Abstract
Background: Irritable bowel syndrome (IBS) is a common gastrointestinal tract disorder, affecting 10–20% of adults worldwide. Mebeverine is an antispasmodic agent indicated for the symptomatic treatment of abdominal pain caused by intestinal smooth muscle spasms and intestinal functional disorders in the course of IBS. The aim of this article was to perform a systematic literature review and update previous overviews of the efficacy and safety of mebeverine treatment in IBS. Methods: Major electronic medical databases, PubMed, EMBASE and Cochrane, were systematically searched from January 1965 to January 2021. Results: Twenty-two studies met our inclusion criteria, including 19 randomised trials, two observational retrospective studies, and one non-randomised, single-blinded study. Six studies reported a significant decrease in abdominal pain after mebeverine treatment (p-values ranging from <0.05 to <0.001). Only three studies showed no improvement after mebeverine treatment in terms of the severity of abdominal pain or discomfort. Some of the included studies also showed significant improvements in abnormal bowel habits, abdominal distension, as well as stool frequency and consistency. Adverse events were rare and associated mainly with IBS symptoms. Conclusions: Mebeverine is an effective treatment option in IBS, with a good safety profile and low frequency of adverse effects.
1. Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal tract disorder, affecting 10–20% of adults worldwide [1]. Almost half of all IBS patients report their first symptoms before age 35, which negatively affects their professional activity [1]. The main clinical manifestation of IBS is abdominal pain related to defecation, in addition to a change of bowel habit or stool consistency [1]. Although the pathogenesis of IBS is not fully understood, gut-brain axis disturbances, intestinal microbiota dysbiosis, abnormal gut motility, visceral hypersensitivity, and local immune system dysfunction are all thought to influence disease development [2].
Diagnosis of IBS is primarily based on clinical symptoms, and additional tests are not routinely recommended. While several diagnostic criteria have been developed over the years (e.g., Manning, Kruis, Rome I–IV), there is still no gold standard for diagnosing IBS [3,4]. Currently, the Rome IV criteria are recommended to establish the diagnosis of the disease [5]. Based on these criteria, IBS with predominant constipation (IBS-C) can be distinguished from IBS with predominant diarrhoea (IBS-D) and IBS with mixed bowel habits (IBS-M), or IBS may be unclassified (IBS-U) [2].
Due to the chronic nature of the disease, treatment remains challenging and depends on the symptoms. Therapeutic options consist of non-pharmacological management like lifestyle and dietary modifications and pharmacological treatment, depending on the predominant symptoms, i.e., abdominal pain, constipation, or diarrhoea [6]. Antispasmodics are currently the recommended treatment of choice for IBS patients with abdominal pain, as previous meta-analyses have reported some advantages over placebo [7]. However, the effectiveness of different antispasmodic agents varies.
Mebeverine is an antispasmodic agent indicated for the symptomatic treatment of abdominal pain caused by intestinal smooth muscle spasms and intestinal functional disorders in the course of IBS. It acts by relaxing the intestinal muscles and regulating bowel function. Studies assessing the effectiveness of mebeverine in IBS date back to the 1960s, even before the Rome I criteria for diagnosing IBS were released in 1992 [8]. The last systematic review and meta-analysis of mebeverine efficacy in IBS was performed more than ten years ago [9]. Therefore, this study aimed to systematically review currently available data to assess the effectiveness and safety of mebeverine in patients who were diagnosed according to IBS diagnostic criteria (i.e., Rome I–IV and other than Rome) and who suffer from bowel symptoms, including abdominal pain and discomfort, abdominal distension, abnormal bowel habits, bloating, constipation, and diarrhoea.
2. Materials and Methods
2.1. Information Sources and Searches
The systematic literature review was performed according to the previously developed, detailed protocol and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. Major electronic medical databases, PubMed, EMBASE and Cochrane, were systematically searched from January 1965 to January 2021 to estimate the treatment effects of mebeverine in patients with IBS. The keywords used for the search were: mebeverine, mebeverin, duspatalin, spasmotalin, and 4-(ethyl-(4-methoxy-alpha-methylphenethyl)aminobutyl) veratrate (Tables S1–S3).
2.2. Study Selection and Quality Evaluation
Titles and abstracts of all obtained articles were assessed for inclusion in the study. We used the PICO framework to develop our search strategy’s inclusion and exclusion criteria (Table 1). Inclusion criteria were: patients with a diagnosis of IBS; mebeverine treatment (regardless of dose and duration); specific outcomes (abdominal pain or discomfort, abdominal distension, abnormal bowel habits, bloating, constipation, diarrhoea, stool frequency and consistency, nausea, anxiety and depression); studies (experimental or observational) including ≥ten patients; and studies published in English. Studies involving mebeverine treatment in combination with another drug, cognitive therapy or diet, as well as case studies or secondary studies (i.e., systematic reviews, reviews), were excluded from the analysis.

Table 1.
Inclusion and exclusion criteria for systematic literature search.
Two authors independently (J.D., B.S.-R) reviewed each title, abstract, and full-text to evaluate study quality and eligibility according to the abovementioned inclusion/exclusion criteria. The same reviewers independently extracted data for measured outcomes. All discrepancies between the reviewers were resolved through discussion.
The risk of bias in experimental studies was assessed by the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [11]. The quality of observational studies was evaluated using the NICE checklist [12]. The Cochrane tool is based on five distinct domains for assessing potential sources of bias: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, (5) selection of the reported results. For each domain, the risk of bias is judged as “low”, “high” or “some concerns”. Because of its comprehensiveness, the RoB2 tool became the standard approach to assess the risk of bias for randomized trials. The NICE checklist contains valid questions about the reliability of the methodology of observational studies, including the clarity of the study purpose and selection criteria, consecutiveness, and the direction of observation. The result is presented on 8-point scale, where the higher score indicates better quality of the study.
2.3. Data Items and Extraction
Data on the criteria used for the diagnosis of IBS, the characteristics of the treated groups, the treatment length and dosages were extracted for each of the studies based on the previously prepared form. Quantitative data on the severity or frequency of the following symptoms (i.e., measured outcomes) were also obtained: abdominal pain or discomfort, abdominal distension, abnormal bowel habits, bloating, constipation, diarrhoea, stool frequency and consistency, nausea, anxiety and depression. The p-value for each specific outcome was also extracted when available.
2.4. Statistical Analysis
Given the qualitative and narrative nature of this systematic review, no statistical analyses were performed.
3. Results
3.1. Overview of Included Studies
The search strategy yielded 871 unique papers (after duplicate removal), with 52 publications warranting further assessment based on their titles and abstracts (Figure 1). Of these, 25 publications (22 studies) met our inclusion criteria (listed in Table 2) [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
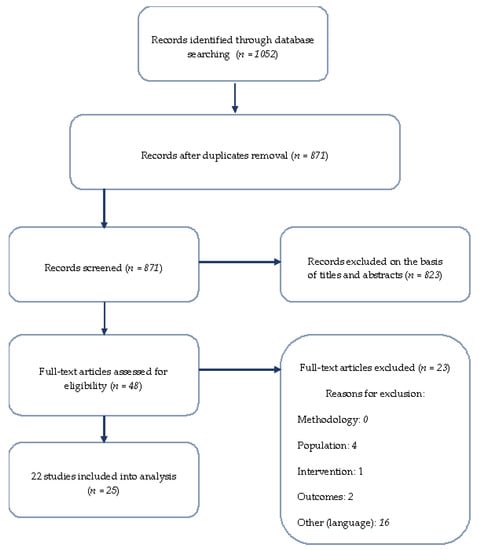
Figure 1.
Flow chart of literature search strategy according to PRISMA.

Table 2.
Characteristics of the included studies.
Among the 22 studies included in our analysis, one used the Manning criteria, two used the Kruis criteria, two used the Rome I criteria, two used the Rome II criteria, five used the Rome III criteria, three used the Rome IV criteria, and seven used different or unspecified criteria for diagnosing IBS.
Of the 22 studies, 19 were randomised trials; two were observational studies [13,14], and one was a non-randomised, single-blinded study [15]. The number of patients included in the studies ranged from 20 to 464 patients, and data about percentages of patients with specific IBS subtypes were present in 12 studies. Mebeverine was compared with placebo (seven studies) [15,16,17,18,19,20,21], trimebutine (two studies) [22,23], octilonium bromide (two studies) [20,24], pinaverium bromide (two studies) [14,25], alosetron (one study) [26], herbal combination (one study) [27], methylcellulose (one study) [16], ramosteron (one study) [28], probiotic (one study) [13], cumin sofouf (one study) [29], Luvos® Healing Earth (one study) [30] and alverine citrate (one study) [31]. Only three studies compared different doses of mebeverine [32,33,34]. The treatment period varied from 2 to 16 weeks across the included studies. The most frequently evaluated symptoms were abdominal pain, bloating, and stool frequency and consistency (Table 2).
Only one of the included in the systematic review trials was at low risk of bias (Table S4) [21]. Some concerns regarding the risk of bias were present in eleven studies [16,20,22,23,24,25,26,28,32,33,34]. These concerns usually resulted from a lack of the description of the randomization process and doubts regarding the concealment of the allocation sequence. Another frequent issue was the high probability of using an inappropriate type of analysis to estimate the effect of assignment to intervention (‘as treated’ or ‘as completed’). The remaining eight studies were judged as being at a high risk of bias [15,17,18,19,27,29,30,31]. The main identified risks for these studies included the possible high impact of missing outcomes on results and the selection of the reported results. The quality of the observational studies was variable (Table S5). An article published by Hou et al. [14] was judged as having a good quality, while Guslandi [13] reported his results only as abstract, so the assessment of the quality of the study may be underestimated.
3.2. Efficacy of Mebeverine
Intestinal symptoms associated with IBS were assessed in all 22 studies, although only two studies [16,29] used the IBS Symptom Severity Scale (IBS-SSS) to measure abdominal pain, its duration, abdominal distension/tightness, bowel habits, and quality of life (QOL). The efficacy of mebeverine on six major intestinal symptoms are detailed below.
3.2.1. Abdominal Pain and Discomfort
Nineteen studies evaluated the effect of mebeverine on the severity, frequency, and intensity of abdominal pain and discomfort in IBS patients, with a total of 1824 patients analysed in the mebeverine arm. A positive effect is presented as a percentage of patients with decreased symptoms or improved abdominal pain score compared to baseline. The treatment period varied from 2 to 12 weeks.
As shown in Table S6, six studies detected a significant decrease in abdominal pain score after mebeverine treatment compared to baseline (p-values ranging from <0.05 to <0.001) [21,23,28,29,30,31]. Eleven more studies showed mebeverine had a beneficial effect on reducing abdominal pain and discomfort, although the authors did not specify the statistical significance of the observed change [13,14,15,18,20,22,26,27,32,33,34]. Additionally, two trials proved the superiority of mebeverine over placebo in terms of abdominal pain reduction [18,20]. In two of nineteen studies, the beneficial effect of mebeverine was uncertain or insignificant [19,25]. Kruis et al. showed that initial abdominal pain improved only in 23% of patients treated with mebeverine; however, the compliance of treatment was below 50% [19]. Lu et al. reported that a similar percentage of patients suffered from abdominal pain before and after mebeverine treatment. However, the lack of significance possibly results from mild baseline pain intensity in about two-thirds of patients [25].
Three articles assessed the frequency of abdominal pain or discomfort after mebeverine treatment. All those studies found that mebeverine treatment reduced numerically abdominal pain frequency compared to baseline [22,27,29]. The improvement from baseline was statistically significant only in one trial [29], as no statistical calculations in the remaining two studies were performed [22,27].
3.2.2. Abdominal Distension
The effect of mebeverine on abdominal distension was assessed in three studies, totalling 109 patients (Table S7) [18,27,32]. All three studies showed that mebeverine had a positive effect on abdominal distension. In particular, Prout et al. found the severity of the distension score was significantly lower in the mebeverine group than the placebo group after eight weeks of treatment (1.692 vs. 1.839; p < 0.05) [18]. The percentage of IBS patients with abdominal distension after mebeverine treatment compared to baseline was also numerically reduced in the two other studies [27,32].
3.2.3. Abnormal Bowel Habits and Bloating
The effect of mebeverine on abnormal bowel habits in IBS patients was evaluated in five studies [15,18,19,28,31], as well as bloating [13,24,25,30,33], totalling 381 patients (Table S8). Four studies showed that mebeverine treatment numerically reduced abnormal bowel habits [15,18,28,31], while benefits in one study were found uncertain [19]. The authors of the two studies performed statistical calculations: Lee et al. reported a significant reduction in the number of abnormal bowel habits among patients taking mebeverine compared to baseline (p < 0.001) [28], and Prout et al. showed a significant reduction in pain during bowel movements in mebeverine-treated groups (i.e., 1.188 and 1.248 for the mebeverine low and high dose, respectively) compared to placebo (1.374; p < 0.05); however, the clinical value of the change was unclear for the authors [18]. Bloating was also reduced in all five studies [13,24,25,30,33]; however, only in one study, the statistics were calculated, i.e., Chang et al. reported that abdominal bloating assessed on a visual analogue scale (VAS) reduced from 4.7 (6.6) at baseline to 1.3 (4.6) at week eight (p < 0.001) [24].
3.2.4. Constipation and Diarrhoea
The effect of mebeverine on constipation and diarrhoea was analysed in three studies for both constipation [20,32,33] and diarrhoea [13,20,30], with all studies showing a reduction of these symptoms (Table S9). Specifically, treatment with mebeverine for 3 to 6 weeks caused the resolution of constipation in 62% to 79% of patients [32,33]. Similarly, six weeks of mebeverine treatment caused diarrhoea to improve or disappear [13]. Finally, one study showed that the severity of both constipation and diarrhoea were significantly lower after mebeverine treatment compared to placebo (p < 0.001) [20].
3.2.5. Stool Frequency and Consistency
Eight studies (totalling 722 patients) evaluated the effect of mebeverine on stool frequency [21,23,24,25,26,27,28,30], with a treatment period ranging from 2 to 12 weeks (Table S10). All eight studies showed a reduction in stool frequency after mebeverine therapy, with five reporting a statistically significant change [21,23,24,25,28]. In one study, the change from baseline was insignificant [30], while in remaining two studies, no statistical calculations were made [26,27]. Five studies, including 608 patients, determined the influence of mebeverine on stool consistency [23,25,26,27,28]. All five studies showed mebeverine had a favourable effect on stool consistency, with three studies [23,25,28] revealing a statistically significant improvement.
3.2.6. Nausea, Anxiety and Depression
The effect of mebeverine on nausea was only evaluated in one study (Table S11), in which the severity of nausea score was significantly lower in the mebeverine group (1.170) than in the placebo (1.311; p < 0.05) [18]. The effect of mebeverine on depression and anxiety, two very common symptoms in IBS patients, was evaluated in the MIBS (Management of IBS in Primary Care) trial (Table S9) [35]. The mean HADS (Hospital Anxiety and Depression Scale) score for anxiety was reduced from 9.23 at baseline to 8.7 at week six and to 8.2 at week 12 in patients treated with mebeverine. Similarly, 85% and 78% of patients reported normal HADS scores for depression after 6 and 12 weeks of treatment, respectively [35]. Furthermore, Prout et al. reported that the severity of anxiety score was significantly lower in the mebeverine high dose group (1.578) than the placebo group (1.704; p < 0.05), nevertheless, the authors were not able to determine if this difference was clinically significant [18].
3.3. Safety Assessment
Nineteen studies examined the prevalence and severity of adverse events after mebeverine treatment (Table S12). Generally, adverse events were rare and associated mainly with IBS symptoms. According to the authors’ opinion, serious adverse events were reported in three studies [24,26,34], although they were at a low prevalence (ranging from 1.8% to 8.6%) and, according to the authors’ opinion, were unlikely to be related to mebeverine.
4. Discussion
Despite its high prevalence, effective treatment for IBS remains challenging. Current guidelines recommend dietary and lifestyle modifications, as well as pharmacological therapies [4,39,40]. The treatment strategy should be based on the most predominant symptoms, patient preferences and expectations. Since IBS is a chronic condition with periods of exacerbation and remission of symptoms, treatment is long-lasting, and therapy outcomes vary between individuals.
Our systematic literature review results demonstrate that mebeverine is an effective and safe therapeutic option in patients with IBS. In the majority of patients included in trials, mebeverine therapy was associated with the reduction of diverse intestinal symptoms, including abdominal pain and discomfort, abdominal distension, abnormal or irregular bowel habits, bloating, and disturbances in stool frequency and consistency.
Mebeverine is an antispasmodic agent that works directly on intestinal smooth muscles and may also have a local anaesthetic effect and weak atropine-like activity. Current guidelines recommend antispasmodics as the drug of choice for IBS patients with a pain predominance [39,40,41]. Indeed, a systematic review of 26 randomised clinical trials (RCTs) including 2811 patients with IBS and 13 different antispasmodics showed significant improvement of IBS symptoms upon antispasmodic treatment compared to placebo (risk ratio [RR] of IBS symptoms not improving 0.65; 95% confidence interval [CI], 0.56–0.76; p < 0.00001; number needed to treat [NNT] 5; 95% CI, 4–8) [39]. However, antispasmodics are a heterogeneous group of drugs with different mechanisms of action. Currently, hyoscine and drotaverine are recommended for IBS treatment over the other antispasmodic agents [39,40]. Other drugs with confirmed efficacy in reducing IBS symptoms are otilonium, pinaverium, cimetropium, and dicyclomine.
A previous literature review indicated that mebeverine has no statistically significant beneficial effect on IBS symptoms (RR 1.18; 95% CI, 0.93–1.50) [39,40]. However, according to the authors, most trials included in the analysis were of poor quality, with a small sample size and significant heterogeneity in the results [40]. Indeed, only six studies totalling 351 patients treated with mebeverine were included in this previous systematic review. The latest meta-analysis published in 2010 also did not show any clinical improvement or reduction of abdominal pain in IBS patients after mebeverine treatment. These results are in contrast to our findings, which showed a beneficial effect of mebeverine on IBS symptoms. This difference may be caused, at least partially, by the inclusion of new studies in our review compared to the previous analysis (which was published almost ten years ago and only included six studies involving 279 patients treated with mebeverine). In addition, abdominal pain was a prevalent symptom in only one of these studies.
Our study added ten recently published articles (eight randomised and two retrospective observational studies), totalling 1945 patients treated with mebeverine. Abdominal pain and discomfort were evaluated in nineteen studies, including 1824 patients in the mebeverine arm. Six studies detected a significant decrease in the abdominal pain score from baseline, and eleven more studies showed a numerical improvement in the reduction of abdominal pain and discomfort after mebeverine (although statistical calculations were not available). Moreover, three studies showed a reduction of the pain frequency from baseline. Our results are similar to those reported by Poynard et al. [42], in which a meta-analysis of showed a significant reduction of pain upon smooth muscle relaxants including mebeverine compared to placebo (odds ratio 1.65; 95% Cl: 1.26–2.17).
Although pain is the major clinical manifestation of IBS, other symptoms are also common due to the multifaceted nature of the disease. Indeed, we showed that mebeverine also had a positive effect on other IBS symptoms, including improvements in abdominal distension, stool frequency, and abnormal bowel habits. These results are in accordance with a previous meta-analysis, which confirmed the beneficial effect of myorelaxants on abdominal distension (OR 1.46: 95% CI: 1.10–1.94, p = 0.008) and a significant global improvement (OR 2.04; 95% Cl: 1.15–3.63); however, antispasmodics had no effect on constipation or bowel transit time [42].
Our analysis included two studies comparing mebeverine with serotonin (5-HT3) receptor antagonists (ramosetron and alosetron), which are new drugs developed for patients with IBS without constipation [26,28,38]. Lee et al. showed mebeverine had comparable effectiveness to ramosetron in male patients with IBS-D [28,38]. Specifically, there were no differences in the global IBS symptoms, abdominal pain/discomfort, abnormal bowel habits, responder rates (37% vs. 38% on the intention-to-treat [ITT] analysis), or safety profiles between the two drugs [28,38]. Conversely, Jones et al. found alosetron was superior to mebeverine in terms of pain relief and improvement of bowel function in non-constipated females with IBS [26]. However, as alosetron is currently unavailable in many countries, mebeverine could still be useful, particularly in treating males and constipated females with IBS.
IBS has a substantial impact on health-related quality of life (QOL): the QOL of IBS patients is lower than that of diabetic patients, individuals suffering from end-stage kidney disease or those with gastroesophageal reflux disease [43]. An observational, prospective study of patients with IBS across four countries (Poland, Egypt, Mexico, and China) showed a significant improvement in IBS-related QOL after eight weeks of mebeverine treatment [14]. The observational study showed that mebeverine had beneficial effects on gastrointestinal symptoms, with a significant decrease in the severity of abdominal pain and discomfort and improvements in stool frequency and consistency, bloating, abdominal distension, and urgency [14].
Our systematic review also showed that mebeverine is a safe drug, with few adverse effects compared to placebo. These results are consistent with previous reports on the safety of this drug [9,44]. For example, the meta-analysis by Poynard et al. found 98% of IBS patients treated with mebeverine have no adverse effects (compared to 99% of patients in the placebo group) [42].
Our study has some limitations. First, current guidelines recommend the use of the Rome IV criteria for the diagnosis of IBS [5], and only three studies in our analysis used Rome IV criteria, while the others were performed on patients with bowel disorders who met older IBS diagnostic criteria (i.e., Rome I–III and other than Rome). Studies comparing the diagnostic IBS criteria (Manning, Rome I, Rome II, Rome III, and Rome IV) suggest Rome IV has a narrower IBS definition (Table S13 and Table S14); therefore, the Rome IV IBS population likely reflects a subgroup of Rome II and III IBS patients with more severe gastrointestinal symptomatology, psychological comorbidities, and lower QOL (Figure 2) [45,46,47,48,49,50,51]. However, there is a large group of patients with less severe intestinal symptoms who, despite fulfilling older Rome criteria, are not considered IBS patients. Hence, the therapeutic effect of mebeverine effect may be different for groups meeting varying diagnostic criteria.
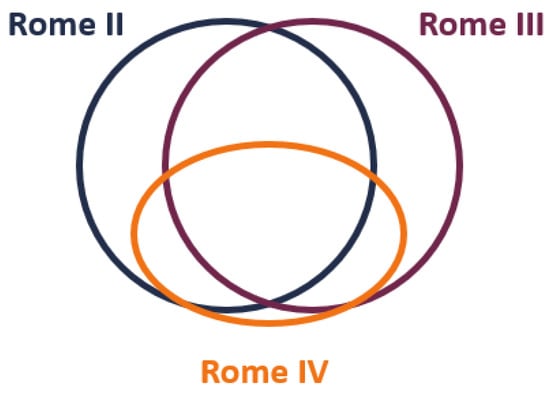
Figure 2.
Venn diagram for range and relationship between IBS diagnostic criteria Rome II, III and IV.
The second limitation is the difficulty in analysing heterogeneous studies with different patient characteristics, treatment durations, and endpoints. Fortunately, most studies included in our analysis were randomised trials with well-characterised outcomes. Our analysis is also limited by the inability to present results for each IBS subtype due to limited data. This particular concerns patients with IBS-C who were a minority in most of the included studies [16,23,24,26,29], so generalization of mebeverine benefits on this population is burdened with uncertainty.
Another limitation of this analysis is the lack of the direct comparison of mebeverine efficacy with placebo, which might be challenging, due to the substantial placebo effect and spontaneous improvement of symptoms in IBS. Pitz et al. showed in the previous meta-analysis that response on placebo in terms of abdominal pain ranges from 24% to 70%, with the mean value of 27.5% [52]. In the studies included in our systematic review, abdominal pain improvement during mebeverine treatment ranged from 23% to 96%, with an average value estimated at 53%, which means that the efficacy of mebeverine is about 25 percentage points higher in comparison with placebo. Furthermore, although we did not perform a formal meta-analysis, our literature search was detailed and followed PRISMA guidelines, and our final analysis included a large number of patients treated with mebeverine.
Despite our results showing that mebeverine may be considered an effective and safe treatment option for patients with IBS, the applicability of our study is limited. Mebeverine is not available in many countries, including the USA.
5. Conclusions
In conclusion, mebeverine is an effective treatment for a wide range of IBS patients who are suffering from abdominal pain and discomfort, distension, abnormal or irregular bowel habits, bloating, constipation and diarrhoea, but who do not necessarily fulfil the recent IBS criteria (Rome IV). We found that mebeverine has a good safety profile, with a low frequency of adverse effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11041044/s1, Table S1: Search strategy for MEDLINE (via Pubmed), Table S2: Search strategy for Embase, Table S3: Search strategy for Cochrane, Table S4: Risk of bias (RoB 2) in experimental studies, Table S5: Quality assessment for observational studies (the NICE checklist), Table S6: Summary of the results of mebeverine studies with respect to the symptoms associated with abdominal pain and discomfort, Table S7: Summary of the results of mebeverine studies with respect to the symptoms associated with abdominal distension, Table S8: Summary of the results of mebeverine studies with respect to the symptoms associated with abnormal bowel habits and bloating, Table S9: Summary of the results of mebeverine studies with respect to the symptoms associated with constipation and diarrhoea, Table S10: Summary of the results of mebeverine studies with respect to the symptoms associated with stool frequency and consistency, Table S11: Summary of the results of mebeverine studies with respect to the symptoms associated with nausea, anxiety and depression, Table S12: Summary of the safety results, Table S13: Comparison of various IBS diagnostic criteria, Table S14: Prevalence of IBS in patients diagnosed by different criteria.
Author Contributions
Conceptualization, G.R. and J.D.; methodology, G.R. and J.D.; formal analysis, E.M.-W. and J.D.; investigation, B.S.-R. and J.D.; writing—original draft preparation, G.R. and J.D.; writing—review and editing, B.S.-R. and E.M.-W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Mylan Healthcare Sp. z o. o. The company did not interfere with the process of data collection and interpretation or in the decision to submit the manuscript for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
J.D. has consulted for Mylan, has received speaker honoraria from Mylan, Ferring, and Takeda. E.M.-W. has received financial support through a grant from the Medical University of Lodz Department of Digestive Tract Diseases, has received compensation from Polpharma, Pfizer, Abbott for service as a consultant, has received payment for lectures including service on speakers bureaus from Janssen, Astellas, Varimed, Polpharma, and Krka, has received payment for the development of educational presentations from Sanofi-Aventis, and has received reimbursements for travel/accommodation/meeting expenses from Abbott and Varimed. Neither funding nor financial support was administered for the current study. B.S.-R. has consulted for Takeda, Sanprobi, Promed, Polfarma, Krka, Adamed, Biocodex, Jansen, Polfa Łódź, Alfasigma and declares no conflict of interests. G.R. has received speaker/advisory honoraria from Adamed, Alfa Sigma, Allergan, Celltrion, Ferring, Janssen, Pfizer, Polpharma, Sandoz, Sanofi, Takeda, Vitama. Neither funding nor financial support was administered for the current study.
References
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef]
- Moayyedi, P.; Mearin, F.; Azpiroz, F.; Andresen, V.; Barbara, G.; Corsetti, M.; Emmanuel, A.; Hungin, A.P.S.; Layer, P.; Stanghellini, V.; et al. Irritable bowel syndrome diagnosis and management: A simplified algorithm for clinical practice. United Eur. Gastroenterol. J. 2017, 5, 773–788. [Google Scholar] [CrossRef] [Green Version]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [Green Version]
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable bowel syndrome: A clinical review. JAMA 2015, 313, 949–958. [Google Scholar] [CrossRef]
- Annaházi, A.; Róka, R.; Rosztóczy, A.; Wittmann, T. Role of antispasmodics in the treatment of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 6031–6043. [Google Scholar] [CrossRef]
- De Groote, J.; Standaert, L. The effect of a new musculotropic subtance 9(Mebeverine) on irritable colon. Tijdschr. Gastroenterol. 1968, 11, 524–528. [Google Scholar]
- Darvish-Damavandi, M.; Nikfar, S.; Abdollahi, M. A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome. World J. Gastroenterol. 2010, 16, 547–553. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.; Savovic, J.; Elbers, R. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.0; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- NICE. Quality of Case Series Form. Available online: http://www.nice.org.uk/guidance/cg3/resources/appendix-4-quality-of-case-series-form2 (accessed on 1 January 2021).
- Guslandi, M. Mebeverine plus saccharomyces boularii versus mebeverine alone in the treatment of irritable bowel syndrome without constipation: A retrospective analysis. Am. J. Gastroenterol. 2011, 106, S495. [Google Scholar] [CrossRef]
- Hou, X.; Chen, S.; Zhang, Y.; Sha, W.; Yu, X.; El Sawah, H.; Afifi, A.F.; El-Khayat, H.R.; Nouh, A.; Hassan, M.F.; et al. Quality of life in patients with Irritable Bowel Syndrome (IBS), assessed using the IBS-Quality of Life (IBS-QOL) measure after 4 and 8 weeks of treatment with mebeverine hydrochloride or pinaverium bromide: Results of an international prospective observational cohort study in Poland, Egypt, Mexico and China. Clin. Drug Investig. 2014, 34, 783–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baume, P. Mebeverine, an effective agent in the irritable colon syndrome. Aust. N. Z. J. Med. 1972, 2, 34–36. [Google Scholar] [CrossRef]
- Everitt, H.; Moss-Morris, R.; Sibelli, A.; Tapp, L.; Coleman, N.; Yardley, L.; Smith, P.W.F.; Little, P. Management of irritable bowel syndrome in primary care: The results of an exploratory randomised controlled trial of mebeverine, methylcellulose, placebo and a self-management website. BMC Gastroenterol. 2013, 13, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, A.M. Physiological and clinical assessment of the effect of the musculotropic agent mebeverine on the human colon. Br. Med. J. 1965, 2, 848–851. [Google Scholar] [CrossRef] [Green Version]
- Prout, B.J. The treatment of irritable bowel syndrome. Two doses of mebeverine compared. Practitioner 1983, 227, 1607–1608. [Google Scholar]
- Kruis, W.; Weinzierl, M.; Schüssler, P.; Holl, J. Comparison of the therapeutic effect of wheat bran, mebeverine and placebo in patients with the irritable bowel syndrome. Digestion 1986, 34, 196–201. [Google Scholar] [CrossRef]
- Capurso, L.; Koch, M.; Tarquini, M.; Dezi, A.; Papi, C.; Fracasso, P. The irritable bowel syndrome. A cross-over study of octilonium bromide, mebeverine and placebo. Clin. Trials J. 1984, 21, 285–291. [Google Scholar]
- Chakraborty, D.S.; Hazra, A.; Sil, A.; Pain, S. Will controlled release mebeverine be able to surpass placebo in treatment of diarrhoea predominant irritable bowel syndrome? J. Fam. Med. Prim. Care 2019, 8, 3173–3178. [Google Scholar] [CrossRef]
- Schaffstein, W.; Panijel, M.; Luettecke, K. Comparative safety and efficacy of trimebutine versus mebeverine in the treatment of irritable bowel syndrome. A multicenter double-blind study. Curr. Res. Clin. Exp. 1990, 47, 136–145. [Google Scholar]
- Rahman, M.Z.; Ahmed, D.S.; Mahmuduzzaman, M.; Chowdhury, M.S.; Barua, R.; Ishaque, S.M. Comparative efficacy and safety of trimebutine versus mebeverine in the treatment of irritable bowel syndrome. Mymensingh Med. J. 2014, 23, 105–113. [Google Scholar] [PubMed]
- Chang, F.Y.; Lu, C.L.; Luo, J.C.; Chen, T.S.; Chen, M.J.; Chang, H.J. The evaluation of otilonium bromide treatment in Asian patients with irritable bowel syndrome. J. Neurogastroenterol. Motil. 2011, 17, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chen, C.; Chang, F.; Chang, S.; Kang, L.; Lu, R.; Lee, S. Effect of a calcium channel blocker and antispasmodic in diarrhoea-predominant irritable bowel syndrome. J. Gastroenterol. Hepatol. 2000, 15, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.H.; Holtmann, G.; Rodrigo, L.; Ehsanullah, R.S.; Crompton, P.M.; Jacques, L.A.; Mills, J.G. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment. Pharm. 1999, 13, 1419–1427. [Google Scholar] [CrossRef]
- Sahib, A.S. Treatment of irritable bowel syndrome using a selected herbal combination of Iraqi folk medicines. J. Ethnopharmacol. 2013, 148, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, N.Y.; Kwon, J.K.; Huh, K.C.; Lee, O.Y.; Lee, J.S.; Choi, S.C.; Sohn, C.I.; Myung, S.J.; Park, H.; et al. Efficacy of ramosetron in the treatment of male patients with irritable bowel syndrome with diarrhea: A multicenter, randomized clinical trial, compared with mebeverine. J. Neurogastroenterol. Motil. 2011, 23, 1098–1104. [Google Scholar] [CrossRef]
- Hatami, K.; Kazemi-Motlagh, A.H.; Ajdarkosh, H.; Zargaran, A.; Karimi, M.; Shamshiri, A.R.; Ghadir, M.R. Comparing the Efficacy of Cumin Sofouf With Mebeverine on Irritable Bowel Syndrome Severity and Quality of Life: A Double-blind Randomized Clinical Trial. Crescent. J. Med. Biol. Sci. 2020, 7, 186–194. [Google Scholar]
- Mokhtare, M.; Asadipanah, M.; Bahardoust, M.; Chaharmahali, A.; Sikaroudi, M.K.; Khoshdelnezamiha, M.; Davanloo, F.A.; Masoodi, M.; Bahadorizadeh, L. Efficacy of adding Luvos® Healing Earth supplementation to mebeverine in improving symptoms and quality of life of patients with diarrhea-predominant irritable bowel syndrome: A randomized clinical trial. Biomed. Res. 2018, 5, 2776–2783. [Google Scholar] [CrossRef]
- Tudor, G.J. A general practice study to compare alverine citrate with mebeverine hydrochloride in the treatment of irritable bowel syndrome. Br. J. Clin. Pract. 1986, 40, 276–278. [Google Scholar]
- Inauen, W.; Halter, F. Clinical Efficacy, Safety and Tolerance of Mebeverine Slow Release (200 mg) vs Mebeverine Tablets in Patients with Irritable Bowel Syndrome. Drug Investig. 1994, 8, 234–240. [Google Scholar] [CrossRef]
- Van Outryve, M.; Mayeur, S.; Meeus, M.A.; Rosillon, D.; Hendrickx, B.; Ceuppens, M. A double-blind crossover comparison study of the safety and efficacy of mebeverine with mebeverine sustained release in the treatment of irritable bowel syndrome. J. Clin. Pharm. 1995, 20, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Gilbody, J.S.; Fletcher, C.P.; Hughes, I.W.; Kidman, S.P. Comparison of two different formulations of mebeverine hydrochloride in irritable bowel syndrome. Int. J. Clin. Pract. 2000, 54, 461–464. [Google Scholar] [PubMed]
- Everitt, H.A.; Moss-Morris, R.E.; Sibelli, A.; Tapp, L.; Coleman, N.S.; Yardley, L.; Smith, P.W.; Little, P.S. Management of irritable bowel syndrome in primary care: Feasibility randomised controlled trial of mebeverine, methylcellulose, placebo and a patient self-management cognitive behavioural therapy website. (MIBS trial). BMC Gastroenterol. 2010, 10, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everitt, H. Management of Irritable Bowel Syndrome in Primary Care (MIBS Trial). ClinicalTrials.gov, Internet. Available online: https://clinicaltrials.gov/ct2/show/NCT00934973 (accessed on 17 June 2021).
- Clinicaltrialsregister.eu Internet. Management of Irritable Bowel Syndrome in Primary Care: Feasibility Randomised Controlled Trial of Mebeverine, Methylcellulose, Placebo and a Patient Self-Management Cognitive Behavioural Therapy Website. (MIBS Trial). Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2009-013426-16/GB (accessed on 17 June 2021).
- Lee, K.J.; Poong-Lyul, R. A Randomized, Open Labeled, Multicenter Clinical Trial on the Effectiveness and Safety of the 5-HT3-Receptor Antagonist Ramosetron in Male Patients with Irritable Bowel Syndrome With Diarrhea: Comparison With Mebeverine. Gastroenterology 2011, 140, S-06. [Google Scholar] [CrossRef]
- Moayyedi, P.; Andrews, C.N.; MacQueen, G.; Korownyk, C.; Marsiglio, M.; Graff, L.; Kvern, B.; Lazarescu, A.; Liu, L.; Paterson, W.G.; et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J. Can. Assoc. Gastroenterol. 2019, 2, 6–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrzak, A.; Skrzydło-Radomańska, B.; Mulak, A.; Lipiński, M.; Małecka-Panas, E.; Regula, J.; Rydzewska, G. Guidelines on the management of irritable bowel syndrome: In memory of Professor Witold Bartnik. Prz. Gastroenterol. 2018, 13, 259–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jailwala, J.; Imperiale, T.F.; Kroenke, K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann. Intern. Med. 2000, 133, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Regimbeau, C.; Benhamou, Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment. Pharm. 2001, 15, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Gralnek, I.M.; Hays, R.D.; Kilbourne, A.; Naliboff, B.; Mayer, E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. [Google Scholar] [CrossRef]
- Poynard, T.; Naveau, S.; Mory, B.; Chaput, J.C. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment. Pharm. 1994, 8, 499–510. [Google Scholar] [CrossRef]
- Vork, L.; Weerts, Z.Z.; Mujagic, Z.; Kruimel, J.W.; Hesselink, M.A.M.; Muris, J.; Keszthelyi, D.; Jonkers, D.M.A.E.; Masclee, A.A.M. Rome III vs Rome IV criteria for irritable bowel syndrome: A comparison of clinical characteristics in a large cohort study. J. Neurogastroenterol. Motil. 2018, 30, e13189. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Abraham, P.; Bhatia, S.J.; Misra, S.P.; Choudhuri, G.; Biswas, K.D.; Chakravartty, K.; Dadhich, S.; Goswami, B.D.; Jayanthi, V.; et al. Comparison of Manning, Rome I, II, and III, and Asian diagnostic criteria: Report of the Multicentric Indian Irritable Bowel Syndrome (MIIBS) study. Indian J. Gastroenterol. 2013, 32, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Shvartzman, P.; Friger, M.; Fich, A. A comparative reappraisal of the Rome II and Rome III diagnostic criteria: Are we getting closer to the ‘true’ prevalence of irritable bowel syndrome? Eur J. Gastroenterol. Hepatol. 2007, 19, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.W.; Lee, O.Y.; Shim, S.G.; Jun, D.W.; Lee, K.N.; Kim, H.Y.; Lee, H.L.; Yoon, B.C.; Choi, H.S. The Differences in Prevalence and Sociodemographic Characteristics of Irritable Bowel Syndrome According to Rome II and Rome III. J. Neurogastroenterol. Motil. 2010, 16, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Xia, J.; Jiang, Y.; Cao, H.; Zhao, Y.; Zhang, L.; Wang, H.; Song, J.; Hou, X. Comparison of the Rome IV and Rome III criteria for IBS diagnosis: A cross-sectional survey. J. Gastroenterol. Hepatol. 2017, 32, 1018–1025. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Thanapirom, K.; Gonlachanvit, S. Application of Rome III vs. Rome IV Diagnostic Criteria for Irritable Bowel Syndrome (IBS) in Clinical Practice: Is the Newer the Better? Gastroenterology 2017, 140, S717. [Google Scholar] [CrossRef]
- Pitz, N.; Cheang, M.; Bernstein, C. Defining the predictors of the placebo response in irritable bowel syndrome. Clin. Gastrol. Hepatol. 2005, 3, 237–247. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).