Prevalence and Impact of Arrhythmia on Outcomes in Restrictive Cardiomyopathy—A Report from the Beijing Municipal Health Commission Information Center (BMHCIC) Database
Abstract
1. Introduction
2. Methods
2.1. Source of Database
2.2. RCM and Atrial Fibrillation, Stroke/Systematic Embolism and Heart Failure
2.3. Statistics Analysis
3. Results
3.1. Baseline Characteristics
3.2. The Influence of Type of Atrial Fibrillation on Prognosis
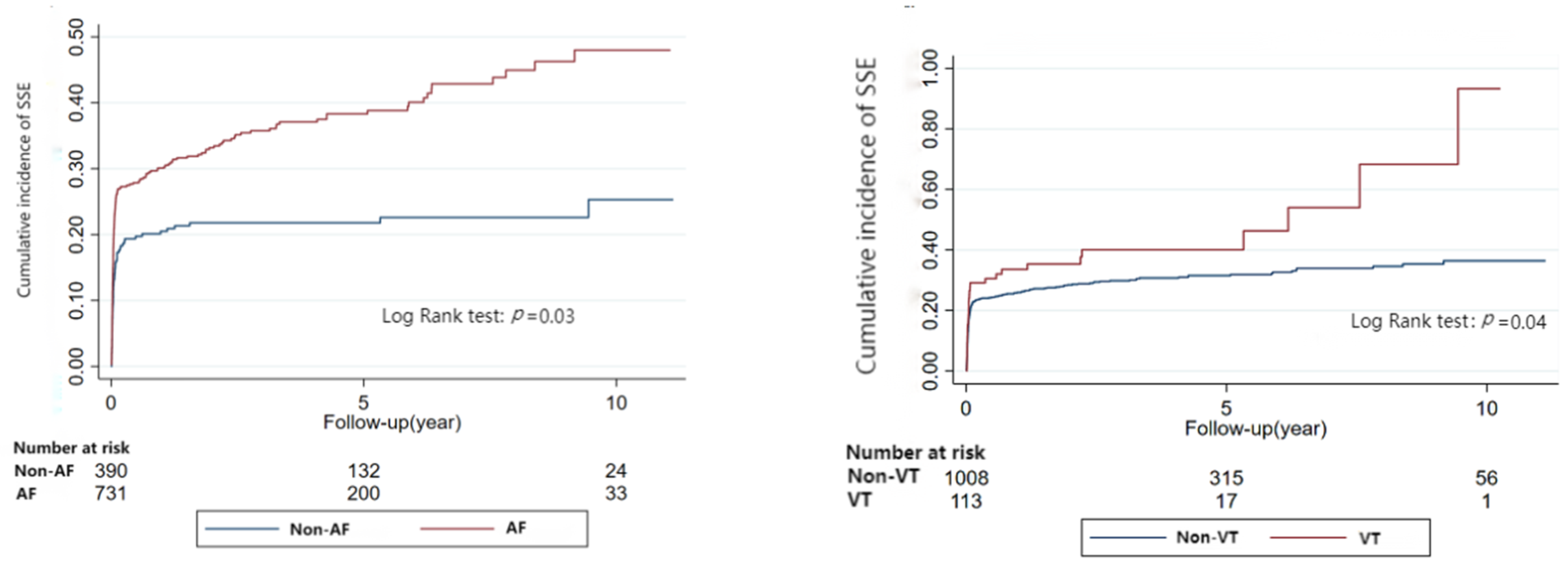
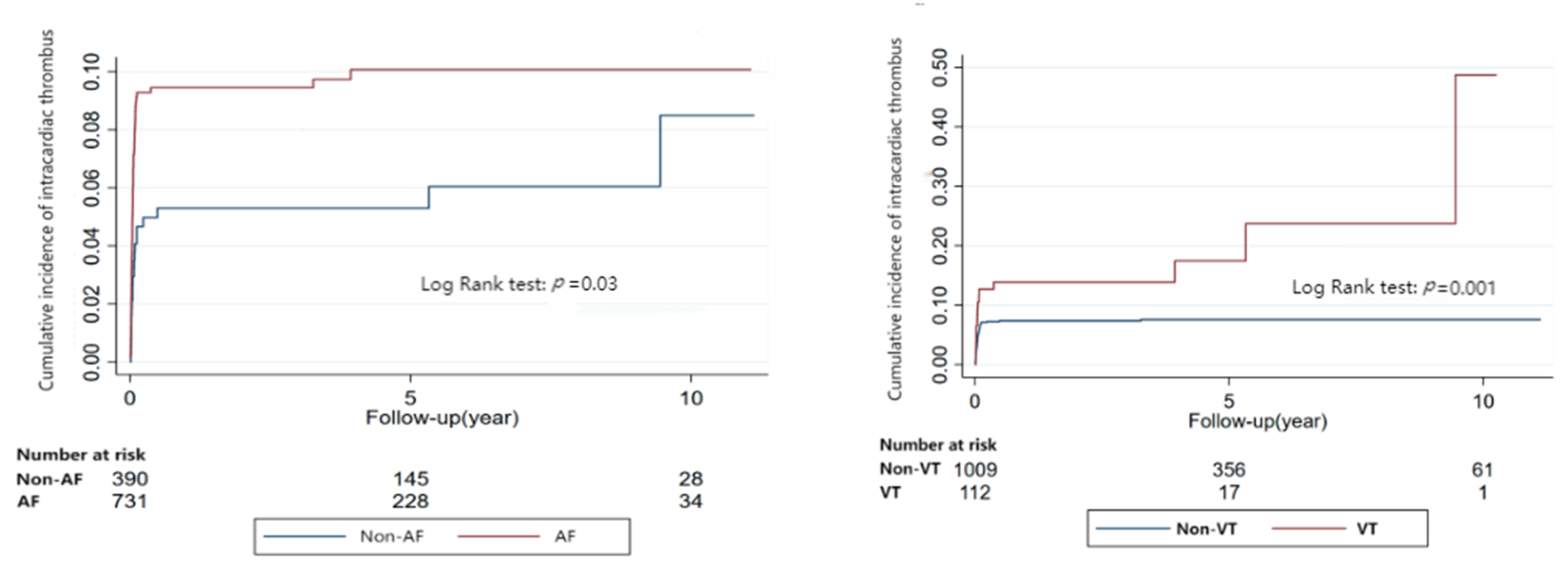
3.3. Risk of Stroke and Systematic Embolism Related to Arrhythmia
3.4. Risk of Heart Failure Related to Arrhythmia
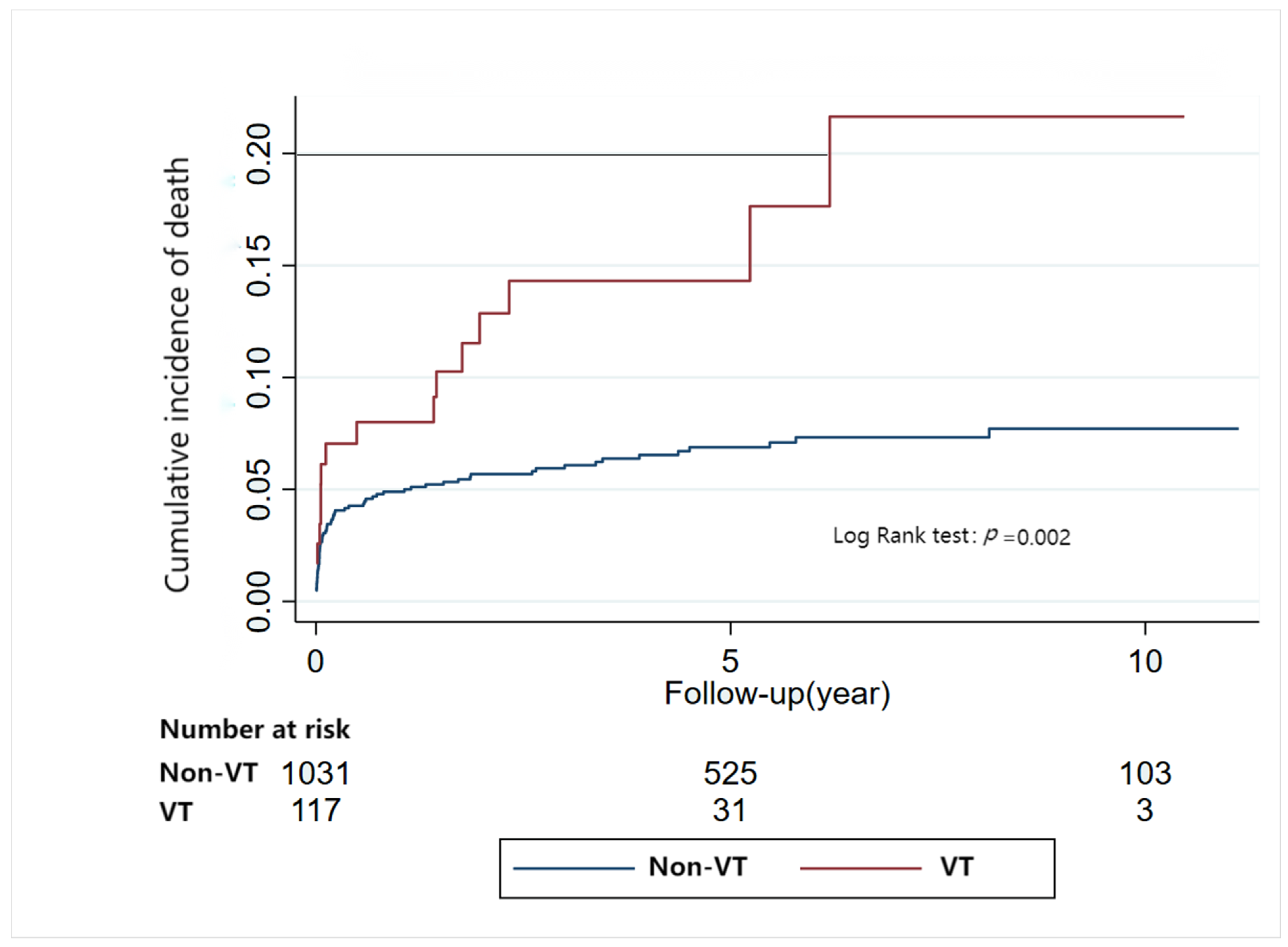
3.5. Risk of Mortality Risk Related to Arrhythmia
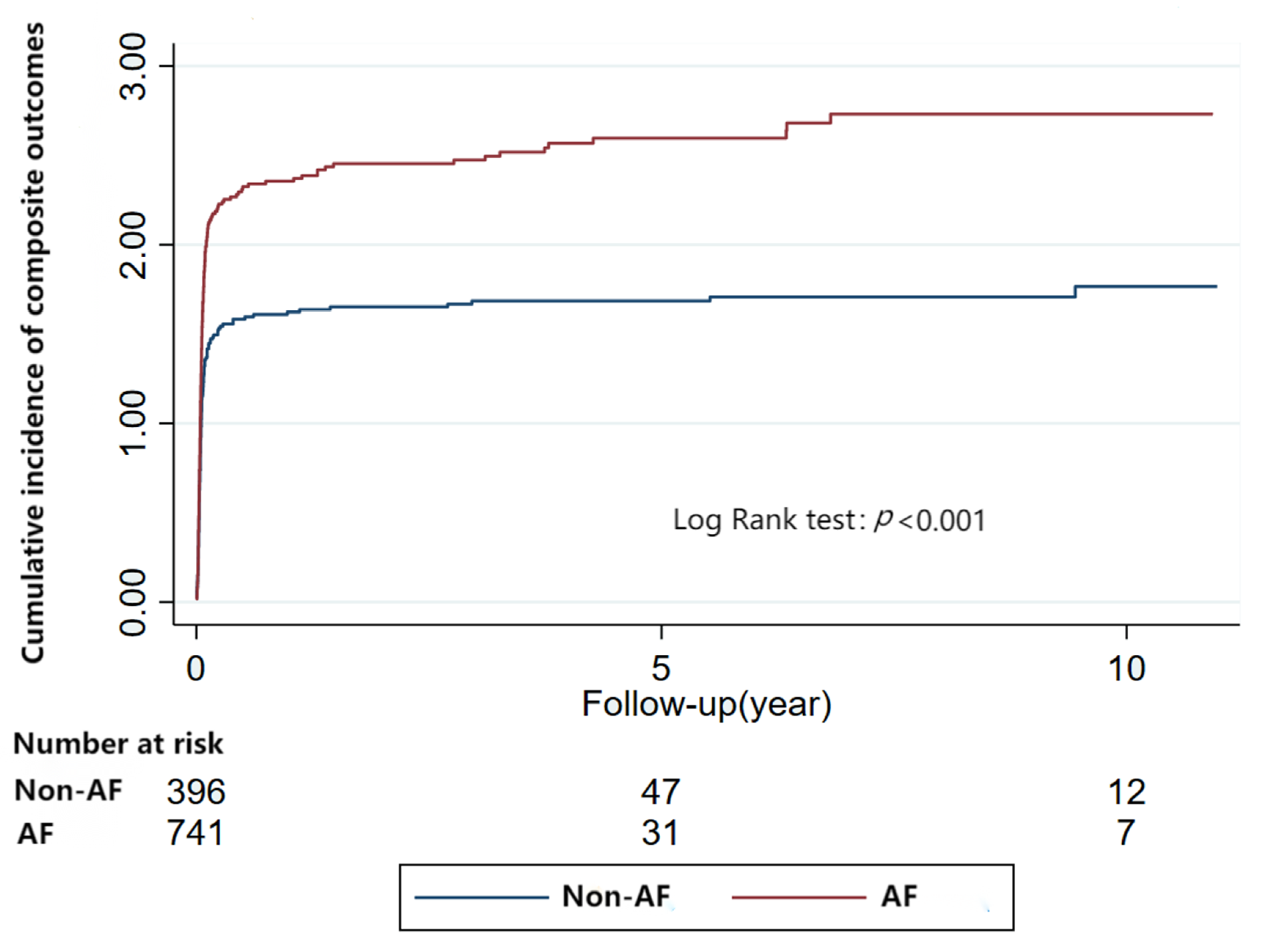
3.6. Risk of Composite Outcomes Related to Arrhythmia
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mogensen, J.; Arbustini, E. Restrictive cardiomyopathy. Curr. Opin. Cardiol. 2009, 24, 214–220. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.S.; Fallon, J.T.; Fuster, V. Restrictive cardiomyopathy. N. Engl. J. Med. 1997, 336, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Tucker, N.R.; McLellan, M.A.; Hu, D.; Ye, J.; Parsons, V.A.; Mills, R.W.; Clauss, S.; Dolmatova, E.; Shea, M.A.; Milan, D.J.; et al. Novel Mutation in FLNC (Filamin C) Causes Familial Restrictive Cardiomyopathy. Circ. Cardiovasc. Genet. 2017, 10, e001780. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.J. Constrictive Pericarditis Versus Restrictive Cardiomyopathy? J. Am. Coll. Cardiol. 2016, 67, 2061–2076. [Google Scholar] [CrossRef]
- Mookadam, F.; Jiamsripong, P.; Raslan, S.F.; Panse, P.M.; Tajik, A.J. Constrictive pericarditis and restrictive cardiomyopathy in the modern era. Future Cardiol. 2011, 7, 471–483. [Google Scholar] [CrossRef]
- Benotti, J.R.; Grossman, W.; Cohn, P.F. Clinical profile of restrictive cardiomyopathy. Circulation 1980, 61, 1206–1212. [Google Scholar] [CrossRef]
- Berger, P.B.; Duffy, J.; Reeder, G.S.; Karon, B.L.; Edwards, W.D. Restrictive cardiomyopathy associated with the eosinophilia-myalgia syndrome. Mayo Clin. Proc. 1994, 69, 162–165. [Google Scholar] [CrossRef]
- Meaney, E.; Shabetai, R.; Bhargava, V.; Shearer, M.; Weidner, C.; Mangiardi, L.M.; Smalling, R.; Peterson, K. Cardiac amyloidosis, contrictive pericarditis and restrictive cardiomyopathy. Am. J. Cardiol. 1976, 38, 547–556. [Google Scholar] [CrossRef]
- Shabetai, R. Pathophysiology and differential diagnosis of restrictive cardiomyopathy. Cardiovasc. Clin. 1988, 19, 123–132. [Google Scholar]
- Siegel, R.J.; Shah, P.K.; Fishbein, M.C. Idiopathic restrictive cardiomyopathy. Circulation 1984, 70, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A. Inherited cardiomyopathies. Circ. J. Off. J. Jpn. Circ. Soc. 2014, 78, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Blauwet, L.A.; Gertz, M.A. Restrictive Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Cimiotti, D.; Budde, H.; Hassoun, R.; Jaquet, K. Genetic Restrictive Cardiomyopathy: Causes and Consequences-An Integrative Approach. Int. J. Mol. Sci. 2021, 22, 558. [Google Scholar] [CrossRef]
- Brodehl, A.; Gerull, B. Genetic Insights into Primary Restrictive Cardiomyopathy. J. Clin. Med. 2022, 11, 2094. [Google Scholar] [CrossRef]
- Ammash, N.M.; Seward, J.B.; Bailey, K.R.; Edwards, W.D.; Tajik, A.J. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation 2000, 101, 2490–2496. [Google Scholar] [CrossRef]
- Sasaki, N.; Yasumura, Y.; Uemura, N.; Hanatani, A.; Nakatani, S.; Yamagishi, M.; Miyatake, K. Restrictive cardiomyopathy with right-sided dominant heart failure after sinus conversion from atrial fibrillation: Case report. Circ. J. Off. J. Jpn. Circ. Soc. 2003, 67, 969–971. [Google Scholar] [CrossRef]
- Fitzpatrick, A.P.; Shapiro, L.M.; Rickards, A.F.; Poole-Wilson, P.A. Familial restrictive cardiomyopathy with atrioventricular block and skeletal myopathy. Br. Heart J. 1990, 63, 114–118. [Google Scholar] [CrossRef]
- Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zheng, J.P.; Lu, F.; Zhang, X.L.; Sun, C.P.; Guo, W.H.; Zou, Y.X.; Lip, G.Y.H.; Shi, X.B. Prevalence, incidence and mortality of hypertrophic cardiomyopathy based on a population cohort of 21.9 million in China. Sci. Rep. 2022, 12, 18799. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.C.; Guo, M.N.; Xin, Z.; Chen, G.J.; Bentley, A.R.; Hua, L.; Zheng, J.P.; Ekoru, K.; Yang, J.K. Incidence of Type 1 Diabetes May Be Underestimated in the Chinese Population: Evidence From 21.7 Million People between 2007 and 2017. Diabetes Care 2021, 44, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 aha/acc/hrs guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Mizia-Stec, K.; Caforio, A.L.P.; Charron, P.; Gimeno, J.R.; Elliott, P.; Kaski, J.P.; Maggioni, A.P.; Tavazzi, L.; Rigopoulos, A.G.; Laroche, C.; et al. Atrial fibrillation, anticoagulation management and risk of stroke in the Cardiomyopathy/Myocarditis registry of the EURObservational Research Programme of the European Society of Cardiology. ESC Heart Fail. 2020, 7, 3601–3609. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef]
- Veselka, J.; Anavekar, N.S.; Charron, P. Hypertrophic obstructive cardiomyopathy. Lancet 2017, 389, 1253–1267. [Google Scholar] [CrossRef]
- Geske, J.B.; Anavekar, N.S.; Nishimura, R.A.; Oh, J.K.; Gersh, B.J. Differentiation of Constriction and Restriction: Complex Cardiovascular Hemodynamics. J. Am. Coll. Cardiol. 2016, 68, 2329–2347. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Gupta, D.; Fazio-Eynullayeva, E.; Underhill, P.; Lip, G.Y.H. Atrial Fibrillation in Patients With Cardiomyopathy: Prevalence and Clinical Outcomes from Real-World Data. J. Am. Heart Assoc. 2021, 10, e021970. [Google Scholar] [CrossRef]
- Aggarwal, A.; Sinha, B.; Rajpal, S.; Dwivedi, S.; Sharma, V. Right ventricular endomyocardial fibrosis presenting with ventricular tachycardia and apical thrombus—An interesting presentation. Indian Pacing Electrophysiol. J. 2009, 9, 360–363. [Google Scholar]
- Seferović, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Gal, T.B.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart failure in cardiomyopathies: A position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef]
- Abdelnabi, M.; Almaghraby, A.; Saleh, Y.; Abd Elsamad, S.; Elfawal, A.S. Endomyocardial fibrosis presented by ventricular tachycardia: Case report. Egypt. Heart J. (EHJ) Off. Bull. Egypt. Soc. Cardiol. 2019, 71, 24. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, Y.B.; Liu, J.; Chou, J.; Hoffman, J.; Comenzo, R.L.; Steingart, R.M. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am. J. Cardiol. 2009, 104, 990–994. [Google Scholar] [CrossRef]
- Palladini, G.; Malamani, G.; Cò, F.; Pistorio, A.; Recusani, F.; Anesi, E.; Garini, P.; Merlini, G. Holter monitoring in AL amyloidosis: Prognostic implications. Pacing Clin. Electrophysiol. PACE 2001, 24, 1228–1233. [Google Scholar] [CrossRef]
- Varr, B.C.; Zarafshar, S.; Coakley, T.; Liedtke, M.; Lafayette, R.A.; Arai, S.; Schrier, S.L.; Witteles, R.M. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 2014, 11, 158–162. [Google Scholar] [CrossRef]
- Giancaterino, S.; Urey, M.A.; Darden, D.; Hsu, J.C. Management of Arrhythmias in Cardiac Amyloidosis. JACC. Clin. Electrophysiol. 2020, 6, 351–361. [Google Scholar] [CrossRef]
- Dubrey, S.W.; Hawkins, P.N.; Falk, R.H. Amyloid diseases of the heart: Assessment, diagnosis, and referral. Heart 2011, 97, 75–84. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Hawkins, P.N.; Fontana, M. Cardiac amyloidosis. Clin. Med. 2018, 18, s30–s35. [Google Scholar] [CrossRef]
- Cappelli, F.; Vignini, E.; Martone, R.; Perlini, S.; Mussinelli, R.; Sabena, A.; Morini, S.; Gabriele, M.; Taborchi, G.; Bartolini, S.; et al. Baseline ECG Features and Arrhythmic Profile in Transthyretin Versus Light Chain Cardiac Amyloidosis. Circ. Heart Fail. 2020, 13, e006619. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Heiss, G.; Rautaharju, P.M.; Shahar, E.; Massing, M.W.; Simpson, R.J., Jr. Premature ventricular complexes and the risk of incident stroke: The Atherosclerosis Risk In Communities (ARIC) Study. Stroke 2010, 41, 588–593. [Google Scholar] [CrossRef]
- Koppikar, S.; Baranchuk, A.; Guzmán, J.C.; Morillo, C.A. Stroke and ventricular arrhythmias. Int. J. Cardiol. 2013, 168, 653–659. [Google Scholar] [CrossRef]
- Worthington, J.M.; Gattellari, M.; Leung, D.Y. ‘Where there’s smoke…’: Are premature ventricular complexes a new risk factor for stroke? Stroke 2010, 41, 572–573. [Google Scholar] [CrossRef]
- Nucci, E.M.; Lisi, M.; Cameli, M.; Baldi, L.; Puccetti, L.; Mondillo, S.; Favilli, R.; Lunghetti, S. The role of 3D and speckle tracking echocardiography in cardiac amyloidosis: A case report. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 74–77. [Google Scholar]
- Ishiwata, S.; Nishiyama, S.; Seki, A.; Kojima, S. Restrictive cardiomyopathy with complete atrioventricular block and distal myopathy with rimmed vacuoles. Jpn. Circ. J. 1993, 57, 928–933. [Google Scholar] [CrossRef]

| Variables | AF | No-AF | p Value | VT | No-VT | p Value | Bradycardia | No-Bradycardia | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Number | 745 (64.90) | 403 (35.10) | NA | 117 (10.19) | 1031 (89.81) | NA | 311 (27.09) | 837 (72.91) | NA |
| Female, n (%) | 361 (48.46) | 168 (41.69) | 0.03 | 45 (38.46) | 484 (46.94) | 0.08 | 144 (46.30) | 385 (46.00) | 0.93 |
| Age (y) | 60.83 ± 16.47 | 42.06 ± 25.77 | <0.001 | 57.06 ± 16.74 | 53.92 ± 22.63 | 0.07 | 57.46 ± 20.02 | 53.04 ± 22.74 | 0.003 |
| Hypertension, n (%) | 295 (39.60) | 75 (18.61) | <0.001 | 41 (35.04) | 329 (31.91) | 0.49 | 102 (32.80) | 268 (32.02) | 0.80 |
| Diabetes mellitus, n (%) | 136 (18.26) | 48 (11.91) | 0.01 | 19 (16.24) | 165 (16.00) | 0.95 | 47 (15.11) | 137 (16.37) | 0.61 |
| Prior stroke/TIA, n (%) | 93 (12.48) | 16 (3.97) | <0.001 | 15 (12.82) | 94 (9.12) | 0.20 | 32 (10.29) | 77 (9.20) | 0.58 |
| Enlarged atrium, n (%) | 146 (19.60) | 58 (14.39) | 0.03 | 19 (16.24) | 185 (17.94) | 0.65 | 62 (19.94) | 142 (16.97) | 0.24 |
| Rheumatic disease, n (%) | 11 (1.48) | 8 (1.99) | 0.52 | 3 (2.56) | 16 (1.55) | 0.67 | 5 (1.61) | 14 (1.67) | 0.94 |
| Hepatic cirrhosis, n (%) | 20 (2.68) | 9 (2.23) | 0.64 | 1 (0.85) | 28 (2.72) | 0.37 | 8 (2.57) | 21 (2.51) | 0.95 |
| All malignancy, n (%) | 26 (3.49) | 26 (6.45) | 0.02 | 5 (4.27) | 47 (4.56) | 0.89 | 13 (4.18) | 39 (4.66) | 0.73 |
| Amyloidosis, n (%) | 56 (7.52) | 80 (19.85) | <0.001 | 16 (13.68) | 120 (11.64) | 0.52 | 40 (12.86) | 96 (11.47) | 0.52 |
| OMI, n (%) | 167 (22.42) | 63 (15.63) | 0.01 | 21 (17.95) | 209 (20.27) | 0.55 | 59 (18.97) | 171 (20.43) | 0.58 |
| Anemia, n (%) | 127 (17.05) | 35 (8.68) | <0.001 | 25 (21.37) | 137 (13.29) | 0.02 | 51 (16.40) | 111 (13.26) | 0.18 |
| Eosinophilia, n (%) | 3 (0.40%) | 742 (99.60%) | 0.41 | 1 (0.90%) | 116 (99.10%) | 0.53 | 0 (0.00%) | 311 (100%) | 0.23 |
| Sarcoidosis, n (%) | 1 (0.10%) | 744 (99.90%) | 1.00 | 0 (0.00%) | 117 (100%) | 1.00 | 0 (0.00%) | 311 (100%) | 1.00 |
| NYHA grading, n (%) | 0.02 | 0.01 | 0.17 | ||||||
| Class II | 23 (4.20%) | 21 (9.30%) | 0 (0.00%) | 44 (6.40%) | 8 (3.50%) | 36 (6.60%) | |||
| Class III | 283 (51.70%) | 105 (46.30%) | 39 (43.80%) | 349 (50.90%) | 112 (49.10%) | 276 (50.50%) | |||
| Class IV | 241 (44.10%) | 101 (44.50%) | 50 (56.20%) | 292 (42.60%) | 108 (47.40%) | 234 (42.90%) |
| Model | AF | VT | Bradycardia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | HR | 95%CI | p Value | |
| Composite Endpoints | |||||||||
| Model 1 | 1.35 | 1.18–1.54 | <0.0001 | 1.13 | 0.93–1.37 | 0.21 | 1.08 | 0.95–1.24 | 0.25 |
| Model 2 | 1.28 | 1.11–1.49 | 0.001 | 1.08 | 0.89–1.32 | 0.44 | 1.06 | 0.92–1.21 | 0.43 |
| Model 3 | 1.28 | 1.10–1.48 | 0.001 | 1.06 | 0.87–1.30 | 0.54 | 1.04 | 0.90–1.19 | 0.60 |
| Death | |||||||||
| Model 1 | 0.80 | 0.51–1.24 | 0.31 | 2.34 | 1.36–4.05 | 0.002 | 1.28 | 0.81–2.03 | 0.29 |
| Model 2 | 0.51 | 0.31–0.83 | 0.01 | 2.07 | 1.19–3.59 | 0.01 | 1.13 | 0.71–1.79 | 0.62 |
| Model 3 | 0.49 | 0.30–0.80 | 0.30–0.80 | 2.16 | 1.23–3.81 | 0.01 | 1.02 | 0.64–1.64 | 0.94 |
| Stroke and systematic embolism | |||||||||
| Model 1 | 1.70 | 1.31–2.21 | <0.0001 | 1.44 | 1.02–2.02 | 0.04 | 1.05 | 0.81–1.34 | 0.73 |
| Model 2 | 1.37 | 1.02–1.83 | 0.04 | 1.41 | 1.00–1.99 | 0.05 | 1.01 | 0.78–1.30 | 0.97 |
| Model 3 | 1.36 | 1.01–1.83 | 0.04 | 1.41 | 1.00–2.00 | 0.05 | 0.98 | 0.75–1.26 | 0.85 |
| Heart failure | |||||||||
| Model 1 | 1.37 | 1.20–1.57 | <0.0001 | 1.13 | 0.93–1.38 | 0.23 | 1.10 | 0.96–1.26 | 0.18 |
| Model 2 | 1.36 | 1.17–1.58 | <0.0001 | 1.08 | 0.88–1.32 | 0.47 | 1.08 | 0.94–1.24 | 0.28 |
| Model 3 | 1.35 | 1.16–1.57 | <0.0001 | 1.05 | 0.86–1.29 | 0.62 | 1.06 | 0.92–1.22 | 0.44 |
| Intracardiac thrombus | |||||||||
| Model 1 | 1.71 | 1.05–2.79 | 0.03 | 2.33 | 1.37–3.95 | 0.002 | 1.13 | 0.72–1.79 | 0.59 |
| Model 2 | 2.76 | 1.57–4.87 | <0.0001 | 2.34 | 1.36–4.01 | 0.002 | 1.16 | 0.73–1.85 | 0.52 |
| Model 3 | 2.76 | 1.57–4.86 | <0.0001 | 2.33 | 1.34–4.05 | 0.003 | 1.00 | 0.62–1.60 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, S.; Zhang, X.; Zheng, J.; Lu, F.; Lip, G.Y.H.; Bai, Y. Prevalence and Impact of Arrhythmia on Outcomes in Restrictive Cardiomyopathy—A Report from the Beijing Municipal Health Commission Information Center (BMHCIC) Database. J. Clin. Med. 2023, 12, 1236. https://doi.org/10.3390/jcm12031236
Wang H, Liu S, Zhang X, Zheng J, Lu F, Lip GYH, Bai Y. Prevalence and Impact of Arrhythmia on Outcomes in Restrictive Cardiomyopathy—A Report from the Beijing Municipal Health Commission Information Center (BMHCIC) Database. Journal of Clinical Medicine. 2023; 12(3):1236. https://doi.org/10.3390/jcm12031236
Chicago/Turabian StyleWang, Haiyan, Sitong Liu, Xilin Zhang, Jianpeng Zheng, Feng Lu, Gregory Y. H. Lip, and Ying Bai. 2023. "Prevalence and Impact of Arrhythmia on Outcomes in Restrictive Cardiomyopathy—A Report from the Beijing Municipal Health Commission Information Center (BMHCIC) Database" Journal of Clinical Medicine 12, no. 3: 1236. https://doi.org/10.3390/jcm12031236
APA StyleWang, H., Liu, S., Zhang, X., Zheng, J., Lu, F., Lip, G. Y. H., & Bai, Y. (2023). Prevalence and Impact of Arrhythmia on Outcomes in Restrictive Cardiomyopathy—A Report from the Beijing Municipal Health Commission Information Center (BMHCIC) Database. Journal of Clinical Medicine, 12(3), 1236. https://doi.org/10.3390/jcm12031236








