Effect of Cervical Transcutaneous Spinal Cord Stimulation on Sensorimotor Cortical Activity during Upper-Limb Movements in Healthy Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
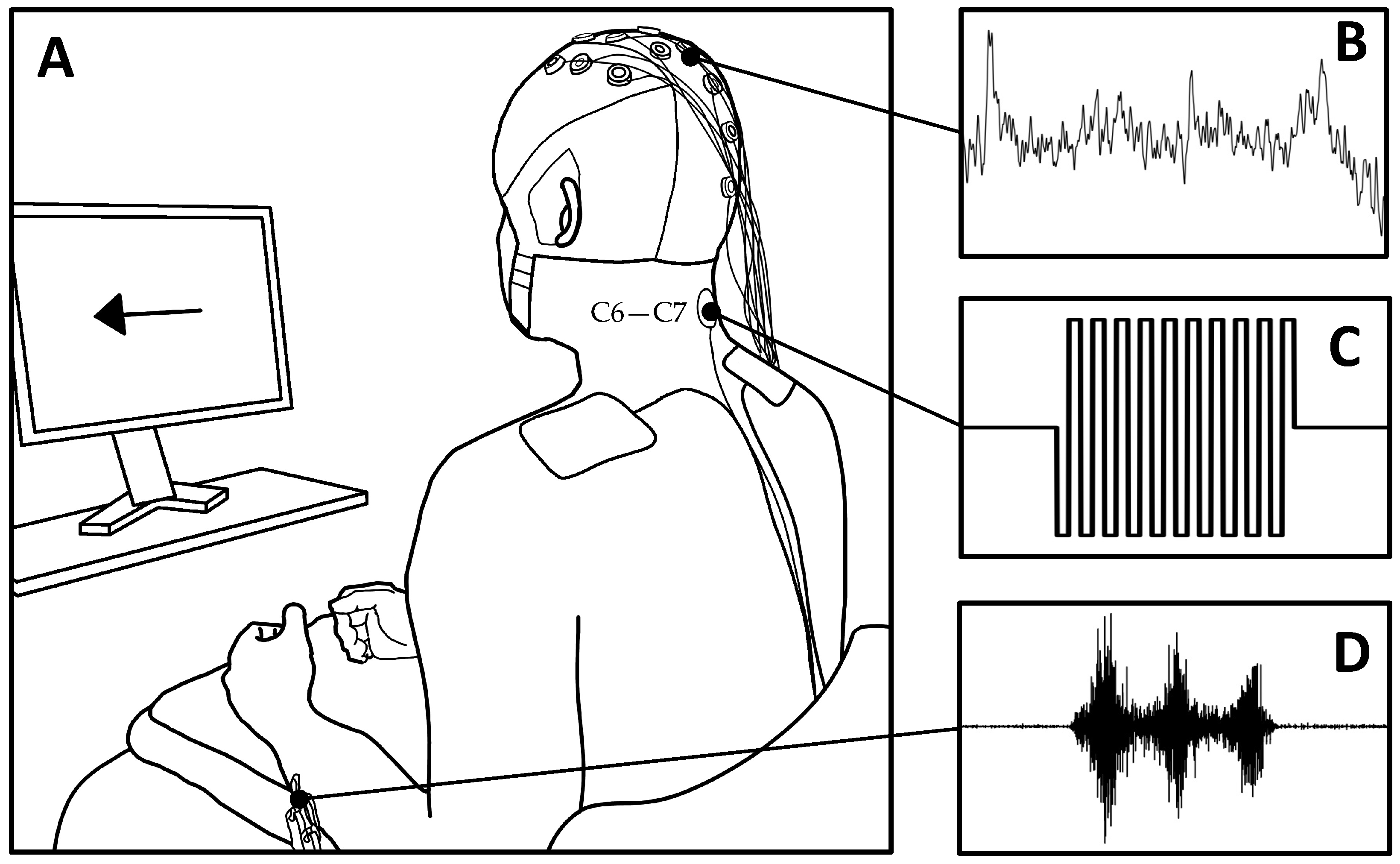
2.2. Experimental Protocol
2.3. Electroencephalography (EEG)
2.4. Electromyography (EMG)
2.5. Transcutaneous Spinal Cord Stimulation (tSCS)
2.6. Quantifying Sensorimotor Cortical Activity during tSCS
2.7. Neuromodulation of Resting-State EEG
3. Results
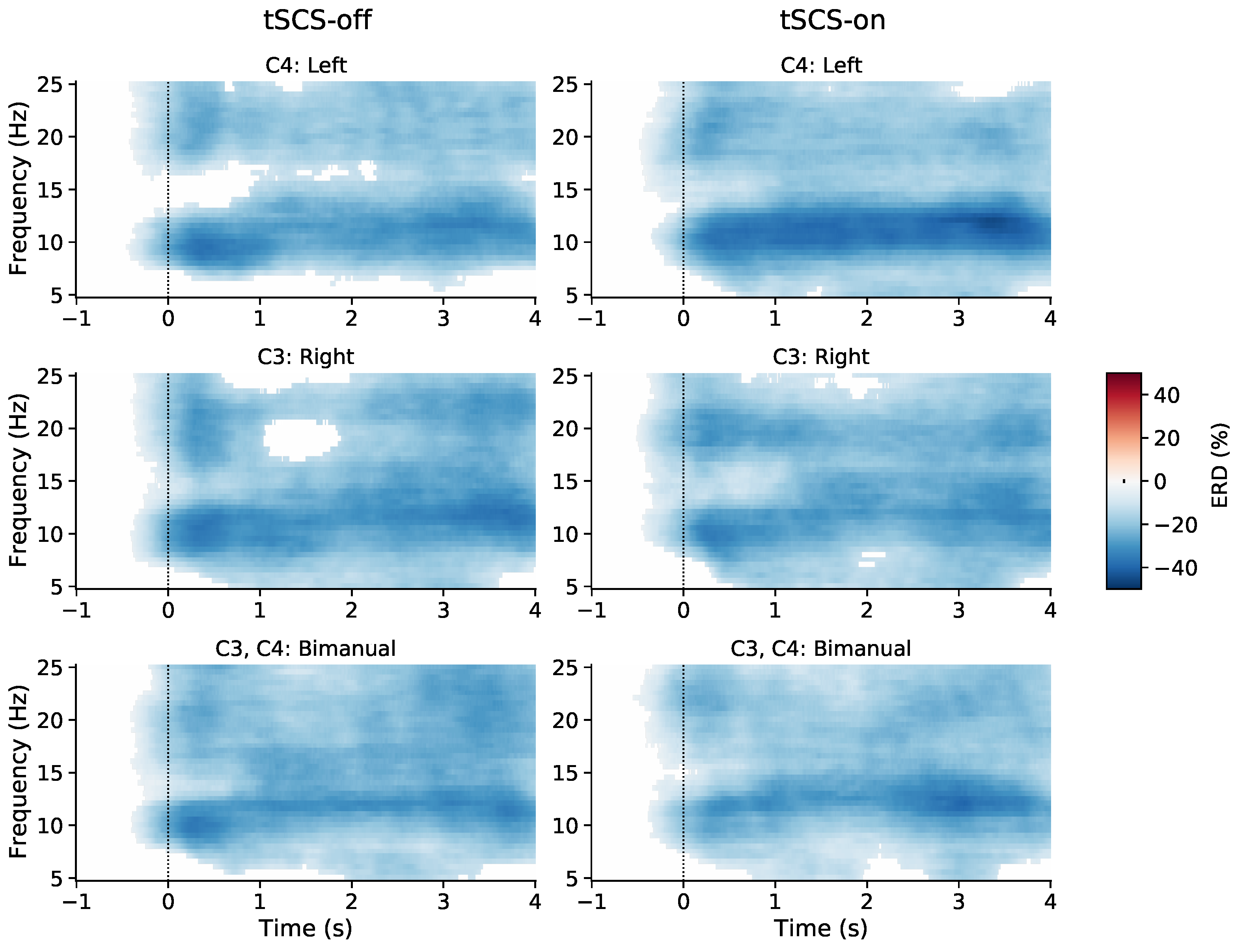
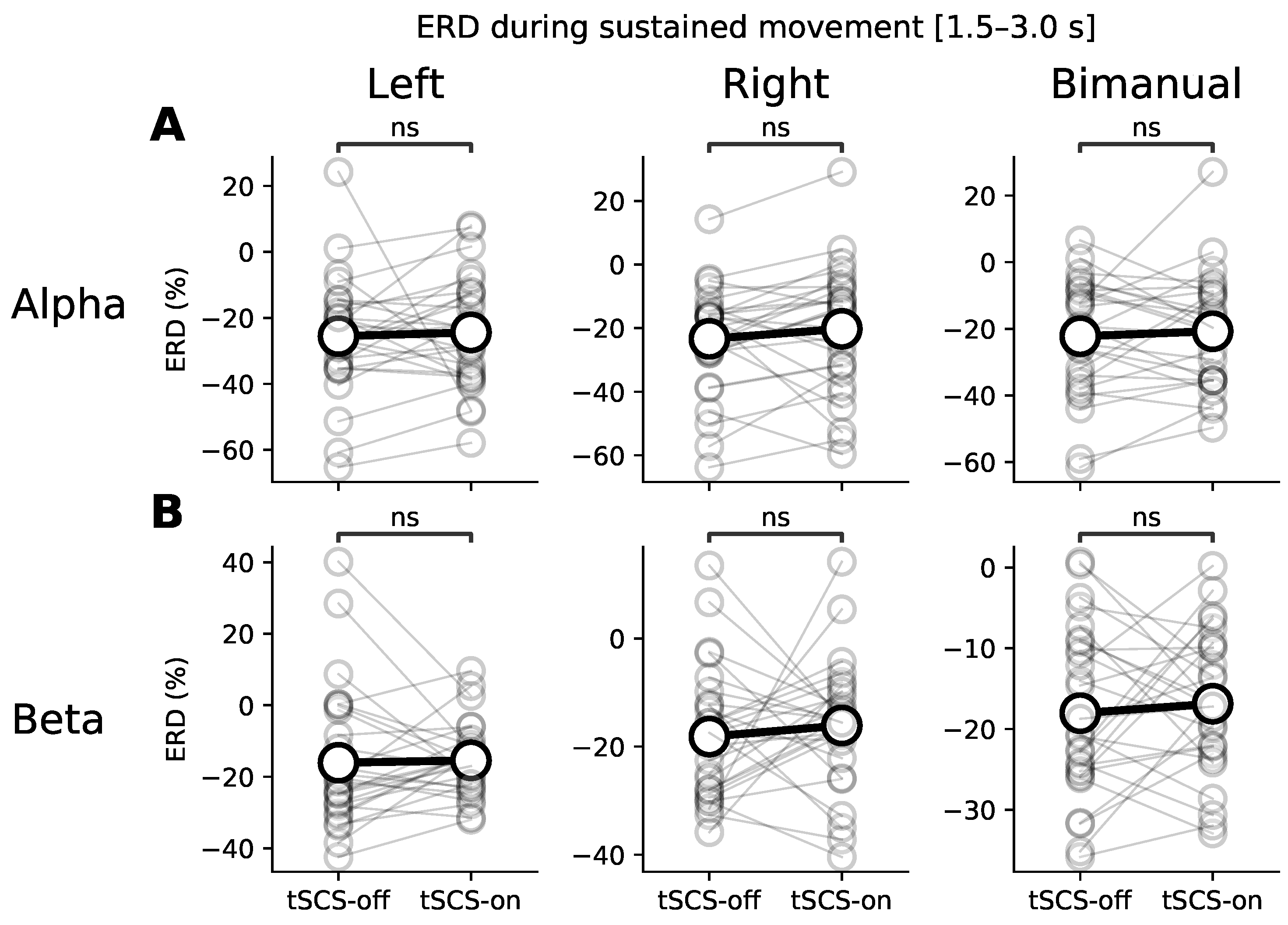
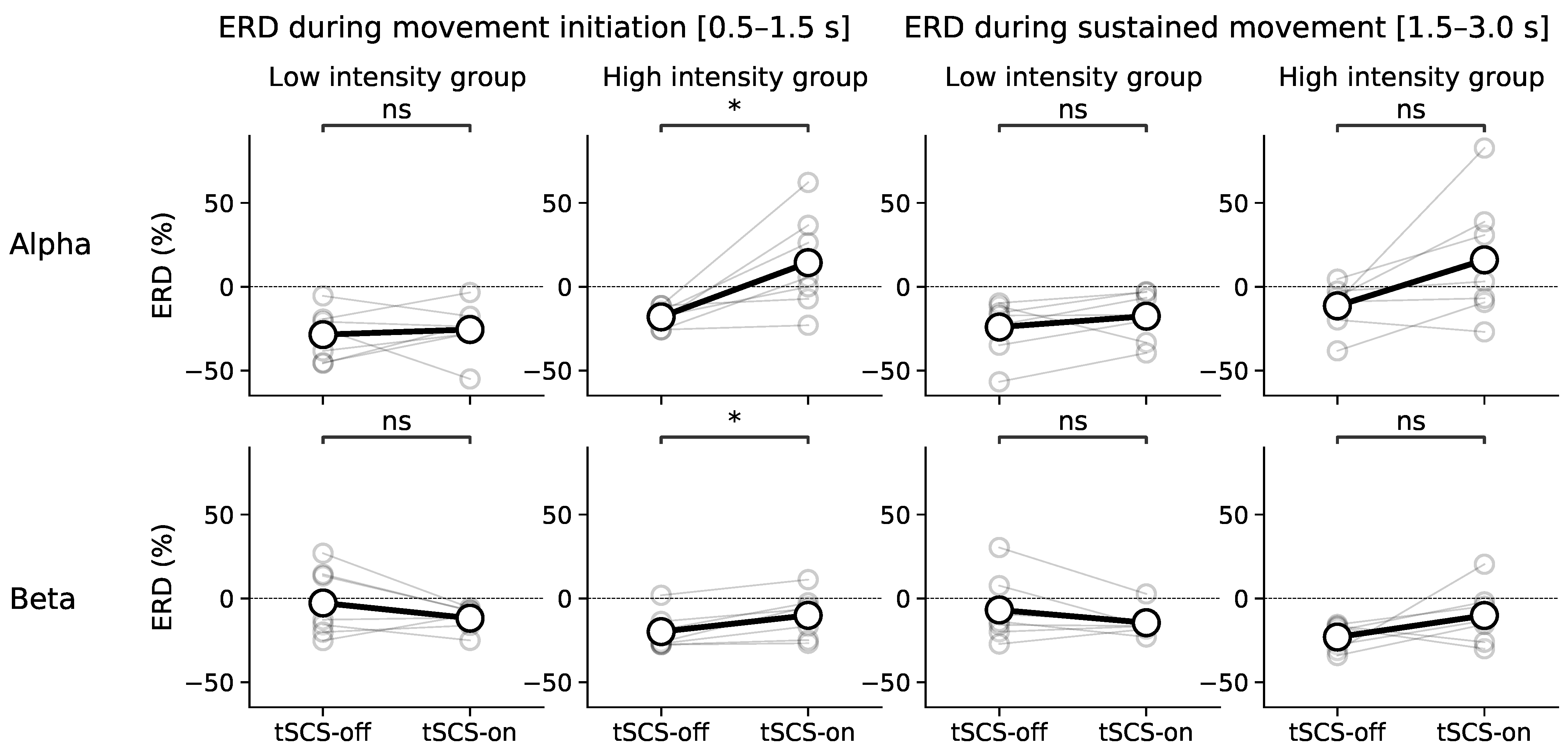
3.1. Event-Related (De)synchronisation (ERD/ERS)
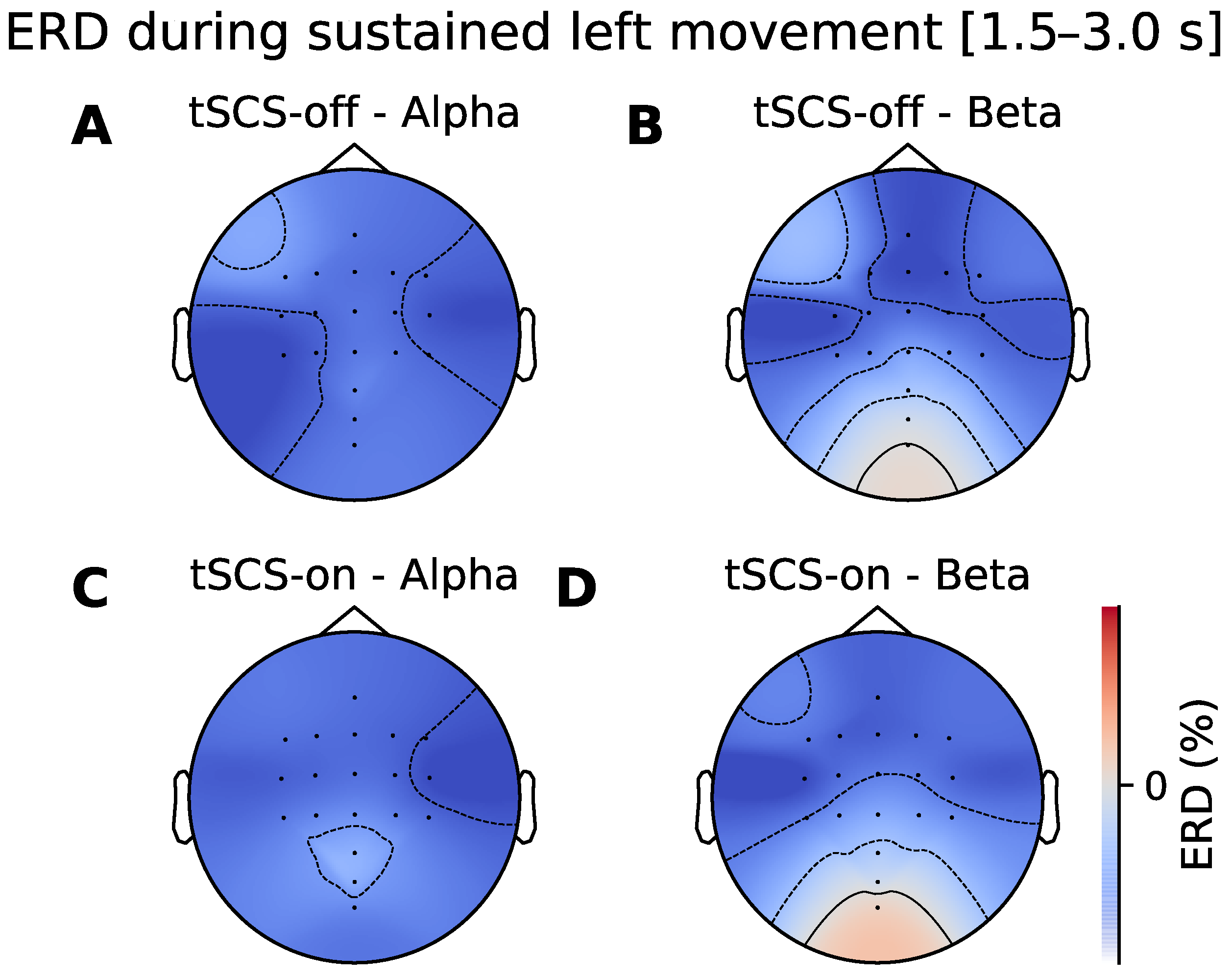
3.2. Topographic Analysis of ERD
3.3. Dose Effect of Event-Related Desynchronisation
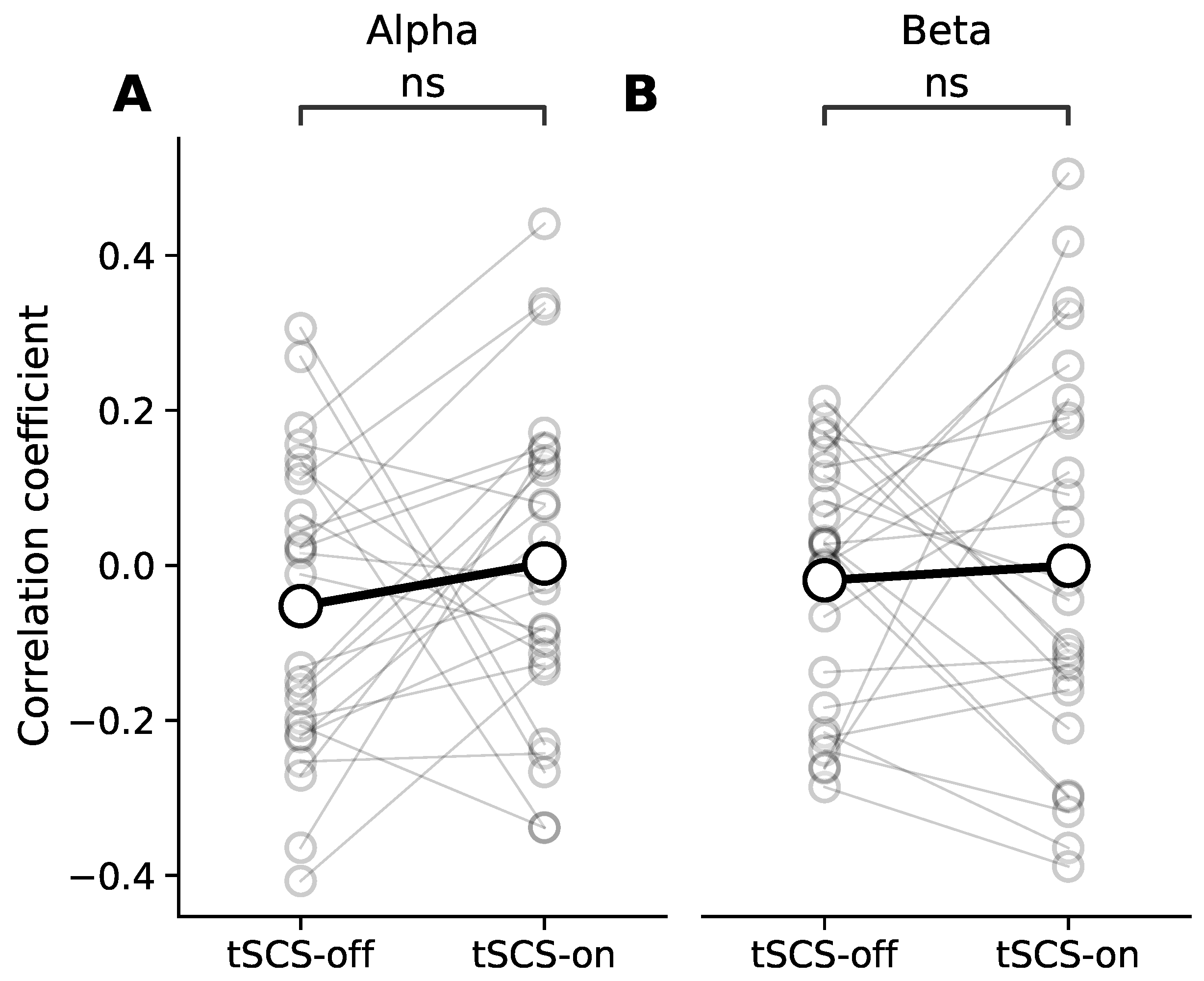
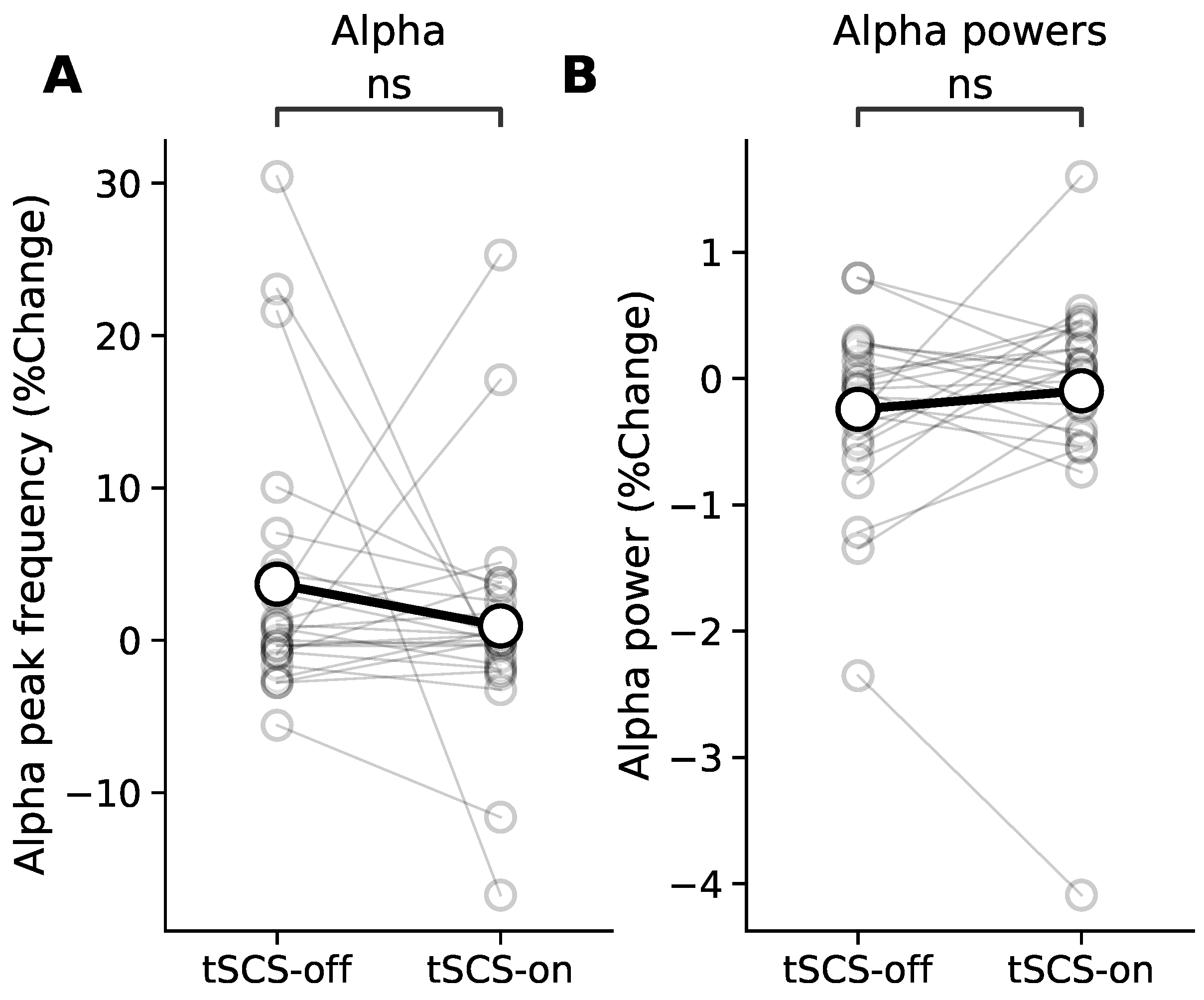
3.4. Resting State Modulation
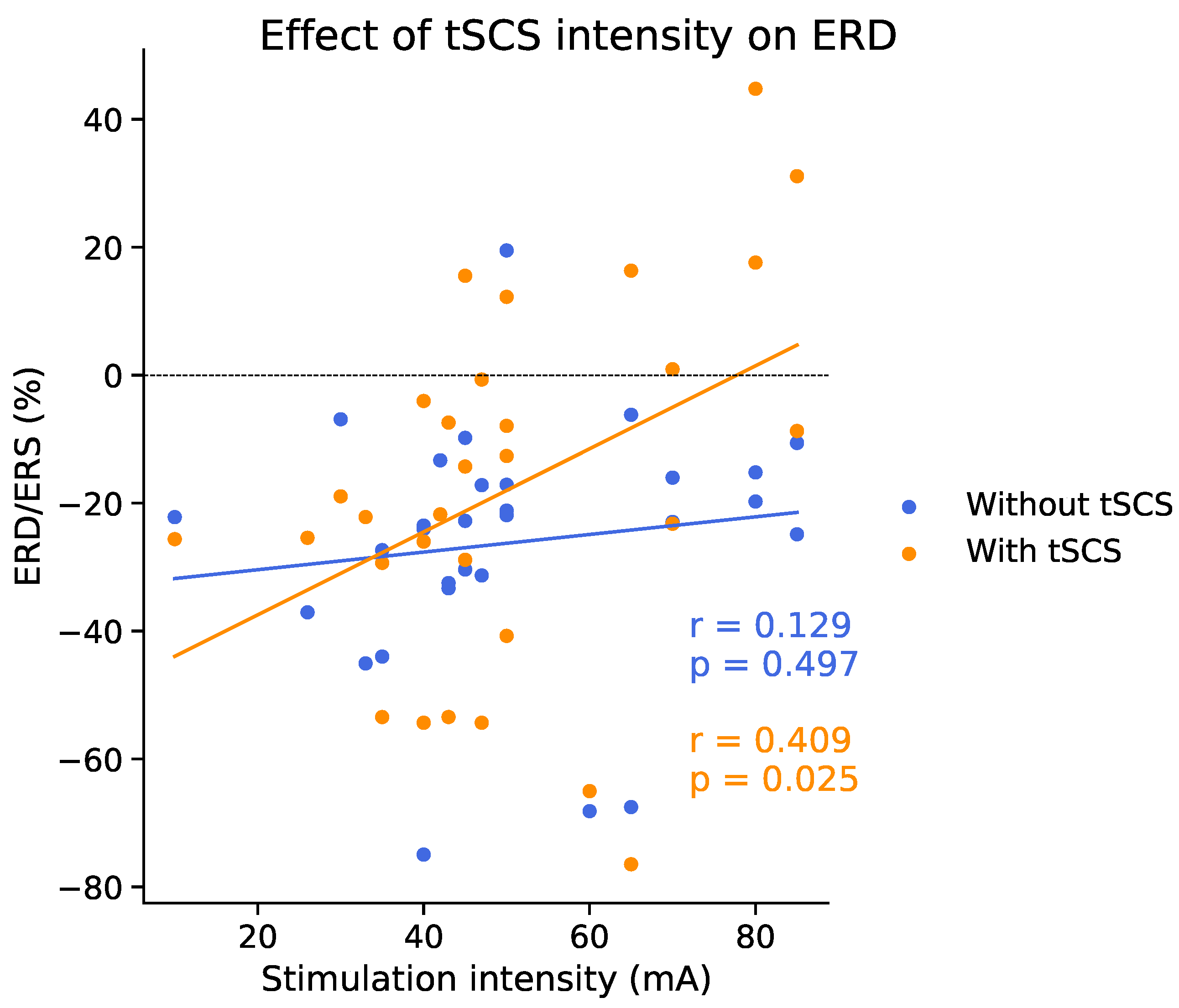
3.5. Effect of tSCS Intensity
3.6. Stimulation Adherence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.; Momeni, K.; Ramanujam, A.; Ravi, M.; Carnahan, J.; Kirshblum, S.; Forrest, G.F. Cervical Spinal Cord Transcutaneous Stimulation Improves Upper Extremity and Hand Function in People With Complete Tetraplegia: A Case Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Recovery cycles of posterior root-muscle reflexes evoked by transcutaneous spinal cord stimulation and of the H reflex in individuals with intact and injured spinal cord. PLoS ONE 2019, 14, e0227057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef]
- Duffell, L.D.; Donaldson, N.D.N. A Comparison of FES and SCS for Neuroplastic Recovery After SCI: Historical Perspectives and Future Directions. Front. Neurol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; McHugh, C.; Mockler, D.; Minogue, C.; Reilly, R.B.; Fleming, N. Transcutaneous spinal cord stimulation and motor responses in individuals with spinal cord injury: A methodological review. PLoS ONE 2021, 16, e0260166. [Google Scholar] [CrossRef]
- Benavides, F.D.; Jo, H.J.; Lundell, H.; Edgerton, V.R.; Gerasimenko, Y.; Perez, M.A. Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia. J. Neurosci. 2020, 40, 2633–2643. [Google Scholar] [CrossRef]
- Kumru, H.; Rodríguez-Cañón, M.; Edgerton, V.R.; García, L.; Flores, Á.; Soriano, I.; Opisso, E.; Gerasimenko, Y.; Navarro, X.; García-Alías, G.; et al. Transcutaneous Electrical Neuromodulation of the Cervical Spinal Cord Depends Both on the Stimulation Intensity and the Degree of Voluntary Activity for Training. A Pilot Study. J. Clin. Med. 2021, 10, 3278. [Google Scholar] [CrossRef]
- Kumru, H.; Flores, Á.; Rodríguez-Cañón, M.; Edgerton, V.R.; García, L.; Benito-Penalva, J.; Navarro, X.; Gerasimenko, Y.; García-Alías, G.; Vidal, J. Cervical Electrical Neuromodulation Effectively Enhances Hand Motor Output in Healthy Subjects by Engaging a Use-Dependent Intervention. J. Clin. Med. 2021, 10, 195. [Google Scholar] [CrossRef]
- Sasaki, A.; de Freitas, R.M.; Sayenko, D.G.; Masugi, Y.; Nomura, T.; Nakazawa, K.; Milosevic, M. Low-Intensity and Short-Duration Continuous Cervical Transcutaneous Spinal Cord Stimulation Intervention Does Not Prime the Corticospinal and Spinal Reflex Pathways in Able-Bodied Subjects. J. Clin. Med. 2021, 10, 3633. [Google Scholar] [CrossRef]
- Megía-García, Á.; Serrano-Muñoz, D.; Taylor, J.; Avendaño-Coy, J.; Comino-Suárez, N.; Gómez-Soriano, J. Transcutaneous Spinal Cord Stimulation Enhances Quadriceps Motor Evoked Potential in Healthy Participants: A Double-Blind Randomized Controlled Study. J. Clin. Med. 2020, 9, 3275. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Reggie Edgerton, V. Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Al’joboori, Y.; Hannah, R.; Lenham, F.; Borgas, P.; Kremers, C.J.P.; Bunday, K.L.; Rothwell, J.; Duffell, L.D. The Immediate and Short-Term Effects of Transcutaneous Spinal Cord Stimulation and Peripheral Nerve Stimulation on Corticospinal Excitability. Front. Neurosci. 2021, 15, 749042. [Google Scholar] [CrossRef]
- Rau, C.; Plewnia, C.; Hummel, F.; Gerloff, C. Event-related desynchronization and excitability of the ipsilateral motor cortex during simple self-paced finger movements. Clin. Neurophysiol. 2003, 114, 1819–1826. [Google Scholar] [CrossRef]
- Mäki, H.; Ilmoniemi, R.J. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin. Neurophysiol. 2010, 121, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Salisbury, D.B.; Parsons, T.D.; Monden, K.R.; Trost, Z.; Driver, S.J. Brain–computer interface for individuals after spinal cord injury. Rehabil. Psychol. 2016, 61, 435–441. [Google Scholar] [CrossRef]
- Daly, J.J.; Huggins, J.E. Brain-Computer Interface: Current and Emerging Rehabilitation Applications. Arch. Phys. Med. Rehabil. 2015, 96, S1–S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, C.; Osuagwu, B.A.; Vuckovic, A. Influence of motor imagination on cortical activation during functional electrical stimulation. Clin. Neurophysiol. 2015, 126, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Lotte, F.; Bougrain, L.; Cichocki, A.; Clerc, M.; Congedo, M.; Rakotomamonjy, A.; Yger, F. A review of classification algorithms for EEG-based brain–computer interfaces: A 10 year update. J. Neural Eng. 2018, 15, 031005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insausti-Delgado, A.; López-Larraz, E.; Omedes, J.; Ramos-Murguialday, A. Intensity and Dose of Neuromuscular Electrical Stimulation Influence Sensorimotor Cortical Excitability. Front. Neurosci. 2021, 14, 1359. [Google Scholar] [CrossRef]
- Sharon, O.; Fahoum, F.; Nir, Y. Transcutaneous Vagus Nerve Stimulation in Humans Induces Pupil Dilation and Attenuates Alpha Oscillations. J. Neurosci. 2021, 41, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Fürst, S. Transmitters involved in antinociception in the spinal cord. Brain Res. Bull. 1999, 48, 129–141. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Birbaumer, N. Brain oscillatory signatures of motor tasks. J. Neurophysiol. 2015, 113, 3663–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graimann, B.; Pfurtscheller, G. Quantification and visualization of event-related changes in oscillatory brain activity in the time–frequency domain. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2006; Volume 159, pp. 79–97. [Google Scholar] [CrossRef]
- Kantak, S.; Jax, S.; Wittenberg, G. Bimanual coordination: A missing piece of arm rehabilitation after stroke. Restor. Neurol. Neurosci. 2017, 35, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.R.; Field-Fote, E.C. Cortical Reorganization Following Bimanual Training and Somatosensory Stimulation in Cervical Spinal Cord Injury: A Case Report. Phys. Ther. 2007, 87, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Kasashima-Shindo, Y.; Fujiwara, T.; Ushiba, J.; Matsushika, Y.; Kamatani, D.; Oto, M.; Ono, T.; Nishimoto, A.; Shindo, K.; Kawakami, M.; et al. Brain-computer interface training combined with transcranial direct current stimulation in patients with chronic severe hemiparesis: Proof of concept study. J. Rehabil. Med. 2015, 47, 318–324. [Google Scholar] [CrossRef] [Green Version]
- McGeady, C.; Vučković, A.; Zheng, Y.P.; Alam, M. EEG Monitoring Is Feasible and Reliable during Simultaneous Transcutaneous Electrical Spinal Cord Stimulation. Sensors 2021, 21, 6593. [Google Scholar] [CrossRef]
- Merletti, R.; Farina, D. Surface Electromyography: Physiology, Engineering, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Wecht, J.R.; Savage, W.M.; Famodimu, G.O.; Mendez, G.A.; Levine, J.M.; Maher, M.T.; Weir, J.P.; Wecht, J.M.; Carmel, J.B.; Wu, Y.K.; et al. Posteroanterior Cervical Transcutaneous Spinal Cord Stimulation: Interactions with Cortical and Peripheral Nerve Stimulation. J. Clin. Med. 2021, 10, 5304. [Google Scholar] [CrossRef]
- Barss, T.S.; Parhizi, B.; Mushahwar, V.K. Transcutaneous spinal cord stimulation of the cervical cord modulates lumbar networks. J. Neurophysiol. 2020, 123, 158–166. [Google Scholar] [CrossRef]
- Espenhahn, S.; de Berker, A.O.; van Wijk, B.C.M.; Rossiter, H.E.; Ward, N.S. Movement-related beta oscillations show high intra-individual reliability. NeuroImage 2017, 147, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Hu, L.; Zhang, Z.; Hu, Y. Changes of spontaneous oscillatory activity to tonic heat pain. PLoS ONE 2014, 9, e91052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.L.; Oswald, M.J.; Kuner, R. Neurobiology of brain oscillations in acute and chronic pain. Trends Neurosci. 2021, 44, 629–642. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Zhou, K.; Wei, W.; Liu, Y. Restoring Sensorimotor Function Through Neuromodulation After Spinal Cord Injury: Progress and Remaining Challenges. Front. Neurosci. 2021, 15, 1312. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.; Edgerton, V.R. Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis. J. Neurotrauma 2018, 35, 2145–2158. [Google Scholar] [CrossRef]
- López-Larraz, E.; Ray, A.M.; Birbaumer, N.; Ramos-Murguialday, A. Sensorimotor rhythm modulation depends on resting-state oscillations and cortex integrity in severely paralyzed stroke patients. In Proceedings of the 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA, USA, 20–23 March 2019; pp. 37–40. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manson, G.A.; Calvert, J.S.; Ling, J.; Tychhon, B.; Ali, A.; Sayenko, D.G. The relationship between maximum tolerance and motor activation during transcutaneous spinal stimulation is unaffected by the carrier frequency or vibration. Physiol. Rep. 2020, 8, e14397. [Google Scholar] [CrossRef] [PubMed]
- Corbet, T.; Iturrate, I.; Pereira, M.; Perdikis, S.; Millán, J.D.R. Sensory threshold neuromuscular electrical stimulation fosters motor imagery performance. NeuroImage 2018, 176, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Takemi, M.; Masakado, Y.; Liu, M.; Ushiba, J. Sensorimotor event-related desynchronization represents the excitability of human spinal motoneurons. Neuroscience 2015, 297, 58–67. [Google Scholar] [CrossRef]
- Kohli, S.; Casson, A.J. Removal of Gross Artifacts of Transcranial Alternating Current Stimulation in Simultaneous EEG Monitoring. Sensors 2019, 19, 190. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, H.; Ke, X.; Liu, X.; Yuan, Y.; Zhang, D.; Xiong, D.; Qiu, Y. Placebo Analgesia Changes Alpha Oscillations Induced by Tonic Muscle Pain: EEG Frequency Analysis Including Data during Pain Evaluation. Front. Comput. Neurosci. 2016, 10, 45. [Google Scholar] [PubMed] [Green Version]
- Turner, C.; Jackson, C.; Learmonth, G. Is the “end-of-study guess” a valid measure of sham blinding during transcranial direct current stimulation? Eur. J. Neurosci. 2021, 53, 1592–1604. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGeady, C.; Alam, M.; Zheng, Y.-P.; Vučković, A. Effect of Cervical Transcutaneous Spinal Cord Stimulation on Sensorimotor Cortical Activity during Upper-Limb Movements in Healthy Individuals. J. Clin. Med. 2022, 11, 1043. https://doi.org/10.3390/jcm11041043
McGeady C, Alam M, Zheng Y-P, Vučković A. Effect of Cervical Transcutaneous Spinal Cord Stimulation on Sensorimotor Cortical Activity during Upper-Limb Movements in Healthy Individuals. Journal of Clinical Medicine. 2022; 11(4):1043. https://doi.org/10.3390/jcm11041043
Chicago/Turabian StyleMcGeady, Ciarán, Monzurul Alam, Yong-Ping Zheng, and Aleksandra Vučković. 2022. "Effect of Cervical Transcutaneous Spinal Cord Stimulation on Sensorimotor Cortical Activity during Upper-Limb Movements in Healthy Individuals" Journal of Clinical Medicine 11, no. 4: 1043. https://doi.org/10.3390/jcm11041043
APA StyleMcGeady, C., Alam, M., Zheng, Y.-P., & Vučković, A. (2022). Effect of Cervical Transcutaneous Spinal Cord Stimulation on Sensorimotor Cortical Activity during Upper-Limb Movements in Healthy Individuals. Journal of Clinical Medicine, 11(4), 1043. https://doi.org/10.3390/jcm11041043






