishiyama, K.; Morimoto, M.; Nakamura, S.; Suzuki, O.; Kawaguchi, Y.; Miyagi, K.; Fujii, T.; Yoshino, K. Definitive radiotherapy for T1-2 hypopharyngeal cancer: A single-institution experience. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e129–e135. [Google Scholar] [CrossRef]
- Rabbani, A.; Amdur, R.J.; Mancuso, A.A.; Werning, J.W.; Kirwan, J.; Morris, C.G.; Mendenhall, W.M. Definitive radiotherapy for T1-T2 squamous cell carcinoma of pyriform sinus. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 351–355. [Google Scholar] [CrossRef]
- Sato, M.P.; Otsuki, N.; Kitano, M.; Ishikawa, K.; Tanaka, K.; Kimura, T.; Doi, K. Up-front neck dissection followed by chemoradiotherapy for T1-T3 hypopharyngeal cancer with advanced nodal involvement. Head Neck 2021, 43, 3810–3819. [Google Scholar] [CrossRef]
- Boros, A.; Blanchard, P.; Dade, A.; Gorphe, P.; Breuskin, I.; Even, C.; Nguyen, F.; Deutsch, E.; Bidault, F.; Janot, F.; et al. Outcomes in N3 head and neck squamous cell carcinoma and role of upfront neck dissection. Laryngoscope 2021, 131, E846–E850. [Google Scholar] [CrossRef]
- Elicin, O.; Nisa, L.; Dal Pra, A.; Bojaxhiu, B.; Caversaccio, M.; Schmücking, M.; Aebersold, D.M.; Giger, R. Up-front neck dissection followed by definitive (chemo)-radiotherapy in head and neck squamous cell carcinoma: Rationale, complications, toxicity rates, and oncological outcomes—A systematic review. Radiother. Oncol. 2016, 119, 185–193. [Google Scholar] [CrossRef]
- Bozec, A.; Culié, D.; Poissonnet, G.; Dassonville, O. Current role of total laryngectomy in the era of organ preservation. Cancers 2020, 12, 584. [Google Scholar] [CrossRef]
- Bradley, P.J.; Liu, L. Open-neck organ preservation surgery for hypopharyngeal cancer: Indications, techniques, limits, and outcomes. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 123–129. [Google Scholar] [CrossRef]
- Xu, S.; Huang, Y.; Huang, H.; Qian, J.; Wang, K.; Wu, Y.; Wang, X.; Xu, Z.; Liu, S.; Liu, J. Organ preservation surgery for pyriform sinus carcinoma with vocal cord fixation: Functional and oncological outcomes. Eur. Arch. Otorhinolaryngol. 2022, 279, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Urken, M.L.; Blackwell, K.; Biller, H.F. Reconstruction of the laryngopharynx after hemicricoid/hemithyroid cartilage resection. Preliminary functional results. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Vandersteen, C.; Benezery, K.; Chamorey, E.; Ettaiche, M.; Dassonville, O.; Poissonnet, G.; Riss, J.C.; Pierre, C.S.; Hannoun-Lévi, J.M.; Chand, M.E.; et al. Contemporary therapeutic management of locally advanced hypopharyngeal cancer: Oncologic and functional outcomes—A report on 100 cases. Acta Otolaryngol. 2015, 135, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Goldstein, D.P.; Brown, D.; Irish, J.; Gullane, P.J.; Gilbert, R.W. Circumferential pharyngeal reconstruction: History, critical analysis of techniques, and current therapeutic recommendations. Head Neck 2010, 32, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Mattei, P.; Thamphya, B.; Chamorey, E.; Scheller, B.; Château, Y.; Dassonville, O.; Poissonnet, G.; Culié, D.; Koulmann, P.H.; Hechema, R.; et al. Therapeutic strategies, oncologic and swallowing outcomes and their predictive factors in patients with locally advanced hypopharyngeal cancer. Eur. Arch. Otorhinolaryngol. 2022, 279, 3629–3637. [Google Scholar] [CrossRef]
- Grasl, S.; Janik, S.; Parzefall, T.; Formanek, M.; Grasl, M.C.; Heiduschka, G.; Erovic, B.M. Lymph node ratio as a prognostic marker in advanced laryngeal and hypopharyngeal carcinoma after primary total laryngopharyngectomy. Clin. Otolaryngol. 2020, 45, 73–82. [Google Scholar] [CrossRef]
- Roux, M.; Dassonville, O.; Ettaiche, M.; Chamorey, E.; Poissonnet, G.; Bozec, A. Primary total laryngectomy and pharyngolaryngectomy in T4 pharyngolaryngeal cancers: Oncologic and functional results and prognostic factors. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 151–154. [Google Scholar] [CrossRef]
- Chung, E.J.; Jeong, W.J.; Jung, Y.H.; Kwon, S.K.; Kwon, T.K.; Ahn, S.H.; Sung, M.W.; Keam, B.; Heo, D.S.; Kim, J.H.; et al. Long-term oncological and functional outcomes of induction chemotherapy followed by (chemo)radiotherapy vs definitive chemoradiotherapy vs surgery-based therapy in locally advanced stage III/IV hypopharyngeal cancer: Multicenter review of 266 cases. Oral Oncol. 2019, 89, 84–94. [Google Scholar] [CrossRef]
- Cabrera, C.I.; Jones, A.J.; Parker, N.P.; Emily Lynn Blevins, A.; Weidenbecher, M.S. Pectoralis major onlay vs interpositional reconstruction fistulation after salvage total laryngectomy: Systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 2021, 164, 972–983. [Google Scholar] [CrossRef]
- Nikolaidou, E.; Pantazi, G.; Sovatzidis, A.; Vakouli, S.; Vardaxi, C.; Evangelopoulos, I.; Gougousis, S. The supraclavicular artery island flap for pharynx reconstruction. J. Clin. Med. 2022, 11, 3126. [Google Scholar] [CrossRef]
- Haidar, Y.M.; Kuan, E.C.; Verma, S.P.; Goddard, J.A.; Armstrong, W.B.; Tjoa, T. Free flap versus pedicled flap reconstruction of laryngopharyngeal defects: A 10-year national surgical quality improvement program analysis. Laryngoscope 2019, 129, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Costantino, A.; Festa, B.M.; Ferreli, F.; Russo, E.; Malvezzi, L.; Giannitto, C.; Spriano, G.; Mercante, G.; De Virgilio, A. Circumferential pharyngeal reconstruction after total laryngopharyngectomy: A systematic review and network meta-analysis. Oral Oncol. 2022, 127, 105809. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.K.; Tan, N.C.; Tan, B.K.; Ooi, A.S.H. Comparison of outcomes of fasciocutaneous free flaps and jejunal free flaps in pharyngolaryngoesophageal reconstruction: A systematic review and meta-analysis. Ann. Plast. Surg. 2019, 82, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Bohlok, A.; Richet, T.; Quiriny, M.; Willemse, E.; Dekeyser, C.; Andry, G.; Donckier, V.; Digonnet, A. The effect of salivary bypass tube use on the prevention of pharyngo-cutaneous fistulas after total laryngectomy. Eur. Arch. Otorhinolaryngol. 2022, 279, 311–317. [Google Scholar] [CrossRef]
- Sharp, D.A.; Theil, D.R.; Cook, R.; Coman, W.B. Long-term functional speech and swallowing outcomes following pharyngolaryngectomy with free jejunal flap reconstruction. Ann. Plast. Surg. 2010, 64, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Lai, W.S.; Lin, Y.Y.; Liu, S.C.; Lee, J.C. Pharyngeal reconstruction using a U-shaped pectoralis major myocutaneous flap: An effective technique that should not be forgotten. Eur. Arch. Otorhinolaryngol. 2020, 277, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Hsiao, J.R.; Lee, W.T.; Ou, C.Y.; Yen, Y.T.; Tseng, Y.L.; Pan, S.C.; Shieh, S.J.; Lee, Y.C. Esophageal reconstruction after oncological total laryngopharyngoesophagectomy: Algorithmic approach. Microsurgery 2019, 39, 6–13. [Google Scholar] [CrossRef]
- Molteni, G.; Fulco, G.; Gazzini, L.; Laura, E.; Paiola, G.; Giacopuzzi, S.; Marchioni, D.; Pighi, G.P. Prosthetic voice rehabilitation after laryngoesophagectomy: Surgical and functional outcomes. Eur. Arch. Otorhinolaryngol. 2022, 279, 4085–4092. [Google Scholar] [CrossRef]
- Bozec, A.; Boscagli, M.; Serris, M.; Chamorey, E.; Dassonville, O.; Poissonnet, G.; Culié, D.; Scheller, B.; Benezery, K.; Gal, J. Long-term functional and quality of life outcomes in laryngectomized patients after successful voice restoration using tracheoesophageal prostheses. Surg. Oncol. 2021, 38, 101580. [Google Scholar] [CrossRef]
- Lavertu, P.; Guay, M.E.; Meeker, S.S.; Kmiecik, J.R.; Secic, M.; Wanamaker, J.R.; Eliachar, I.; Wood, B.G. Secondary tracheoesophageal puncture: Factors predictive of voice quality and prosthesis use. Head Neck. 1996, 18, 393–398. [Google Scholar] [CrossRef]
- Bozec, A.; Poissonnet, G.; Chamorey, E.; Demard, F.; Santini, J.; Peyrade, F.; Ortholan, C.; Benezery, K.; Thariat, J.; Sudaka, A.; et al. Results of vocal rehabilitation using tracheoesophageal voice prosthesis after total laryngectomy and their predictive factors. Eur. Arch. Otorhinolaryngol. 2010, 267, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Gitomer, S.A.; Hutcheson, K.A.; Christianson, B.L.; Samuelson, M.B.; Barringer, D.A.; Roberts, D.B.; Hessel, A.C.; Weber, R.S.; Lewin, J.S.; Zafereo, M.E. Influence of timing, radiation, and reconstruction on complications and speech outcomes with tracheoesophageal puncture. Head Neck 2016, 38, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Department of Veterans Affairs Laryngeal Cancer Study Group; Wolf, G.T.; Fisher, S.G.; Hong, W.K.; Hillman, R.; Spaulding, M.; Laramore, G.E.; Endicott, J.W.; McClatchey, K.; Henderson, W.G. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar] [PubMed]
- Lefebvre, J.L.; Andry, G.; Chevalier, D.; Luboinski, B.; Collette, L.; Traissac, L.; de Raucourt, D.; Langendijk, J.A.; for the EORTC Head and Neck Cancer Group. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann. Oncol. 2012, 23, 2708–2714. [Google Scholar] [CrossRef]
- Henriques De Figueiredo, B.; Fortpied, C.; Menis, J.; Lefebvre, J.L.; Barzan, L.; de Raucourt, D.; Geoffrois, L.; Giurgea, L.; Hupperets, P.; Leemans, C.R.; et al. Long-term update of the 24954 EORTC phase III trial on larynx preservation. Eur. J. Cancer 2016, 65, 109–112. [Google Scholar] [CrossRef]
- Janoray, G.; Pointreau, Y.; Garaud, P.; Chapet, S.; Alfonsi, M.; Sire, C.; Jadaud, E.; Calais, G. Long-term results of a multicenter randomized phase iii trial of induction chemotherapy with cisplatin, 5-fluorouracil, ± docetaxel for larynx preservation. J. Natl. Cancer Inst. 2015, 108, djv368. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Lefebvre, J.L.; Pointreau, Y.; Rolland, F.; Alfonsi, M.; Baudoux, A.; Sire, C.; de Raucourt, D.; Malard, O.; Degardin, M.; Tuchais, C.; et al. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: The TREMPLIN randomized phase II study. J. Clin. Oncol. 2013, 31, 853–859. [Google Scholar] [CrossRef]
- Dietz, A.; Wichmann, G.; Kuhnt, T.; Pfreundner, L.; Hagen, R.; Scheich, M.; Kölbl, O.; Hautmann, M.G.; Strutz, J.; Schreiber, F.; et al. Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy-final results of the larynx organ preservation trial DeLOS-II. Ann. Oncol. 2018, 29, 2105–2114. [Google Scholar] [CrossRef]
- Choi, Y.S.; Park, S.G.; Song, E.K.; Cho, S.H.; Park, M.R.; Park, K.U.; Lee, K.H.; Song, I.C.; Lee, H.J.; Jo, D.Y.; et al. Comparison of the therapeutic effects of total laryngectomy and a larynx-preservation approach in patients with T4a laryngeal cancer and thyroid cartilage invasion: A multicenter retrospective review. Head Neck 2016, 38, 1271–1277. [Google Scholar] [CrossRef]
- Chen, A.; Halpern, M. Factors predictive of survival in advanced laryngeal cancer. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 1270–1276. [Google Scholar] [CrossRef]
- Grover, S.; Swisher-McClure, S.; Mitra, N.; Li, J.; Cohen, R.B.; Ahn, P.H.; Lukens, J.N.; Chalian, A.A.; Weinstein, G.S.; O’Malley, B.W.; et al. Total laryngectomy versus larynx preservation for T4a larynx cancer: Patterns of care and survival outcomes. Int. J. Radiat. Oncol. 2015, 92, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.F.; Timmermans, A.J.; van Dijk, B.A.C.; Overbeek, L.I.H.; Smit, L.A.; Hilgers, F.J.M.; Stuiver, M.M.; van den Brekel, M.W.M. Trends in treatment, incidence and survival of hypopharynx cancer: A 20-year population-based study in the Netherlands. Eur. Arch. Otorhinolaryngol. 2018, 275, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; Navran, A.; Walraven, I.; Schreuder, W.H.; Tesselaar, M.E.T.; Klop, W.M.C. Organ-preservation (chemo)radiotherapy for T4 laryngeal and hypopharyngeal cancer: Is the effort worth? Eur. Arch. Otorhinolaryngol. 2019, 276, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; O’Neill, A.; Rabinowits, G.; Tishler, R.; Khuri, F.; Adkins, D.; Clark, J.; Sarlis, N.; Lorch, J.; Beitler, J.J.; et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 257–264. [Google Scholar] [CrossRef]
- Hamoir, M.; Schmitz, S.; Suarez, C.; Strojan, P.; Hutcheson, K.A.; Rodrigo, J.P.; Mendenhall, W.M.; Simo, R.; Saba, N.F.; D’Cruz, A.K.; et al. The current role of salvage surgery in recurrent head and neck squamous cell carcinoma. Cancers 2018, 10, 267. [Google Scholar] [CrossRef]
- Fakhry, N.; Chamorey, E.; Michel, J.; Collet, C.; Santini, L.; Poissonnet, G.; Santini, J.; Dessi, P.; Giovanni, A.; Dassonville, O.; et al. Salvage circular laryngopharyngectomy and radial forearm free flap for recurrent hypopharyngeal cancer. Laryngoscope 2013, 123, 910–915. [Google Scholar] [CrossRef]
- Meulemans, J.; Debacker, J.; Demarsin, H.; Vanclooster, C.; Neyt, P.; Mennes, T.; Vauterin, T.; Huvenne, W.; Laenen, A.; Delaere, P.; et al. Oncologic outcomes after salvage laryngectomy for squamous cell carcinoma of the larynx and hypopharynx: A multicenter retrospective cohort study. Ann. Surg. Oncol. 2021, 28, 1751–1761. [Google Scholar] [CrossRef]
- Saada-Bouzid, E.; Peyrade, F.; Guigay, J. Systemic treatment of recurrent and/or metastatic squamous cell carcinomas of the head and neck: What is the best therapeutic sequence? Curr. Opin. Oncol. 2022, 34, 196–203. [Google Scholar] [CrossRef]
- Szturz, P.; Nevens, D.; Vermorken, J.B. Oligometastatic disease management: Finding the sweet spot. Front. Oncol. 2020, 10, 617793. [Google Scholar] [CrossRef]
- Guigay, J.; Aupérin, A.; Fayette, J.; Saada-Bouzid, E.; Lafond, C.; Taberna, M.; Geoffrois, L.; Martin, L.; Capitain, O.; Cupissol, D.; et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021, 22, 463–475. [Google Scholar] [PubMed]
- Burtness, B.; Harrington, K.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Elaldi, R.; Roussel, L.M.; Gal, J.; Scheller, B.; Chamorey, E.; Schiappa, R.; Lasne-Cardon, A.; Louis, M.Y.; Culié, D.; Dassonville, O.; et al. Correlations between long-term quality of life and patient needs and concerns following head and neck cancer treatment and the impact of psychological distress. A multicentric cross-sectional study. Eur. Arch. Otorhinolaryngol. 2021, 278, 2437–2445. [Google Scholar] [CrossRef]
- Hanna, E.; Sherman, A.; Cash, D.; Adams, D.; Vural, E.; Fan, C.Y.; Suen, J.Y. Quality of life for patients following total laryngectomy vs chemoradiation for laryngeal preservation. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 875–879. [Google Scholar] [CrossRef]
- Chang, D.C.; Chen, A.W.; Lo, Y.S.; Chuang, Y.C.; Chen, M.K. Factors associated with suicidal ideation risk in head and neck cancer: A longitudinal study. Laryngoscope 2019, 129, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozec, A.; Poissonnet, G.; Dassonville, O.; Culié, D. Current Therapeutic Strategies for Patients with Hypopharyngeal Carcinoma: Oncologic and Functional Outcomes. J. Clin. Med. 2023, 12, 1237. https://doi.org/10.3390/jcm12031237
Bozec A, Poissonnet G, Dassonville O, Culié D. Current Therapeutic Strategies for Patients with Hypopharyngeal Carcinoma: Oncologic and Functional Outcomes. Journal of Clinical Medicine. 2023; 12(3):1237. https://doi.org/10.3390/jcm12031237
Chicago/Turabian StyleBozec, Alexandre, Gilles Poissonnet, Olivier Dassonville, and Dorian Culié. 2023. "Current Therapeutic Strategies for Patients with Hypopharyngeal Carcinoma: Oncologic and Functional Outcomes" Journal of Clinical Medicine 12, no. 3: 1237. https://doi.org/10.3390/jcm12031237
APA StyleBozec, A., Poissonnet, G., Dassonville, O., & Culié, D. (2023). Current Therapeutic Strategies for Patients with Hypopharyngeal Carcinoma: Oncologic and Functional Outcomes. Journal of Clinical Medicine, 12(3), 1237. https://doi.org/10.3390/jcm12031237





