Long-Term Outcome of Dental Implants in Immediate Function Inserted on Autogenous Grafted Bone
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Surgical and Prosthetic Protocols
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Covani, U.; Ricci, M.; Bozzolo, G.; Mangano, F.; Zini, A.; Barone, A. Analysis of the pattern of the alveolar ridge remodelling following single tooth extraction. Clin. Oral Implant. Res. 2011, 22, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. Interventions for replacing missing teeth: Horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst. Rev. 2009, 2009, CD003607. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C.; Shao, M. Simultaneous implant placement with autogenous onlay bone grafts: A systematic review and meta-analysis. Int. J. Implant. Dent. 2021, 7, 61. [Google Scholar] [CrossRef]
- Raghoebar, G.M.; Onclin, P.; Boven, G.C.; Vissink, A.; Meijer, H.J.A. Long-term effectiveness of maxillary sinus floor augmentation: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, S307–S318. [Google Scholar] [CrossRef]
- Buser, D.; Dula, K.; Hess, D.; Hirt, H.P.; Belser, U.C. Localized ridge augmentation with autografts and barrier membranes. Periodontol 2000 1999, 19, 151–163. [Google Scholar] [CrossRef]
- Buser, D.; Weber, H.P.; Lang, N.P. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin. Oral Implant. Res. 1990, 1, 33–40. [Google Scholar] [CrossRef]
- Naert, I.; Quirynen, M.; van Steenberghe, D.; Darius, P. A six-year prosthodontic study of 509 consecutively inserted implants for the treatment of partial edentulism. J. Prosthet. Dent. 1992, 67, 236–245. [Google Scholar] [CrossRef]
- Maló, P.; Nobre, M.A. A new approach for maxilla reconstruction. Eur. J. Oral Implantol. 2009, 2, 101–114. [Google Scholar]
- Mordenfeld, A.; Albrektsson, T.; Hallman, M. A 10-year clinical and radiographic study of implants placed after maxillary sinus floor augmentation with an 80:20 mixture of deproteinized bovine bone and autogenous bone. Clin. Implant. Dent. Relat. Res. 2014, 16, 435–446. [Google Scholar] [CrossRef]
- Soehardi, A.; Meijer, G.J.; Hoppenreijs, T.J.M.; Brouns, J.J.A.; de Koning, M.; Stoelinga, P.J.W. Stability, complications, implant survival, and patient satisfaction after Le Fort I osteotomy and interposed bone grafts: Follow-up of 5–18 years. International J. Oral Maxillofac. Surg. 2015, 44, 97–103. [Google Scholar] [CrossRef]
- de Moraes, P.H.; Olate, S.; Lauria, A.; Asprino, L.; de Moraes, M.; de Albergaria-Barbosa, J.R. 8–10 year follow-up survival of dental implants in maxillae with or without autogenous bone graft reconstruction. Int. J. Clin. Exp. Med. 2015, 8, 19282–19289. [Google Scholar]
- Fuglsig, J.M.C.E.S.; Thorn, J.J.; Ingerslev, J.; Wenzel, A.; Spin-Neto, R. Long term follow-up of titanium implants installed in block-grafted areas: A systematic review. Clin. Implant. Dent. Relat. Res. 2018, 20, 1036–1046. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Emtiaz, S.; Classi, A. Immediate loading of threaded implants at stage 1 surgery in edentulous arches: Ten consecutive case reports with 1- to 5-year data. Int. J. Oral Maxillofac. Implant. 1997, 12, 319–324. [Google Scholar]
- Maló, P.S.; de Araújo Nobre, M.A.; Ferro, A.S.; Parreira, G.G. Five-year outcome of a retrospective cohort study comparing smokers vs. Nonsmokers with full-arch mandibular implant-supported rehabilitation using the All-on-4 concept. J. Oral Sci. 2018, 60, 177–186. [Google Scholar] [CrossRef]
- de Araújo Nobre, M.; Mano Azul, A.; Rocha, E.; Maló, P.; Salvado, F. Attributable fractions, modifiable risk factors and risk stratification using a risk score for peri-implant pathology. J. Prosthodont. Researc 2017, 61, 43–53. [Google Scholar] [CrossRef]
- Nyström, E.; Nilson, H.; Gunne, J.; Lundgren, S. A 9–14 year follow-up of onlay bone grafting in the atrophic maxilla. Int. J. Oral Maxillofac. Surg. 2009, 38, 111–116. [Google Scholar] [CrossRef]
- Trbakovic, A.; Toljanic, J.A.; Kumar, V.V.; Thor, A. Eight to eleven-year follow-up of immediately loaded implants placed in edentulous maxillae with compromised bone volume and poor bone quality: A prospective cohort study. Clin. Implant. Dent. Relat. Res. 2020, 22, 69–76. [Google Scholar] [CrossRef]
- Cassetta, M. Immediate loading of implants inserted in edentulous arches using multiple mucosa-supported stereolithographic surgical templates: A 10-year prospective cohort study. Int. J. Oral Maxillofac. Surg. 2016, 45, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Ferro, A.; Nunes, M. The All-on-4 concept for full-arch rehabilitation of the edentulous maxillae: A longitudinal study with 5–13 years of follow-up. Clin. Implant. Dent. Relat. Res. 2019, 21, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Pozzi, A.; Romeo, D.; de Araújo Nobre, M.; Agliardi, E. Outcomes of Fixed Full-Arch Rehabilitations Supported by Tilted and Axially Placed Implants: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2022, 37, 1003–1025. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Nack, C.; Al-Ghrairi, M.; Raguse, J.D.; Stricker, A.; Schmelzeisen, R.; Nelson, K.; Nahles, S. Long-term retrospective evaluation of the peri-implant bone level in onlay grafted patients with iliac bone from the anterior superior iliac crest. J. Cranio-Maxillofac. Surg. 2015, 43, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Strietzel, F.P.; Reichart, P.A.; Kale, A.; Kulkarni, M.; Wegner, B.; Küchler, I. Smoking interferes with the prognosis of dental implant treatment: A systematic review and meta-analysis. J. Clin. Periodontol. 2007, 34, 523–544. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Smoking and dental implants: A systematic review and meta-analysis. J. Dent. 2015, 43, 487–498. [Google Scholar] [CrossRef]
- Moraschini, V.; Barboza, E.D.S.P. Success of dental implants in smokers and non-smokers: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 205–215. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, T.J.; Misch, C.E.; Wang, H.L. Occlusal considerations in implant therapy: Clinical guidelines with biomechanical rationale. Clin. Oral Implant. Res. 2005, 16, 26–35. [Google Scholar] [CrossRef]
- Kinsel, R.P.; Lin, D. Retrospective analysis of porcelain failures of metal ceramic crowns and fixed partial dentures supported by 729 implants in 152 patients: Patient-specific and implant-specific predictors of ceramic failure. J. Prosthet. Dent. 2009, 101, 388–394. [Google Scholar] [CrossRef]
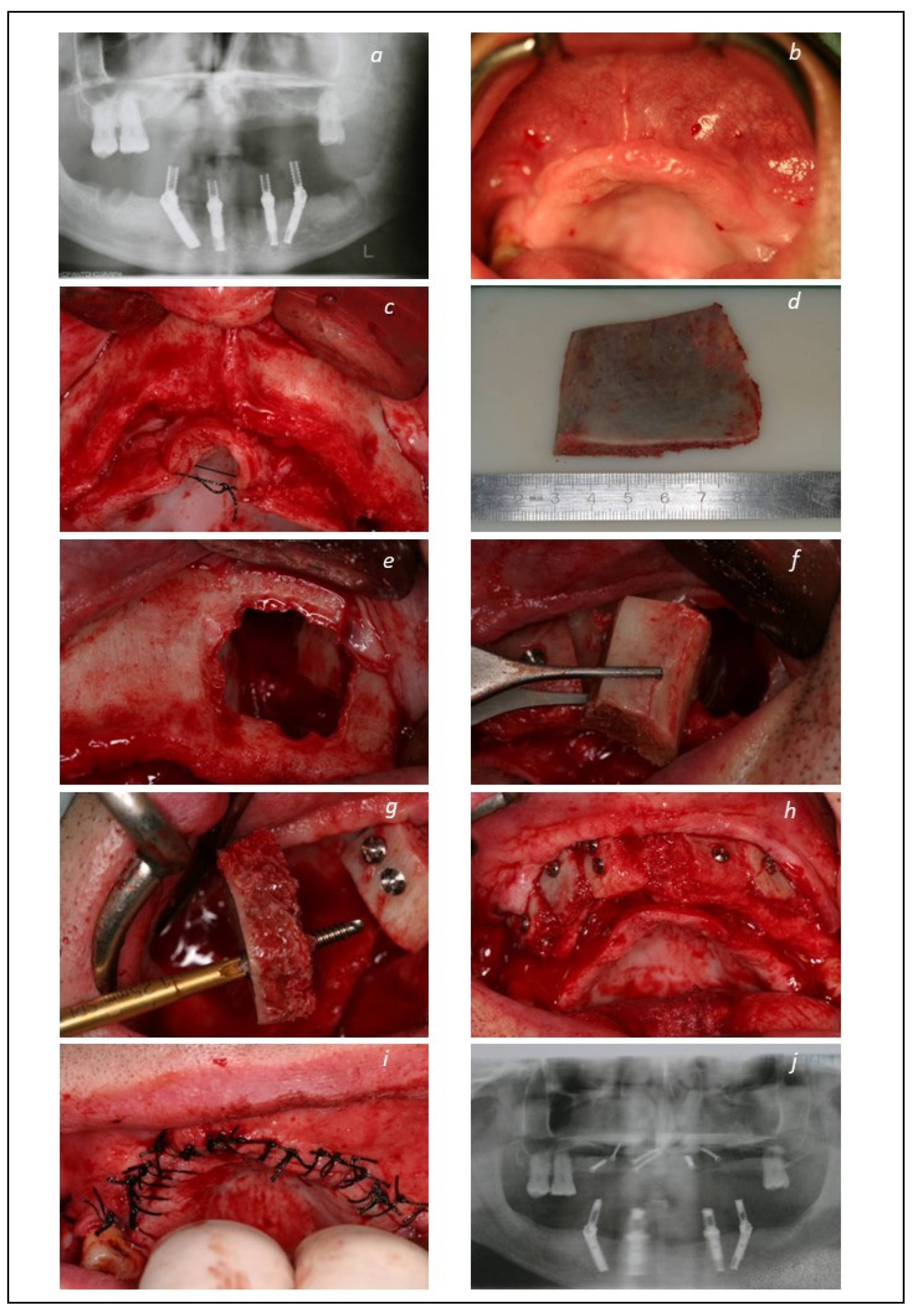
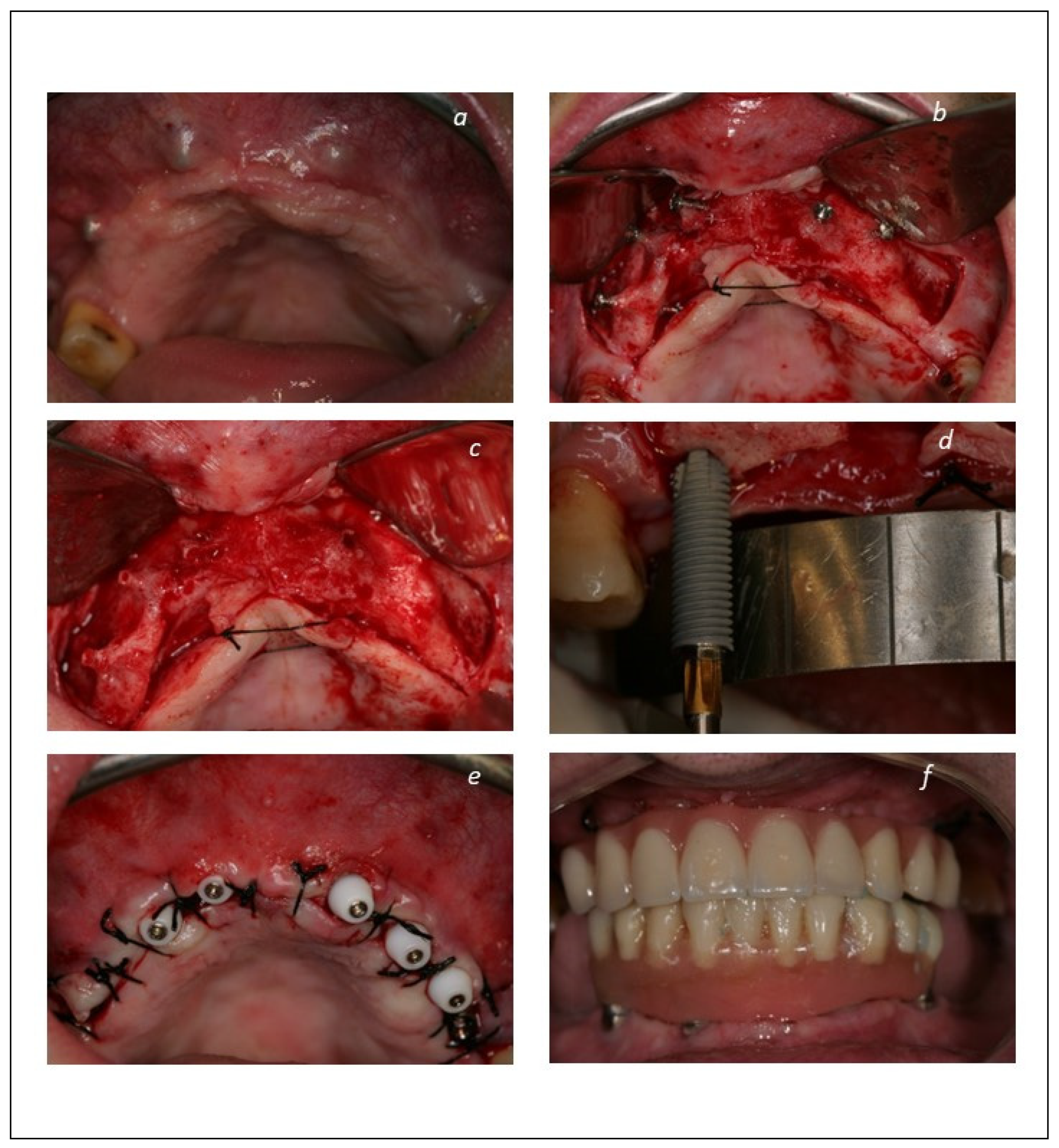
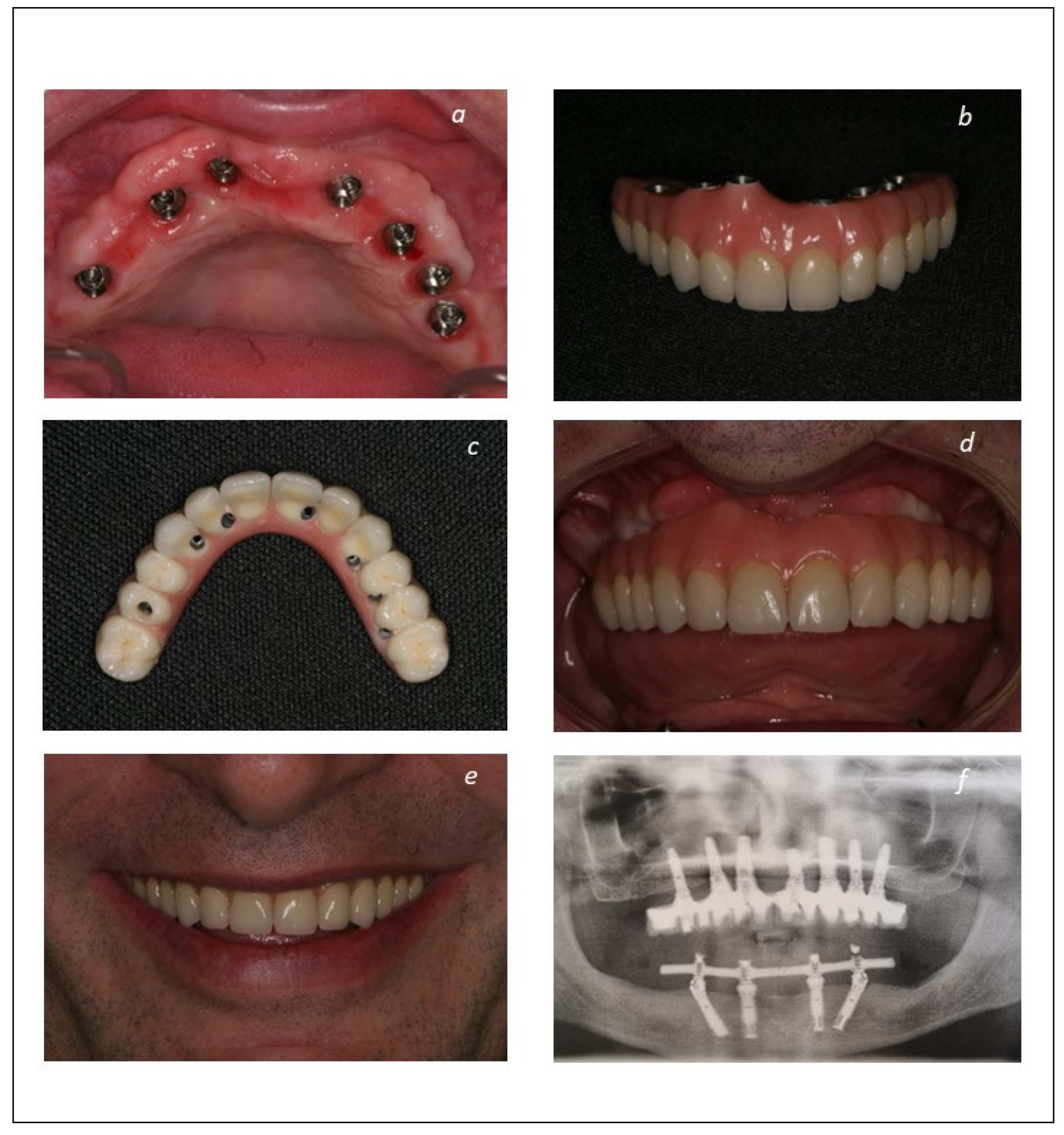
| Total Sample | Group 1 (Implant-Supported Fixed Prosthesis) | Group 2 (Mucosa-Retained Removable Prosthesis) | Group 3 (Tooth-Retained Fixed Prosthesis) | Grupo 4 (Palatal-Implant Retained Prosthesis) | |
|---|---|---|---|---|---|
| Number of patients (male/female) | 36 (12/24) | 10 (6/4) | 7 (0/7) | 6 (4/2) | 13 (2/11) |
| Average age in years | 54 | 48 | 55 | 54 | 57 |
| Smoking habits (n patients) | 12 | 5 | 1 | 1 | 5 |
| Systemic condition (n patients) | 12 * | 2 | 3 | 2 | 5 |
| Cardiovascular condition | 9 | 2 | 1 | 2 | 4 |
| Thyroid condition | 2 | 1 | 1 | 0 | 0 |
| Rheumatologic condition | 2 | 0 | 2 | 0 | 1 |
| Oncologic condition | 1 | 0 | 0 | 0 | 1 |
| Inflammatory condition | 1 | 0 | 0 | 0 | 1 |
| Number of implants inserted at step 1 (bone graft) in non-grafted bone | 55 | 38 | 0 | 0 | 17 (palatal) |
| Number of failed grafts | 0 | 0 | 0 | 0 | 0 |
| Number of implants inserted in immediate loading on grafted bone | 225 | 48 | 48 | 39 | 90 |
| Implant Distribution according to Type | ||||
| Type | Number of Implants (Number of Failures) | |||
| Mk III | 17 (0) | |||
| Mk IV | 95 (11) | |||
| NobelSpeedy Groovy | 113 (4) | |||
| Total | 225 (15) | |||
| Implant failure analysis | ||||
| Patient no. and characteristics | Implant position | Implant type | Follow-up in months | Reason for failure |
| 1 (male, 45y) Cardiovascular disease; smoker | #21 | MkIV | 8 | Loss of osseointegration |
| 2 (female, 49y) | #11 | MkIV | 22 | Loss of osseointegration |
| 3 (female, 70y) | #21 | MkIV | 4 | Loss of osseointegration |
| #23 | MkIV | 12 | ||
| #11 | MkIV | 23 | ||
| 4 (female, 62y) Heavy bruxer | #12 | MkIV | 4 | Loss of osseointegration |
| #23 | MkIV | 73 | ||
| 5 (female, 56y) Cardiovascular disease; smoker | #23 | MkIV | 144 | Loss of integration |
| #25 | MkIV | 144 | Peri-implant pathology | |
| 6 (female, 49y) Cardiovascular disease; smoker | #24 | MkIV | 4 | Loss of osseointegration |
| #21 | MkIV | 5 | ||
| 7 (female, 65y) Smoker | #25 | NobelSpeedy Groovy | 116 | Peri-implant pathology |
| #15 | NobelSpeedy Groovy | 117 | ||
| 8 (female, 35y) | #14 | NobelSpeedy Groovy | 14 | Loss of osseointegration |
| #21 | NobelSpeedy Groovy | 14 | ||
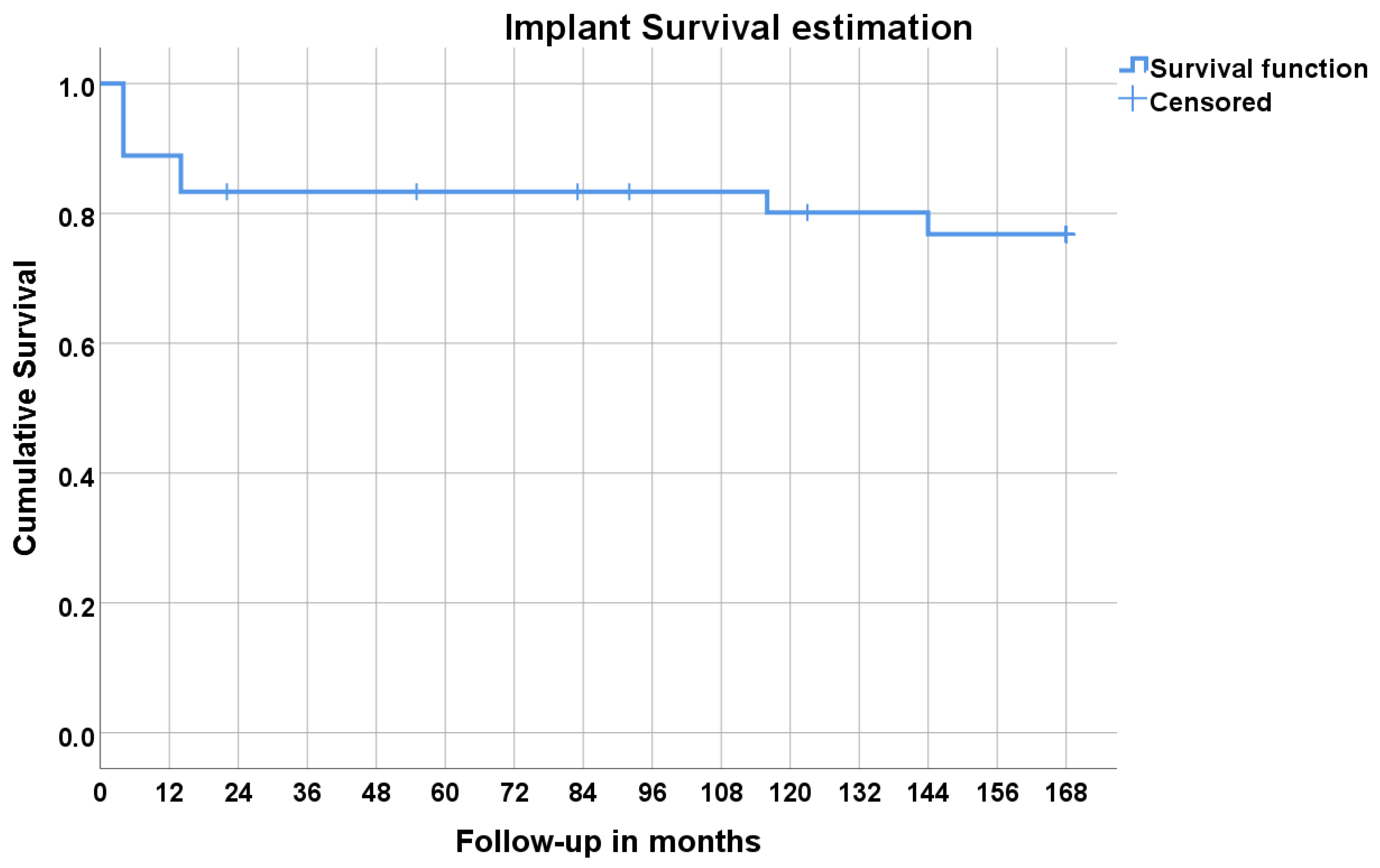
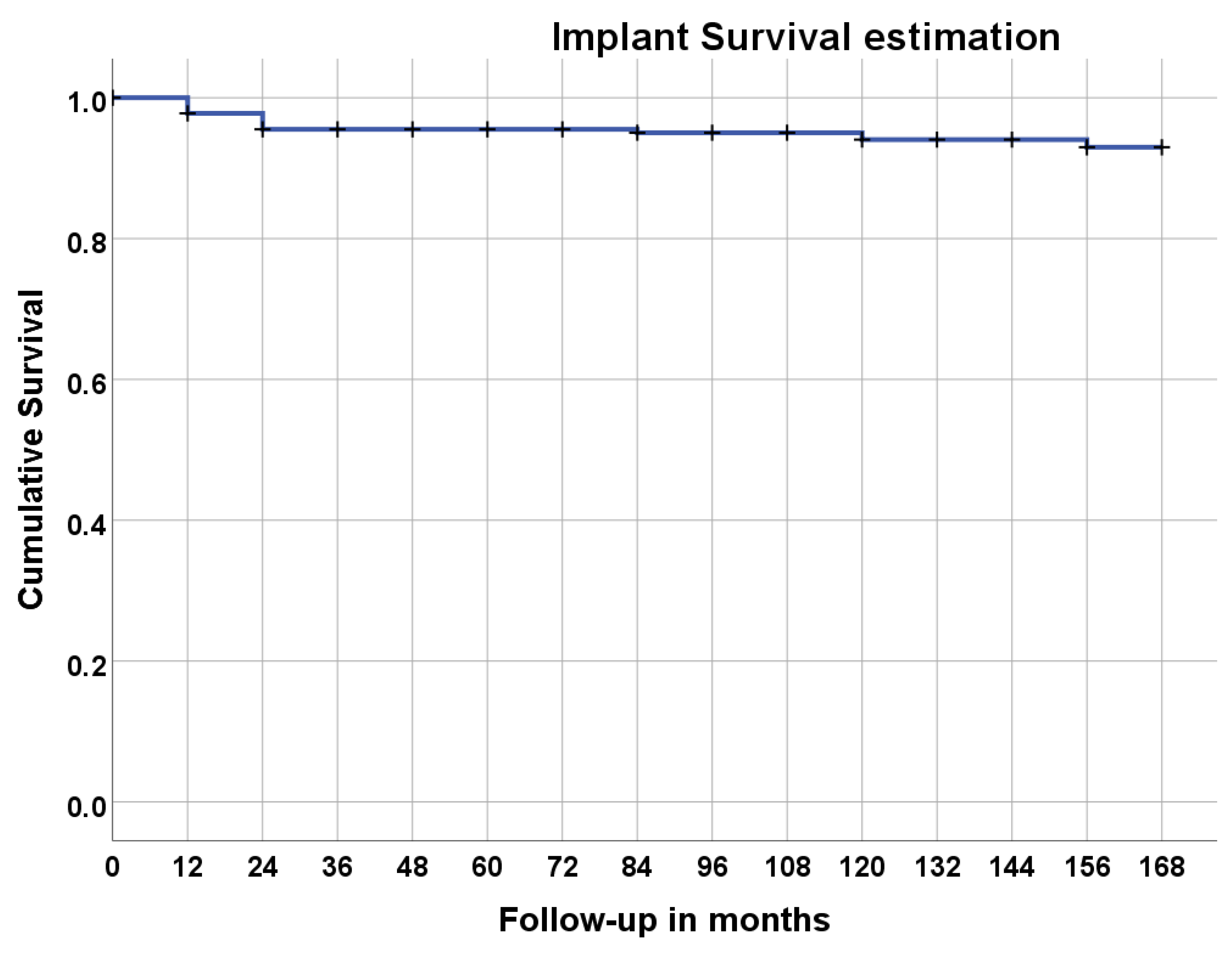
| Time (Months) | Status (0 = non-Failure, 1 = Failure *) | Cumulative Proportion Surviving | Cumulative Events (n) | Patients at Risk (n) | |
|---|---|---|---|---|---|
| Estimate | Standard Error | ||||
| 0 | 0 | 0 | 36 | ||
| 4 | 1 | 0.889 | 0.052 | 4 | 32 |
| 12 | 0 | 4 | 32 | ||
| 14 | 1 | 0.833 | 0.062 | 6 | 30 |
| 24 | 0 | 6 | 29 | ||
| 36 | 0 | 6 | 29 | ||
| 48 | 0 | 6 | 29 | ||
| 60 | 0 | 6 | 28 | ||
| 72 | 0 | 6 | 28 | ||
| 84 | 0 | 6 | 27 | ||
| 96 | 0 | 6 | 26 | ||
| 108 | 0 | 6 | 26 | ||
| 116 | 1 | 0.801 | 0.067 | 7 | 25 |
| 120 | 0 | 7 | 25 | ||
| 132 | 0 | 7 | 24 | ||
| 144 | 1 | 0.768 | 0.072 | 8 | 23 |
| 168 | 0 | 8 | 23 | ||
| Duration | Total Implants | Failed | Lost to Follow-Up | Survival Rate % | Cumulative Survival Rate% |
|---|---|---|---|---|---|
| Placement–1 year | 225 | 5 | 0 | 97.8% | 97.8% |
| 1 year–2 years | 220 | 5 | 8 | 97.7% | 95.5% |
| 2 years–3 years | 207 | 0 | 0 | 100% | 95.5% |
| 3 years–4 years | 207 | 0 | 0 | 100% | 95.5% |
| 4 years–5 years | 207 | 0 | 6 | 100% | 95.5% |
| 5 years–6 years | 201 | 0 | 0 | 100% | 95.5% |
| 6 years–7 years | 201 | 1 | 6 | 99.5% | 95.0% |
| 7 years–8 years | 194 | 0 | 0 | 100% | 95.0% |
| 8 years–9 years | 194 | 0 | 0 | 100% | 95.0% |
| 9 years–10 years | 194 | 2 | 0 | 99.0% | 94.1% |
| 10 years–11 years | 192 | 0 | 12 | 100% | 94.1% |
| 11 years–12 years | 180 | 0 | 6 | 100% | 94.1% |
| 12 years–13 years | 174 | 2 | 0 | 98.9% | 93.0% |
| 13 years–14 years | 172 | 0 | 0 | 100% | 93.0% |
| Mean (mm) | 2.01 | |
| SD (mm) | 1.58 | |
| Number | 176 | |
| Frequencies | N | % |
| 0 mm | 9 | 5.1% |
| 0.1–1.0 mm | 37 | 21.0% |
| 1.1–2.0 mm | 62 | 35.2% |
| 2.1–3.0 mm | 40 | 22.7% |
| >3.0 mm | 28 | 15.9% |
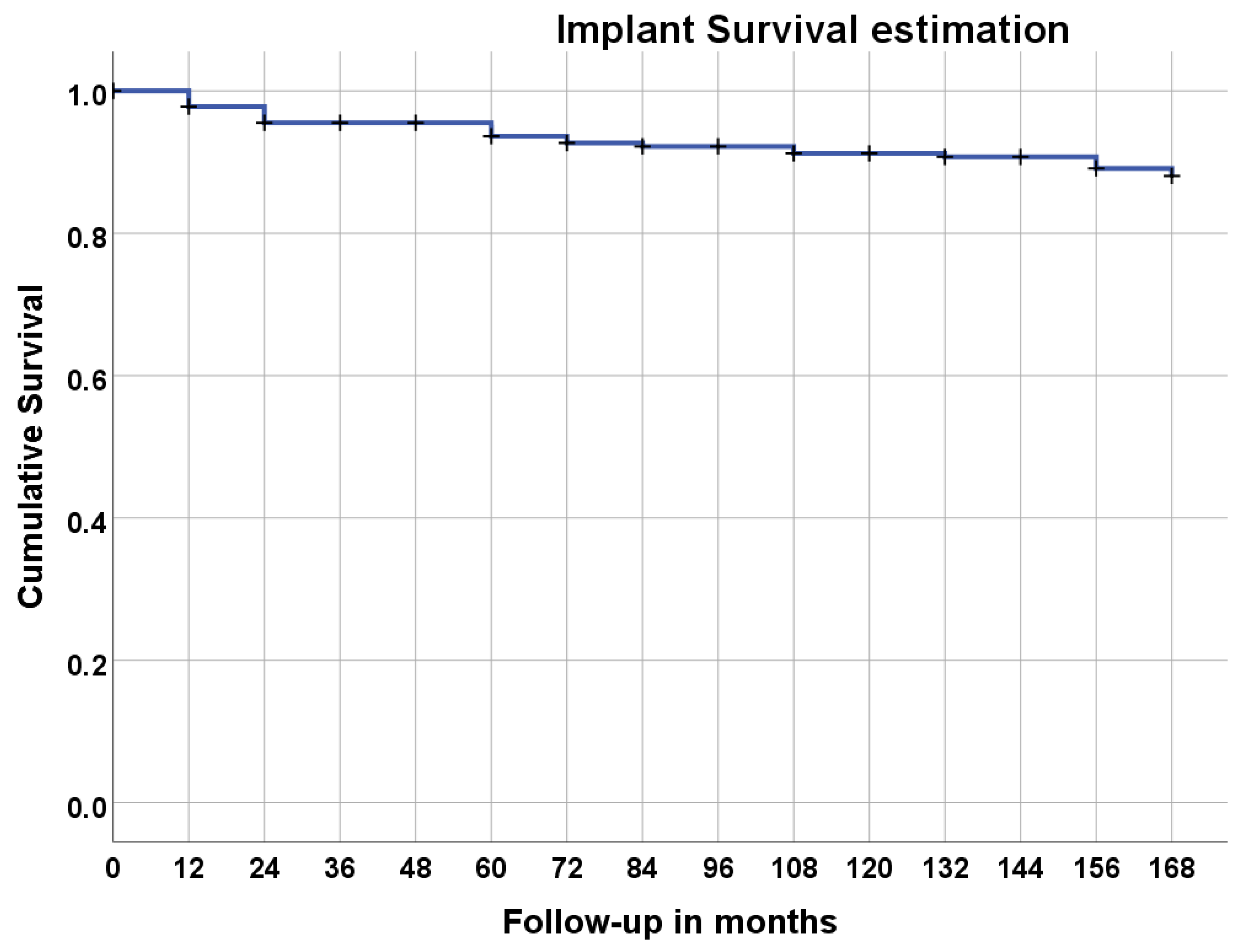
| Duration | Total Implants | Unsuccessful | Lost to Follow-Up | Survival Rate % | Cumulative Survival Rate% |
|---|---|---|---|---|---|
| Placement–1 year | 225 | 5 | 0 | 97.8% | 97.8% |
| 1 year–2 years | 220 | 5 | 8 | 97.7% | 95.5% |
| 2 years–3 years | 207 | 0 | 0 | 100% | 95.5% |
| 3 years–4 years | 207 | 0 | 0 | 100% | 95.5% |
| 4 years–5 years | 207 | 4 | 6 | 98.0% | 93.6% |
| 5 years–6 years | 197 | 2 | 0 | 99.0% | 92.7% |
| 6 years–7 years | 195 | 1 | 6 | 99.5% | 92.2% |
| 7 years–8 years | 188 | 0 | 0 | 100% | 92.2% |
| 8 years–9 years | 188 | 2 | 0 | 98.9% | 91.2% |
| 9 years–10 years | 186 | 0 | 0 | 100% | 91.2% |
| 10 years–11 years | 176 | 1 | 9 | 99.4% | 90.7% |
| 11 years–12 years | 170 | 0 | 6 | 100% | 90.7% |
| 12 years–13 years | 167 | 3 | 0 | 98.2% | 89.1% |
| 13 years–14 years | 165 | 2 | 0 | 98.8% | 88.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo Nobre, M.; Salvado, F.; Correia, J.A.; Teixeira, M.C.F.; Coutinho, F.A. Long-Term Outcome of Dental Implants in Immediate Function Inserted on Autogenous Grafted Bone. J. Clin. Med. 2023, 12, 261. https://doi.org/10.3390/jcm12010261
de Araújo Nobre M, Salvado F, Correia JA, Teixeira MCF, Coutinho FA. Long-Term Outcome of Dental Implants in Immediate Function Inserted on Autogenous Grafted Bone. Journal of Clinical Medicine. 2023; 12(1):261. https://doi.org/10.3390/jcm12010261
Chicago/Turabian Stylede Araújo Nobre, Miguel, Francisco Salvado, João André Correia, Maria Cristina Faria Teixeira, and Francisco Azevedo Coutinho. 2023. "Long-Term Outcome of Dental Implants in Immediate Function Inserted on Autogenous Grafted Bone" Journal of Clinical Medicine 12, no. 1: 261. https://doi.org/10.3390/jcm12010261
APA Stylede Araújo Nobre, M., Salvado, F., Correia, J. A., Teixeira, M. C. F., & Coutinho, F. A. (2023). Long-Term Outcome of Dental Implants in Immediate Function Inserted on Autogenous Grafted Bone. Journal of Clinical Medicine, 12(1), 261. https://doi.org/10.3390/jcm12010261








