Myosteatosis Is Not Associated with Complications or Survival in HCC Patients Undergoing Trans Arterial Embolization
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Assessment of Myosteatosis
2.3. Statistical Analysis
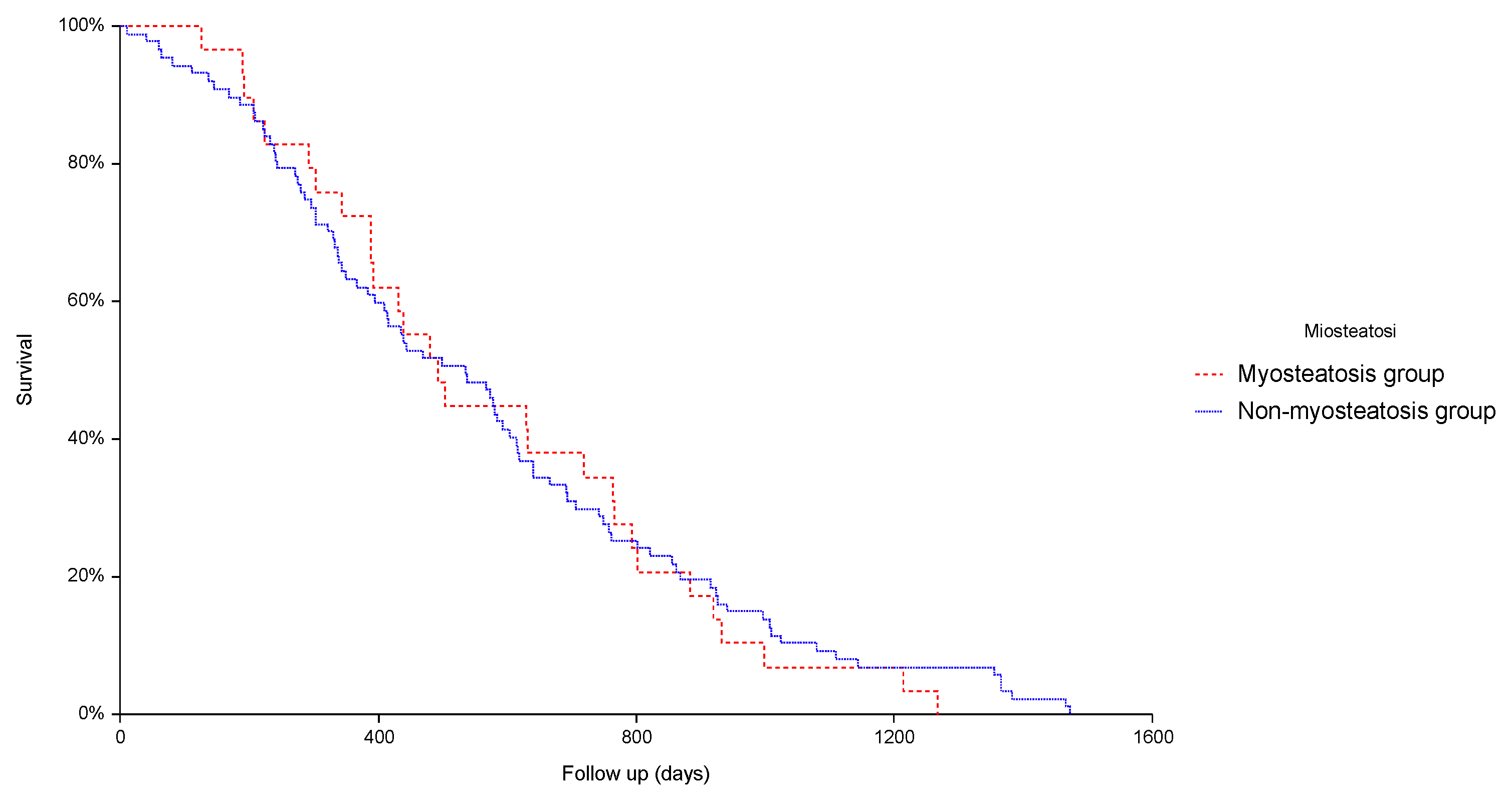
3. Results
3.1. Patients Characteristics
3.2. HCC Treatment
3.3. Prevalence of Myosteatosis and Its Impact on Safety and Efficacy of TAE
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Sarcopenia and Frailty in Liver Cirrhosis. Life 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.; Lanza, E.; Aghemo, A. Sarcopenia in chronic liver disease: Easy to diagnose but hard to treat. Liver Int. 2020, 40, 2627–2629. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients with Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021, 74, 1611–1644, Erratum in Hepatology 2021, 74, 3563. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.; Shachar, S.; Nyrop, K.; Muss, H.; Malpica, L.; Williams, G. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. 2019, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, G.; Chen, S.; Li, N. Myosteatosis reduces overall survival in patients with digestive system malignancies: A meta-analysis with trial sequential analysis. Nutr. Res. 2021, 94, 25–33. [Google Scholar] [CrossRef]
- Cespiati, A.; Meroni, M.; Lombardi, R.; Oberti, G.; Dongiovanni, P.; Fracanzani, A.L. Impact of Sarcopenia and Myosteatosis in Non-Cirrhotic Stages of Liver Diseases: Similarities and Differences across Aetiologies and Possible Therapeutic Strategies. Biomedicines 2022, 10, 182. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Angulo, P.; Meza-Junco, J.; Prado, C.M.M.; Sawyer, M.B.; Beaumont, C.; Esfandiari, N.; Ma, M.; Baracos, V.E. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachex-Sarcopenia Muscle 2015, 7, 126–135. [Google Scholar] [CrossRef]
- Irwin, N.E.A.; Fabian, J.; Hari, K.R.; Lorentz, L.; Mahomed, A.; Botha, J.F. Myosteatosis, the More Significant Predictor of Outcome: An Analysis of the Impact of Myosteatosis, Sarcopenia, and Sarcopenic Obesity on Liver Transplant Outcomes in Johannesburg, South Africa. Exp. Clin. Transplant. 2021, 19, 948–955. [Google Scholar] [CrossRef]
- Czigany, Z.; Kramp, W.; Bednarsch, J.; van der Kroft, G.; Boecker, J.; Strnad, P.; Zimmermann, M.; Koek, G.; Neumann, U.P.; Lurje, G. Myosteatosis to predict inferior perioperative outcome in patients undergoing orthotopic liver transplantation. Am. J. Transplant. 2019, 20, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Ishizaki, M.; Iida, H.; Matsui, K.; Sakaguchi, T.; Inoue, K.; Mizuta, T.; Ide, Y.; Iwasaka, J.; Kimura, Y.; et al. Effect of Intramuscular Adipose Tissue Content on Prognosis in Patients Undergoing Hepatocellular Carcinoma Resection. J. Gastrointest. Surg. 2015, 19, 1315–1323. [Google Scholar] [CrossRef]
- Meister, F.A.; Lurje, G.; Verhoeven, S.; Wiltberger, G.; Heij, L.; Liu, W.-J.; Jiang, D.; Bruners, P.; Lang, S.A.; Ulmer, T.F.; et al. The Role of Sarcopenia and Myosteatosis in Short- and Long-Term Outcomes Following Curative-Intent Surgery for Hepatocellular Carcinoma in a European Cohort. Cancers 2022, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Masetti, C.; Messana, G.; Muglia, R.; Pugliese, N.; Ceriani, R.; de Nalda, A.L.; Rimassa, L.; Torzilli, G.; Poretti, D.; et al. Sarcopenia as a predictor of survival in patients undergoing bland transarterial embolization for unresectable hepatocellular carcinoma. PLoS ONE 2020, 15, e0232371, Erratum in PLoS ONE 2020, 15, e0241715. [Google Scholar] [CrossRef]
- Eslamparast, T.; Montano-Loza, A.J.; Raman, M.; Tandon, P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018, 38, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-C.; Joo, S.K.; Koo, B.K.; Lin, H.-C.; Lee, D.H.; Chang, M.S.; Park, J.H.; So, Y.H.; Kim, W. Myosteatosis, but not Sarcopenia, Predisposes NAFLD Subjects to Early Steatohepatitis and Fibrosis Progression. Clin. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Kuznia, P.; Heshka, S.; Albu, J.; Heymsfield, S.B.; Goodpaster, B.; Visser, M.; Harris, T.B. Adipose tissue in muscle: A novel depot similar in size to visceral adipose tissue. Am. J. Clin. Nutr. 2005, 81, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Choi, G.H.; Hwang, S.H.; Jang, E.S.; Kim, J.W.; Ahn, J.M.; Choi, Y.; Cho, J.Y.; Han, H.S.; Lee, J.; et al. Sarcopenia and visceral adiposity predict poor overall survival in hepatocellular carcinoma patients after curative hepatic resection. Transl. Cancer Res. 2021, 10, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
| Variable | All Patients (n = 151) | Myosteatosis Y (n = 115) | Myosteatosis N (n = 36) | p Value |
|---|---|---|---|---|
| Age (yr) | 73.2 ± 9.3 | 74.8 ± 7.8 | 68.0 ± 11.7 | 0.0001 |
| Gender | M = 76.8% F = 23.2% | 80.9% 19.1% | 63.9% 36.1% | 0.035 |
| BMI (kg/m2) | 25.8 ± 4.9 | 26.6 ± 5.1 | 23.4 ± 3.3 | 0.0006 |
| AST (U/L) | 67 ± 50 | 66 ± 52 | 71 ± 39 | 0.54 |
| ALT (U/L) | 57 ± 55 | 56 ± 58 | 60 ± 48 | 0.68 |
| Total bilirubin (mg/dL) | 1.4 ± 0.8 | 1.3 ± 0.7 | 1.6 ± 1 | 0.027 |
| Serum albumin (g/dL) | 3.62 ± 0.51 | 3.62 ± 0.53 | 3.62 ± 0.49 | 0.98 |
| CPT | A = 82% B = 17.2% C = 0.8% | 86% 12.9% 1.1% | 69% 31% 0.0% | 0.07 |
| MELD score | 10 ± 2.8 | 9.8 ± 22 | 10.5 ± 4.1 | 0.23 |
| ALBI score | 1 = 22.6% 2 = 69.3% 3 = 8.1% | 23.2% 72.1% 4.7% | 22.6% 61.3% 16.1% | 0.11 |
| BCLC stage | Very early = 8% Early 28.5% Intermediate 63.5% | 9.5% 29.5% 61% | 5.5% 22.2% 72.3% | 0.46 |
| Cirrhosis etiology | HCV = 45.3% HBV = 5.3% Alcohol = 22.7% NASH = 15.3% Other = 11.4% | 43.8% 6.1% 26.3% 16.7% 7.1% | 50% 2.7% 11.1% 11.1% 25.1% | 0.029 |
| Sarcopenia | Y 85.3% N 14.7% | 85.1% 14.9% | 86.1% 13.9% | 0.88 |
| Performance status | 0 = 43.3% 1 = 46% 2 = 10.7% | 41.7% 45.2% 13.1% | 48.6% 48.6% 2.8% | 0.22 |
| Previous treatments | Y = 41.1% N = 58.9% | Y = 40.9% N = 59.1% | Y = 41.6% N = 58.4% | 0.93 |
| Number of nodules | Monofocal = 28% Bifocal = 22% Multifocal = 50% | 28.9% 21.9% 49.2% | 25% 22.2% 52.8% | 0.89 |
| Major lesion diameter (mm) | 32.8 ± 22.4 | 33 ± 22.4 | 32.3 ± 22.5 | 0.88 |
| Length of hospitalization (days) | 2 ± 1.7 | 2.1 ± 1.8 | 1.8 ± 1.3 | 0.36 |
| Complications after procedure | Y = 7.3% N = 92.7% | 7.8% 92.2% | 5.5% 94.5% | 0.64 |
| Rehospitalization in 30 days | Y = 6% N = 94% | 6% 94% | 5.5% 94.5% | 0.90 |
| Complete response | Y = 27.1% N = 72.9% | 29.3% 70.7% | 20% 80% | 0.27 |
| Survival (days) | 808 ± 673 | 816 ± 682 | 804 ± 650 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masetti, C.; Pugliese, N.; Lofino, L.; Colapietro, F.; Ceriani, R.; Lleo, A.; Poretti, D.; Pedicini, V.; De Nicola, S.; Torzilli, G.; et al. Myosteatosis Is Not Associated with Complications or Survival in HCC Patients Undergoing Trans Arterial Embolization. J. Clin. Med. 2023, 12, 262. https://doi.org/10.3390/jcm12010262
Masetti C, Pugliese N, Lofino L, Colapietro F, Ceriani R, Lleo A, Poretti D, Pedicini V, De Nicola S, Torzilli G, et al. Myosteatosis Is Not Associated with Complications or Survival in HCC Patients Undergoing Trans Arterial Embolization. Journal of Clinical Medicine. 2023; 12(1):262. https://doi.org/10.3390/jcm12010262
Chicago/Turabian StyleMasetti, Chiara, Nicola Pugliese, Ludovica Lofino, Francesca Colapietro, Roberto Ceriani, Ana Lleo, Dario Poretti, Vittorio Pedicini, Stella De Nicola, Guido Torzilli, and et al. 2023. "Myosteatosis Is Not Associated with Complications or Survival in HCC Patients Undergoing Trans Arterial Embolization" Journal of Clinical Medicine 12, no. 1: 262. https://doi.org/10.3390/jcm12010262
APA StyleMasetti, C., Pugliese, N., Lofino, L., Colapietro, F., Ceriani, R., Lleo, A., Poretti, D., Pedicini, V., De Nicola, S., Torzilli, G., Rimassa, L., Aghemo, A., & Lanza, E. (2023). Myosteatosis Is Not Associated with Complications or Survival in HCC Patients Undergoing Trans Arterial Embolization. Journal of Clinical Medicine, 12(1), 262. https://doi.org/10.3390/jcm12010262









