Value of Left Ventricular Indexed Ejection Time to Characterize the Severity of Aortic Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Groups
2.2. Echocardiographic Examination
2.3. AS Severity Definition
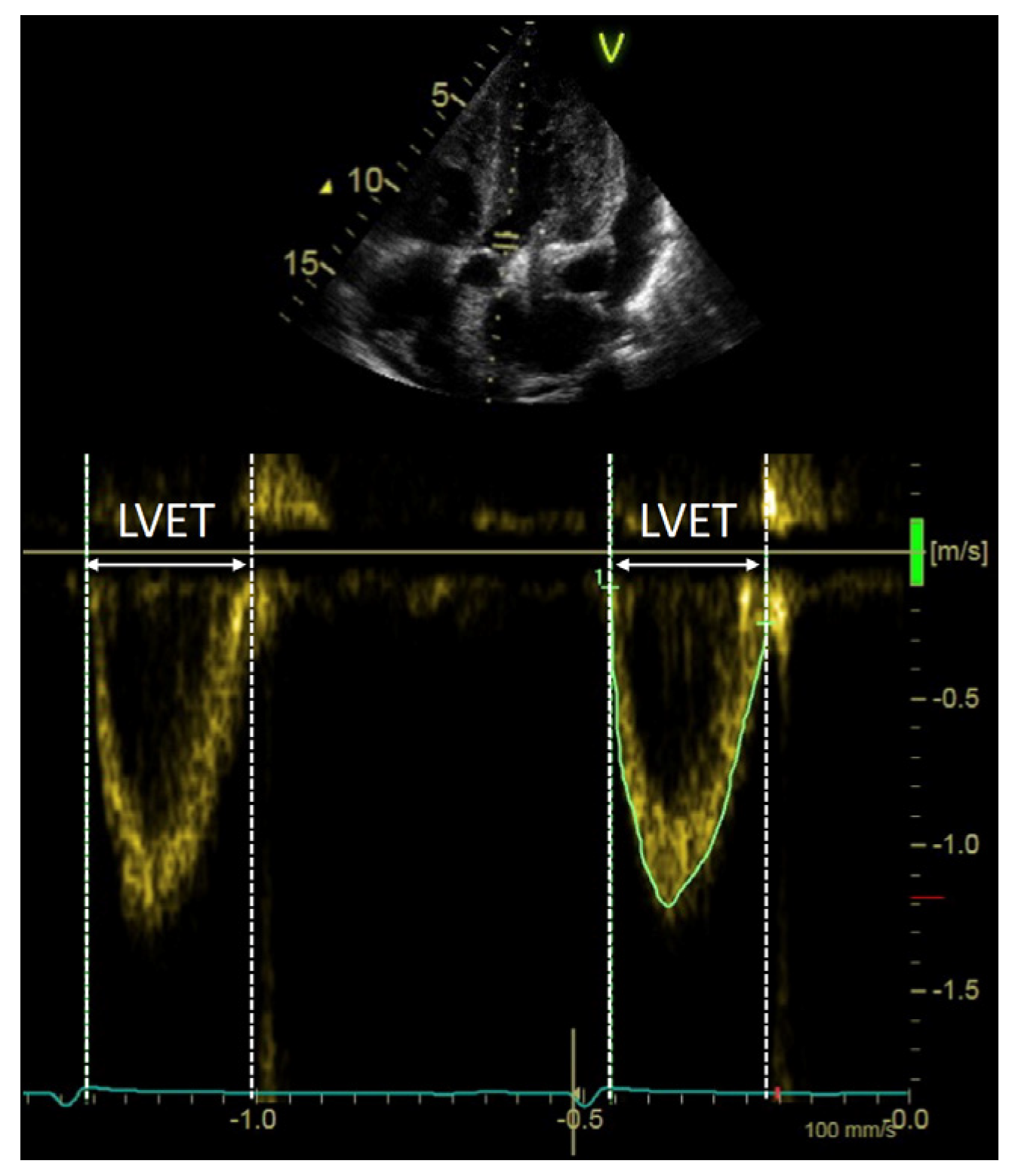
2.4. LV Systolic Output Measures
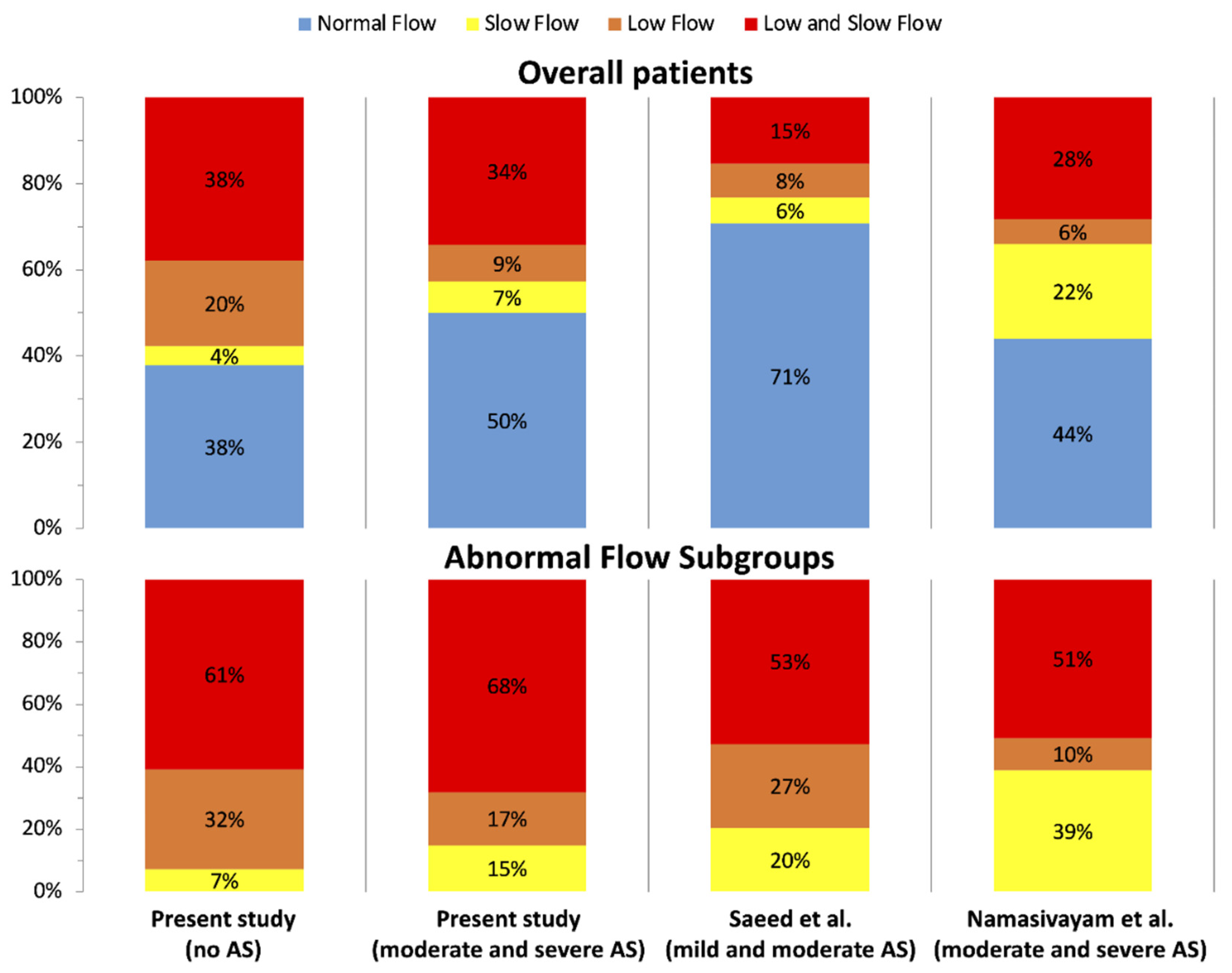
2.5. Classification of Flow Status
2.6. Statistical Analysis and Study Endpoints
3. Results
3.1. Patient Characteristics
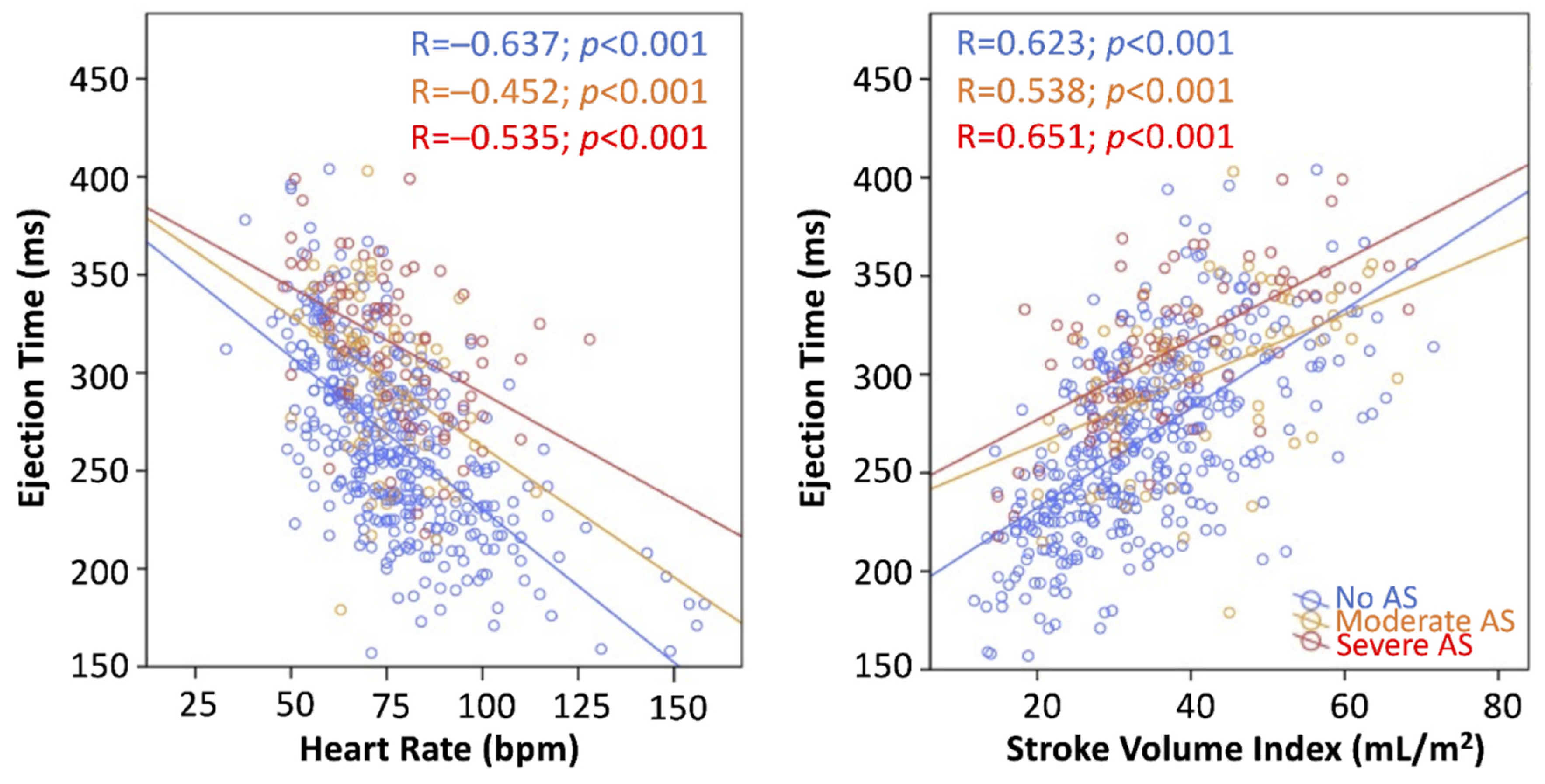
3.2. Determinants of LVET and LVETI Derivation
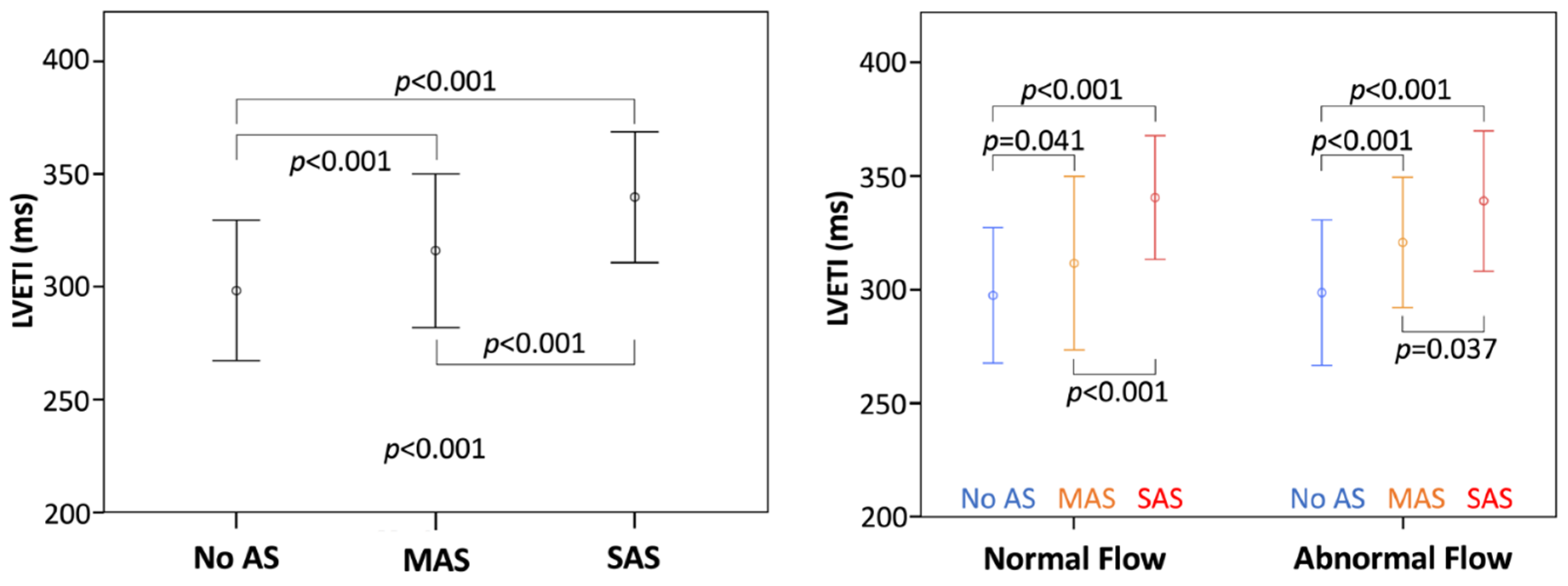
3.3. Analysis with LVETI
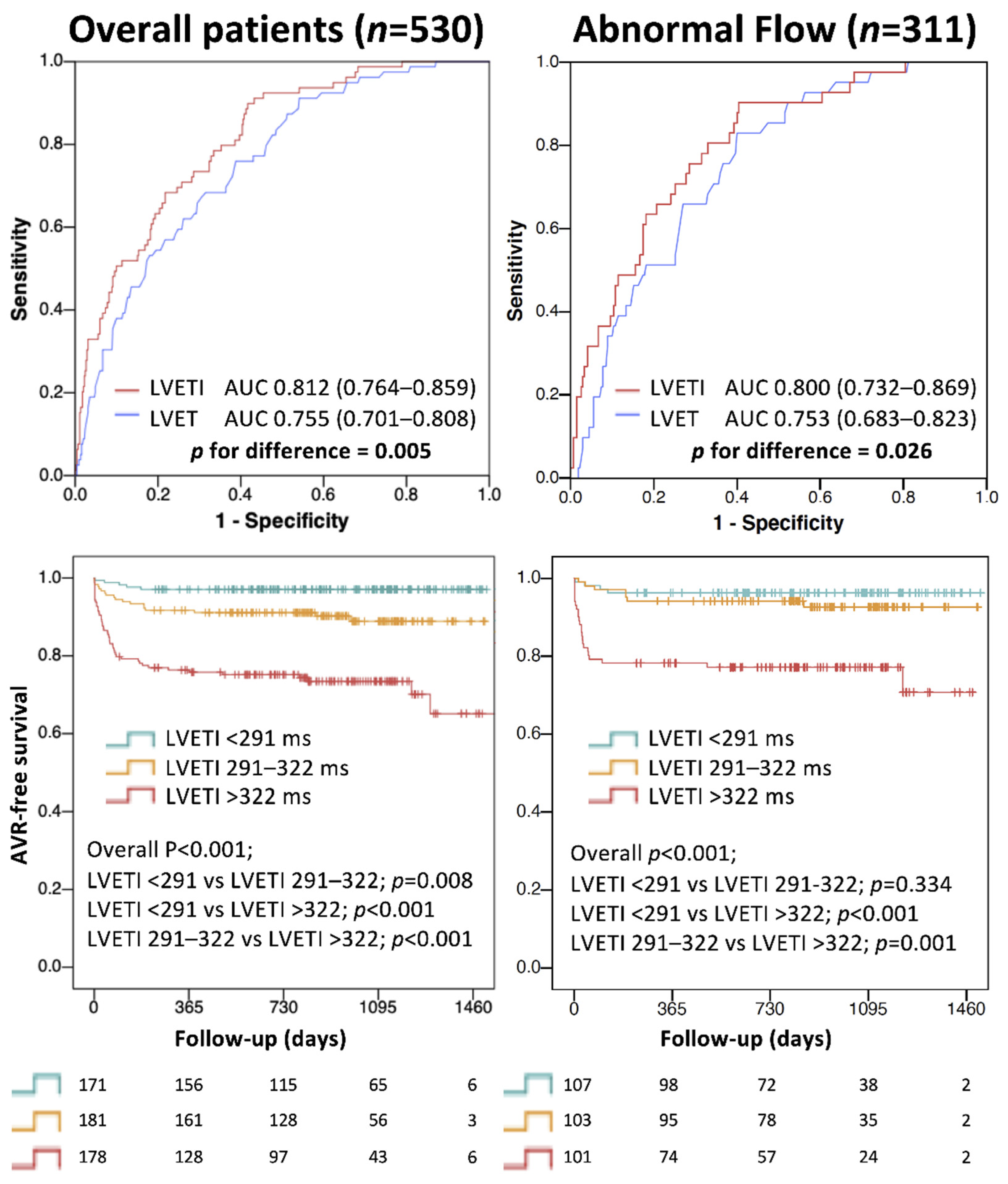
3.4. LVETI and Outcome
4. Discussion
4.1. Pathophysiology of LVET
4.2. LVET in Aortic Stenosis
4.3. Assessment of Forward Flow Status
4.4. LVETI for SAS Identification
4.5. LVETI and Outcome
4.6. Study Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Hear. J.-Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, V.; Clavel, M.-A.; Hahn, R.T.; Gillam, L.; Bax, J.; Sengupta, P.P.; Pibarot, P. How Do We Reconcile Echocardiography, Computed Tomography, and Hybrid Imaging in Assessing Discordant Grading of Aortic Stenosis Severity? JACC Cardiovasc. Imaging 2019, 12, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Ringle Griguer, A.; Tribouilloy, C.; Truffier, A.; Castel, A.-L.; Delelis, F.; Levy, F.; Vincentelli, A.; Bohbot, Y.; Maréchaux, S. Clinical Significance of Ejection Dynamics Parameters in Patients with Aortic Stenosis: An Outcome Study. J. Am. Soc. Echocardiogr. 2018, 31, 551–560.e2. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Querejeta Roca, G.; Hegde, S.M.; Shah, A.M.; Claggett, B.; Mosley, T.H.; Butler, K.R.; Solomon, S.D. Left ventricular ejection time is an independent predictor of incident heart failure in a community-based cohort. Eur. J. Heart Fail. 2018, 20, 1106–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorlin, R.; Gorlin, S.G. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. I. Am. Heart J. 1951, 41, 1–29. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Rosenhek, R.; Binder, T.; Porenta, G.; Lang, I.; Christ, G.; Schemper, M.; Maurer, G.; Baumgartner, H. Predictors of Outcome in Severe, Asymptomatic Aortic Stenosis. N. Engl. J. Med. 2000, 343, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Altes, A.; Thellier, N.; Bohbot, Y.; Ringle Griguer, A.; Verdun, S.; Levy, F.; Castel, A.L.; Delelis, F.; Mailliet, A.; Tribouilloy, C.; et al. Relationship Between the Ratio of Acceleration Time/Ejection Time and Mortality in Patients With High-Gradient Severe Aortic Stenosis. J. Am. Heart Assoc. 2021, 10, e021873. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, M.; He, W.; Churchill, T.W.; Capoulade, R.; Liu, S.; Lee, H.; Danik, J.S.; Picard, M.H.; Pibarot, P.; Levine, R.A.; et al. Transvalvular Flow Rate Determines Prognostic Value of Aortic Valve Area in Aortic Stenosis. J. Am. Coll. Cardiol. 2020, 75, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.P.; Rittgers, S.E.; Forester, W.F.; Boudoulas, H. A critical review of the systolic time intervals. Circulation 1977, 56, 146–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kligfield, P.; Goldberg, H.; Kline, S.A.; Scheidt, S. Effect of additional valve lesions on left ventricular ejection time in aortic stenosis. Br. Heart J. 1977, 39, 1259–1264. [Google Scholar] [CrossRef] [Green Version]
- Weissler, A.M.; Peeler, R.G.; Roehll, W.H. Relationships between left ventricular ejection time, stroke volume, and heart rate in normal individuals and patients with cardiovascular disease. Am. Heart J. 1961, 62, 367–378. [Google Scholar] [CrossRef]
- Kligfield, P.; Okin, P. Effect of ventricular function on left ventricular ejection time in aortic stenosis. Br. Heart J. 1979, 42, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Kligfield, P.; Okin, P.; Devereux, R.B.; Goldberg, H.; Borer, J.S. Duration of ejection in aortic stenosis: Effect of stroke volume and pressure gradient. J. Am. Coll. Cardiol. 1984, 3, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Chahal, N.S.; Drakopoulou, M.; Gonzalez-Gonzalez, A.M.; Manivarmane, R.; Khattar, R.; Senior, R. Resting Aortic Valve Area at Normal Transaortic Flow Rate Reflects True Valve Area in Suspected Low-Gradient Severe Aortic Stenosis. JACC Cardiovasc. Imaging 2015, 8, 1133–1139. [Google Scholar] [CrossRef] [Green Version]
- Saeed, S.; Senior, R.; Chahal, N.S.; Lønnebakken, M.T.; Chambers, J.B.; Bahlmann, E.; Gerdts, E. Lower Transaortic Flow Rate Is Associated With Increased Mortality in Aortic Valve Stenosis. JACC Cardiovasc. Imaging 2017, 10, 912–920. [Google Scholar] [CrossRef]


| Overall Patients n = 530 | AS (Group A) n = 152 | Moderate AS n = 73 | Severe AS n = 79 | No-AS (Group B) n = 378 | p * | p ** | |
|---|---|---|---|---|---|---|---|
| Age (years) | 80 (72–86) | 82 (77–86) | 83 (77–87) | 81 (76–85) | 78 (70–85) | <0.001 | 0.001 |
| Male (n) | 270 (51) | 77 (51) | 35 (48) | 42 (53) | 193 (51) | 0.934 | 0.810 |
| BSA (m2) | 1.85 (1.7–1.99) | 1.83 (1.71–1.97) | 1.83 (1.71–1.97) | 1.85 (1.71–1.96) | 1.86 (1.69–2) | 0.364 | 0.468 |
| HR (bpm) | 74 (64–85) | 73 (65–85) | 71 (66–80) | 76 (64–90) | 75 (64–85) | 0.807 | 0.216 |
| SBP (mmHg) | 140 (120–160) | 130 (120–150) | 130 (120–150) | 130 (115–145) | 140 (120–160) | 0.005 | 0.011 |
| History of HF (n) | 154 (29) | 61 (40) | 24 (33) | 37 (47) | 93 (25) | <0.001 | <0.001 |
| AF (n) | 223 (42) | 67 (44) | 32 (44) | 35 (44) | 156 (41) | 0.554 | 0.838 |
| Hypertension (n) | 397 (75) | 121 (80) | 56 (77) | 65 (82) | 276 (73) | 0.114 | 0.209 |
| DM (n) | 151 (29) | 46 (30) | 22 (30) | 24 (30) | 105 (28) | 0.566 | 0.848 |
| CKD (n) | 161 (30) | 37 (24) | 18 (25) | 19 (24) | 124 (33) | 0.055 | 0.159 |
| CAD (n) | 182 (34) | 65 (43) | 27 (38) | 38 (48) | 117 (31) | 0.008 | 0.012 |
| Loop diuretic (n) | 454 (86) | 120 (79) | 59 (81) | 61 (77) | 334 (88) | 0.001 | 0.003 |
| Beta-blockers (n) | 357 (67) | 83 (54) | 40 (55) | 43 (54) | 274 (73) | <0.001 | <0.001 |
| Renin-angiotensin system blockers | 366 (69) | 111 (73) | 53 (73) | 58 (73) | 255 (68) | 0.210 | 0.453 |
| Mineralcorticoid receptor antagonist | 215 (41) | 53 (35) | 29 (40) | 24 (30) | 162 (43) | 0.076 | 0.089 |
| LVEDV (mL) | 113 (88–145) | 115 (90–141) | 114 (83–135) | 117 (94–145) | 111 (86–145) | 0.713 | 0.493 |
| LVESV (mL) | 55 (36–91) | 52 (37–78) | 49 (36–72) | 53 (37–89) | 56 (36–98) | 0.211 | 0.389 |
| LVEF (%) | 50 (34–59) | 55 (39–61) | 53 (39–60) | 56 (38–62) | 48 (32–58) | 0.002 | 0.007 |
| SVI (mL/m2) | 34 (27–42) | 37 (31–49) | 38 (31–49) | 37 (29–48) | 32 (26–40) | <0.001 | <0.001 |
| Low Flow (n) | 283 (53) | 65 (43) | 29 (40) | 36 (46) | 218 (58) | 0.002 | 0.006 |
| FR (mL/s) | 224 (187–274) | 226 (190–276) | 233 (200–293) | 222 (178–261) | 223 (186–268) | 0.628 | 0.23 |
| Slow Flow (n) | 223 (42) | 63 (41) | 28 (38) | 35 (44) | 160 (42) | 0.853 | 0.746 |
| LVET (ms) | 278 (48) | 306 (40) | 298 (41) | 314 (39) | 267 (46) | <0.001 | <0.001 |
| LAVI (mL/m2) | 49 (40–58) | 50 (43–63) | 53 (44–65) | 49 (41–62) | 49 (39–57) | 0.013 | 0.013 |
| MR (n) | 251 (47) | 56 (37) | 33 (45) | 23 (29) | 195 (52) | 0.002 | 0.001 |
| SPAP (mmHg) | 43 (35–53) | 38 (30–50) | 40 (31–50) | 37 (30–47) | 45 (38–53) | <0.001 | <0.001 |
| AT/ET | - | 0.35 (0.31–0.38) | 0.33 (0.29–0.36) | 0.37 (0.34–0.41) | - | - | <0.001 |
| AVA (cm2) | - | 0.9 (0.7–1.1) | 1.2 (1–1.5) | 0.7 (0.6–0.9) | - | - | <0.001 |
| Mean Gradient (mmHg) | - | 29 (21–44) | 21 (16–26) | 43 (30–56) | - | - | <0.001 |
| V Max (m/s) | - | 3.7 (3.2–4.6) | 3.2 (3–3.4) | 4.3 (3.6–4.8) | - | - | <0.001 |
| R | p | Univariate Beta | p | Multivariate Beta Model with SVI | p | Multivariate Beta Model with FR | p | |
|---|---|---|---|---|---|---|---|---|
| SVI | 0.618 | <0.001 | 0.612 | <0.001 | 0.354 | <0.001 | Not tested | |
| HR | −0.539 | <0.001 | −0.546 | <0.001 | −0.385 | <0.001 | −0.497 | <0.001 |
| AS grade | 0.394 | <0.001 | 0.383 | <0.001 | 0.301 | <0.001 | 0.349 | <0.001 |
| LVEF | 0.372 | <0.001 | 0.385 | <0.001 | 0.108 | 0.001 | 0.247 | <0.001 |
| SBP | 0.4 | 0.052 | 0.254 | |||||
| MR | −0.226 | <0.001 | −0.231 | <0.001 | 0.427 | 0.166 | ||
| FR | 0.145 | 0.001 | 0.11 | 0.011 | Not tested | 0.42 | ||
| Regression equation for indexing LVET LVETI = LVET – (1.452 × SVI) + (1.05 × HR) | ||||||||
| Global R-Square | Change in R-Square from Previous Model | p for Difference from Previous Model | |
|---|---|---|---|
| Model with SVI | |||
| SVI | 0.374 | 0.374 | <0.001 |
| SVI + HR | 0.485 | 0.111 | <0.001 |
| SVI + HR + AS grade | 0.575 | 0.090 | <0.001 |
| SVI + HR + AS grade + LVEF | 0.584 | 0.009 | 0.001 |
| Model with FR | |||
| HR | 0.298 | 0.298 | <0.001 |
| HR + AS grade | 0.442 | 0.144 | <0.001 |
| HR + AS grade + LVEF | 0.500 | 0.058 | <0.001 |
| R | p | Uni Beta | p | Multi Beta | p | |
|---|---|---|---|---|---|---|
| Low Flow (SVI ≤ 35 mL/m2) (n = 283) | ||||||
| SVI | 0.52 | <0.001 | 0.539 | <0.001 | 0.304 | <0.001 |
| HR | −0.504 | <0.001 | −0.526 | <0.001 | −0.396 | <0.001 |
| LVEF | 0.25 | <0.001 | 0.236 | <0.001 | 0.123 | 0.005 |
| AS grade | 0.388 | <0.001 | 0.389 | <0.001 | 0.342 | <0.001 |
| SBP | 0.759 | 0.007 | 0.905 | |||
| MR | −0.144 | 0.015 | −0.137 | 0.021 | 0.478 | |
| Slow Flow (FR ≤ 210 mL/s) (n = 223) | ||||||
| SVI | 0.766 | <0.001 | 0.781 | <0.001 | 0.607 | <0.001 |
| HR | −0.563 | <0.001 | −0.575 | <0.001 | −0.288 | <0.001 |
| LVEF | 0.394 | <0.001 | 0.403 | <0.001 | 0.061 | |
| AS grade | 0.358 | <0.001 | 0.327 | <0.001 | 0.222 | <0.001 |
| SBP | 0.523 | 0.073 | 0.3 | |||
| MR | −0.2 | 0.003 | −0.195 | 0.004 | 0.566 | |
| Normal Flow (n = 219) | ||||||
| SVI | 0.462 | <0.001 | 0.436 | <0.001 | 0.265 | <0.001 |
| HR | −0.403 | <0.001 | −0.388 | <0.001 | −0.367 | <0.001 |
| LVEF | 0.242 | <0.001 | 0.211 | 0.002 | 0.74 | |
| AS grade | 0.432 | <0.001 | 0.417 | <0.001 | 0.399 | <0.001 |
| SBP | 0.613 | −0.053 | 0.454 | |||
| MR | 0.051 | −0.162 | 0.016 | 0.129 | ||
| Abnormal Flow (n = 311) | ||||||
| SVI | 0.611 | <0.001 | 0.66 | <0.001 | 0.423 | <0.001 |
| HR | −0.549 | <0.001 | −0.562 | <0.001 | −0.359 | <0.001 |
| LVEF | 0.314 | <0.001 | 0.321 | <0.001 | 0.113 | 0.004 |
| AS grade | 0.379 | <0.001 | 0.353 | <0.001 | 0.279 | <0.001 |
| SBP | 0.889 | 0.043 | 0.469 | |||
| MR | −0.185 | 0.001 | −0.179 | 0.001 | 0.611 | |
| LVEF 50% (n = 263) | ||||||
| SVI | 0.567 | <0.001 | 0.553 | <0.001 | 0.352 | <0.001 |
| HR | −0.471 | <0.001 | −0.492 | <0.001 | −0.382 | <0.001 |
| LVEF | 0.158 | 0.047 | 0.451 | |||
| AS grade | 0.417 | <0.001 | 0.401 | <0.001 | 0.314 | <0.001 |
| SBP | 0.987 | −0.019 | 0.766 | |||
| MR | 0.097 | −0.105 | 0.090 | |||
| LVEF < 50% (n = 267) | ||||||
| SVI | 0.571 | <0.001 | 0.548 | <0.001 | 0.329 | <0.001 |
| HR | −0.536 | <0.001 | −0.539 | <0.001 | −0.431 | <0.001 |
| LVEF | 0.282 | <0.001 | 0.277 | <0.001 | 0.15 | 0.001 |
| AS grade | 0.339 | <0.001 | 0.321 | <0.001 | 0.332 | <0.001 |
| SBP | 0.677 | 0.081 | 0.204 | |||
| MR | −0.135 | 0.028 | −0.139 | 0.023 | 0.422 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pestelli, G.; Pergola, V.; Totaro, G.; Previtero, M.; Aruta, P.; Cecchetto, A.; Fiorencis, A.; Palermo, C.; Iliceto, S.; Mele, D. Value of Left Ventricular Indexed Ejection Time to Characterize the Severity of Aortic Stenosis. J. Clin. Med. 2022, 11, 1877. https://doi.org/10.3390/jcm11071877
Pestelli G, Pergola V, Totaro G, Previtero M, Aruta P, Cecchetto A, Fiorencis A, Palermo C, Iliceto S, Mele D. Value of Left Ventricular Indexed Ejection Time to Characterize the Severity of Aortic Stenosis. Journal of Clinical Medicine. 2022; 11(7):1877. https://doi.org/10.3390/jcm11071877
Chicago/Turabian StylePestelli, Gabriele, Valeria Pergola, Giuseppe Totaro, Marco Previtero, Patrizia Aruta, Antonella Cecchetto, Andrea Fiorencis, Chiara Palermo, Sabino Iliceto, and Donato Mele. 2022. "Value of Left Ventricular Indexed Ejection Time to Characterize the Severity of Aortic Stenosis" Journal of Clinical Medicine 11, no. 7: 1877. https://doi.org/10.3390/jcm11071877
APA StylePestelli, G., Pergola, V., Totaro, G., Previtero, M., Aruta, P., Cecchetto, A., Fiorencis, A., Palermo, C., Iliceto, S., & Mele, D. (2022). Value of Left Ventricular Indexed Ejection Time to Characterize the Severity of Aortic Stenosis. Journal of Clinical Medicine, 11(7), 1877. https://doi.org/10.3390/jcm11071877








