Changing Paradigms in the Diagnosis of Ischemic Heart Disease by Multimodality Imaging
Abstract
:1. Introduction
2. Last Updates Regarding Available Second-Line Non-Invasive Tests
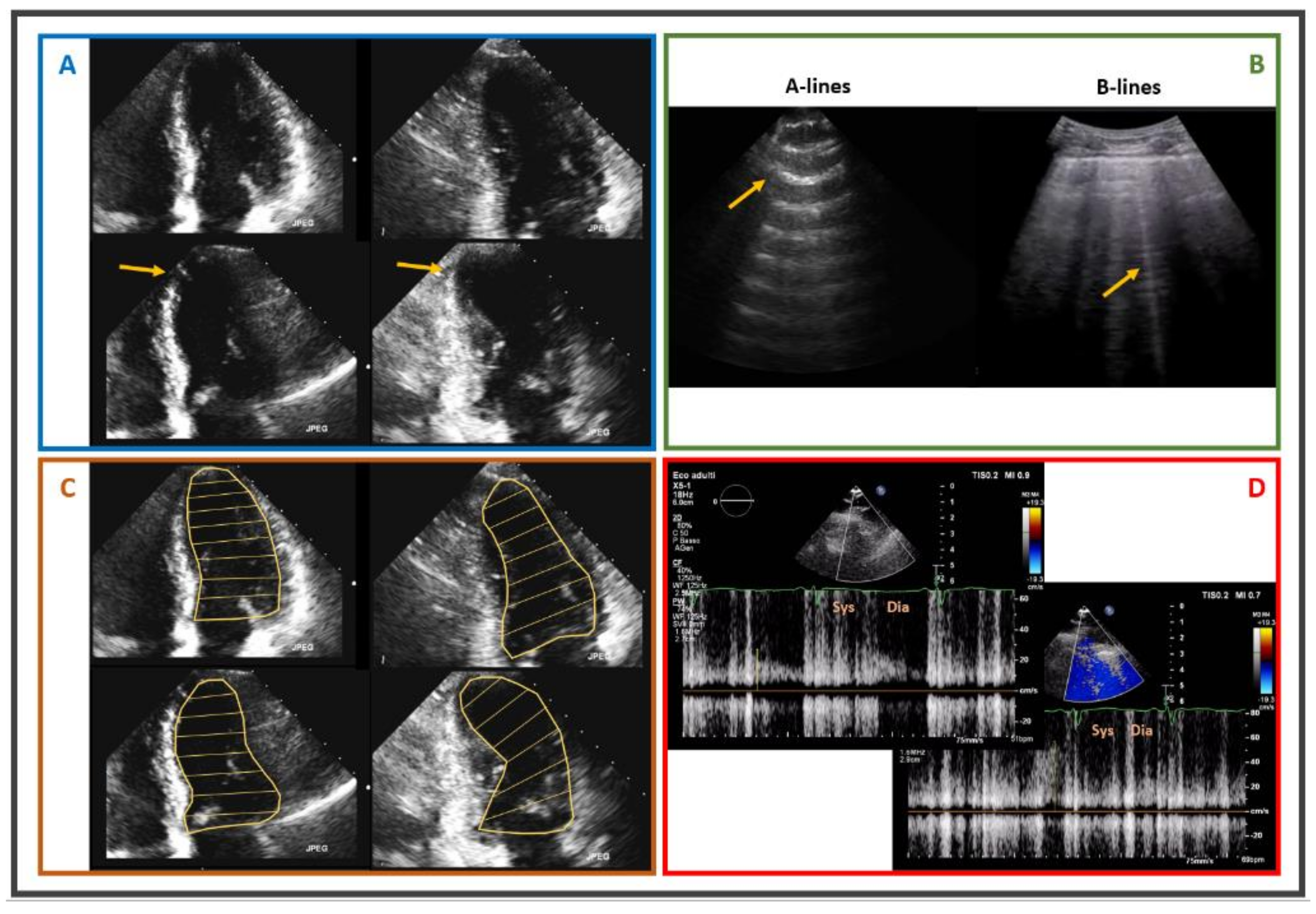
2.1. Stress Echocardiography
2.2. Single-Photon Emission Computed Tomography
2.3. Positron Emission Tomography
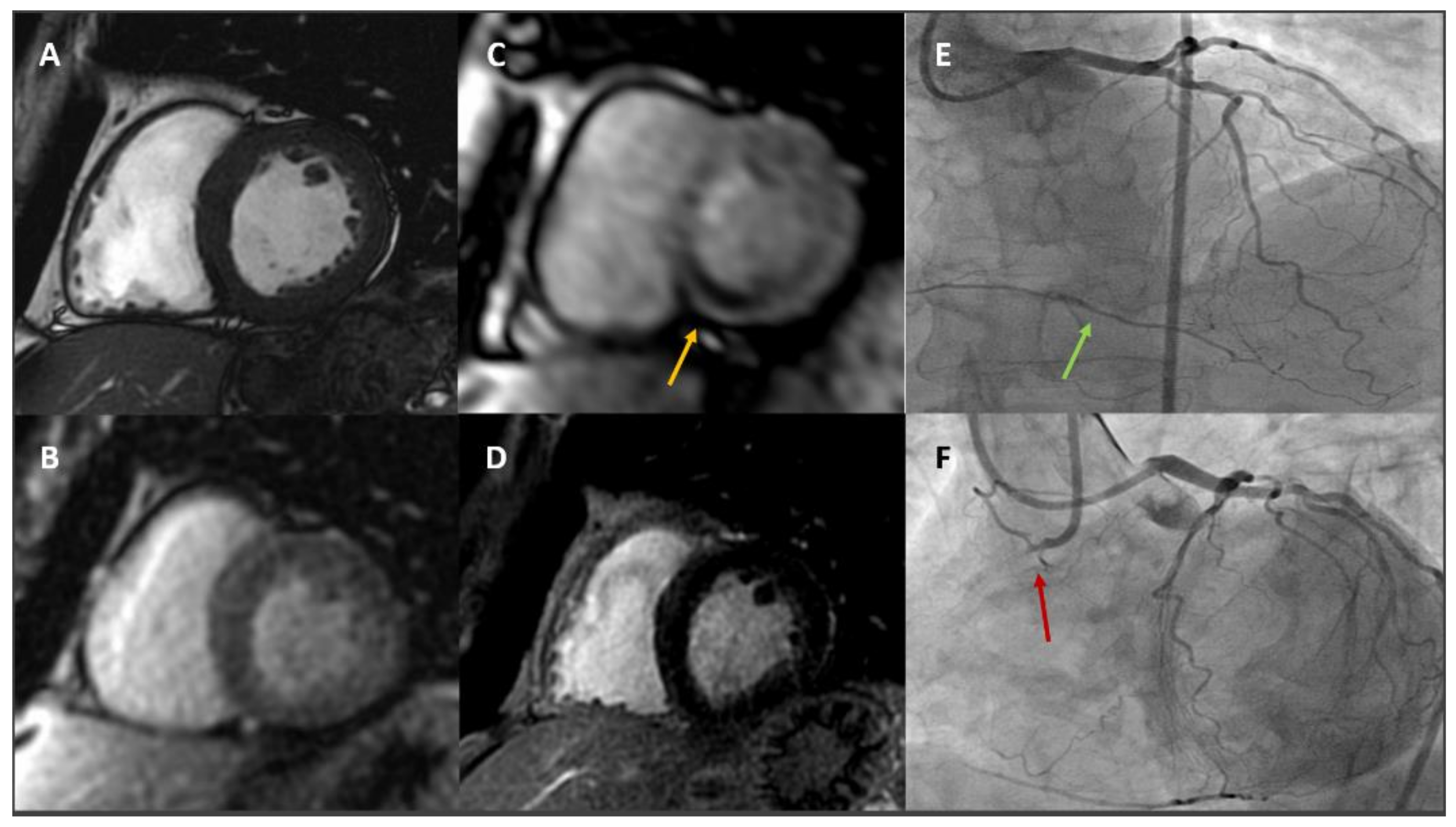
2.4. Cardiovascular Magnetic Resonance
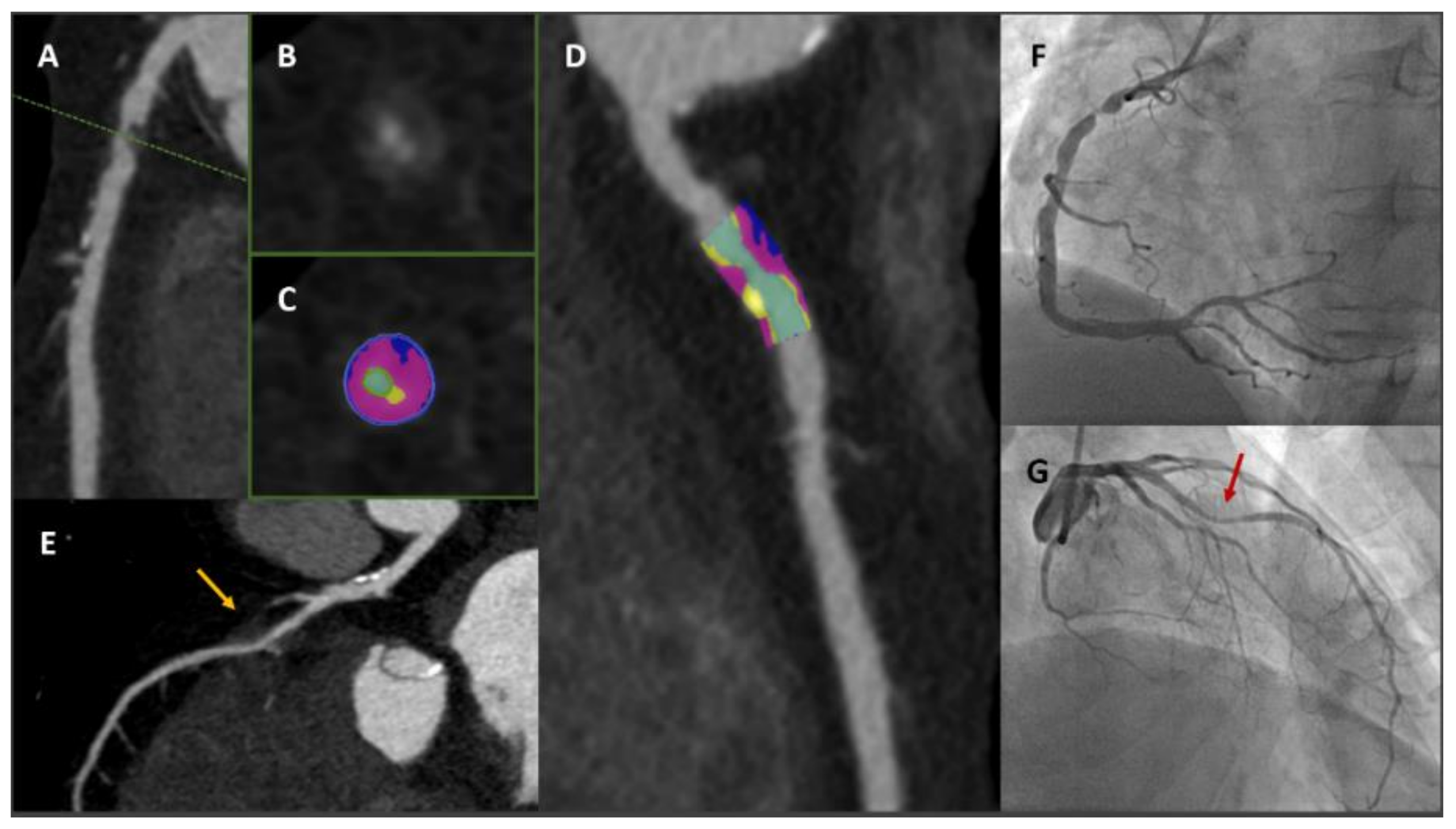
2.5. Cardiac Computed Tomography Angiography and Plaque Imaging
3. Function vs. Anatomy
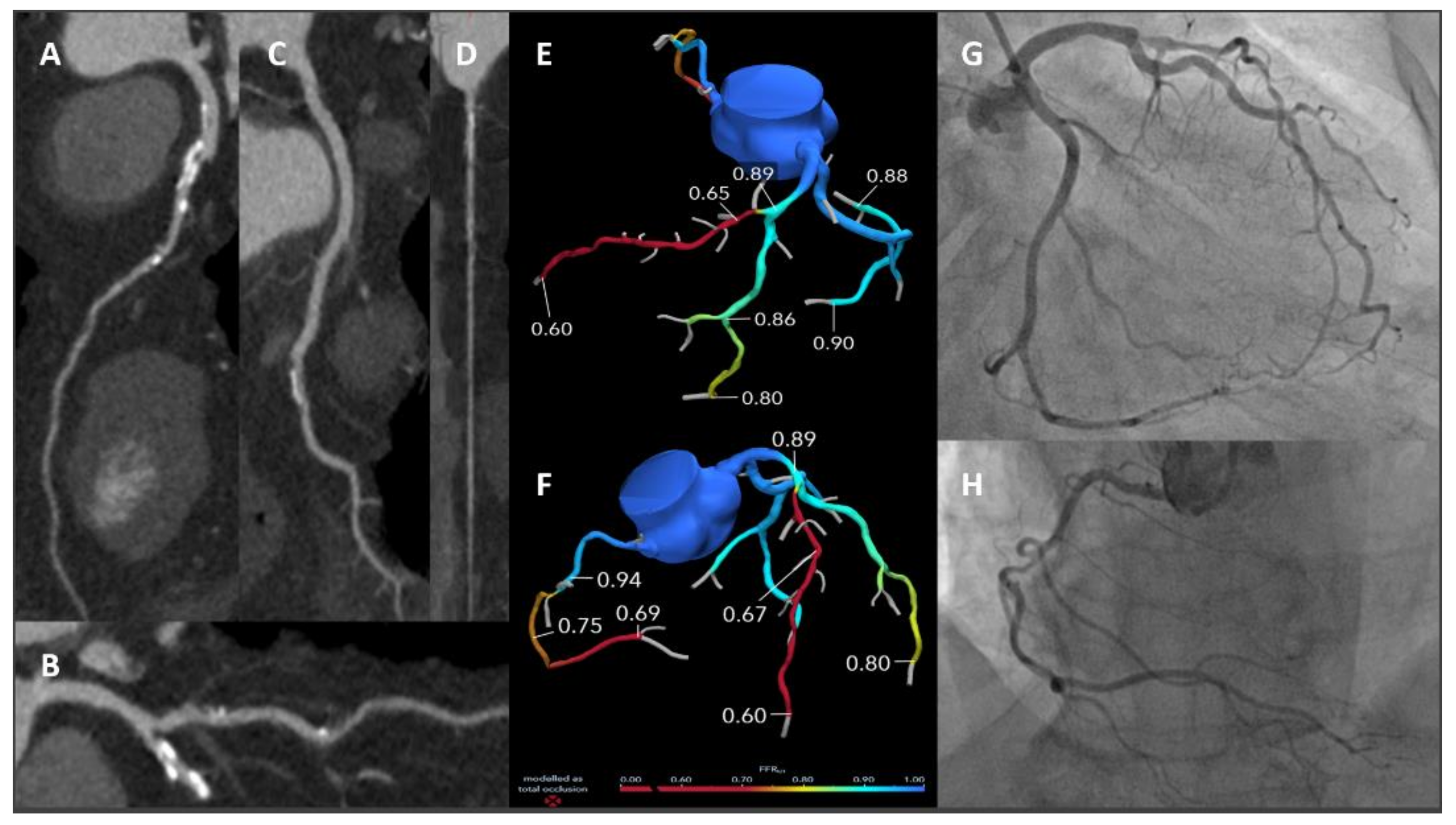
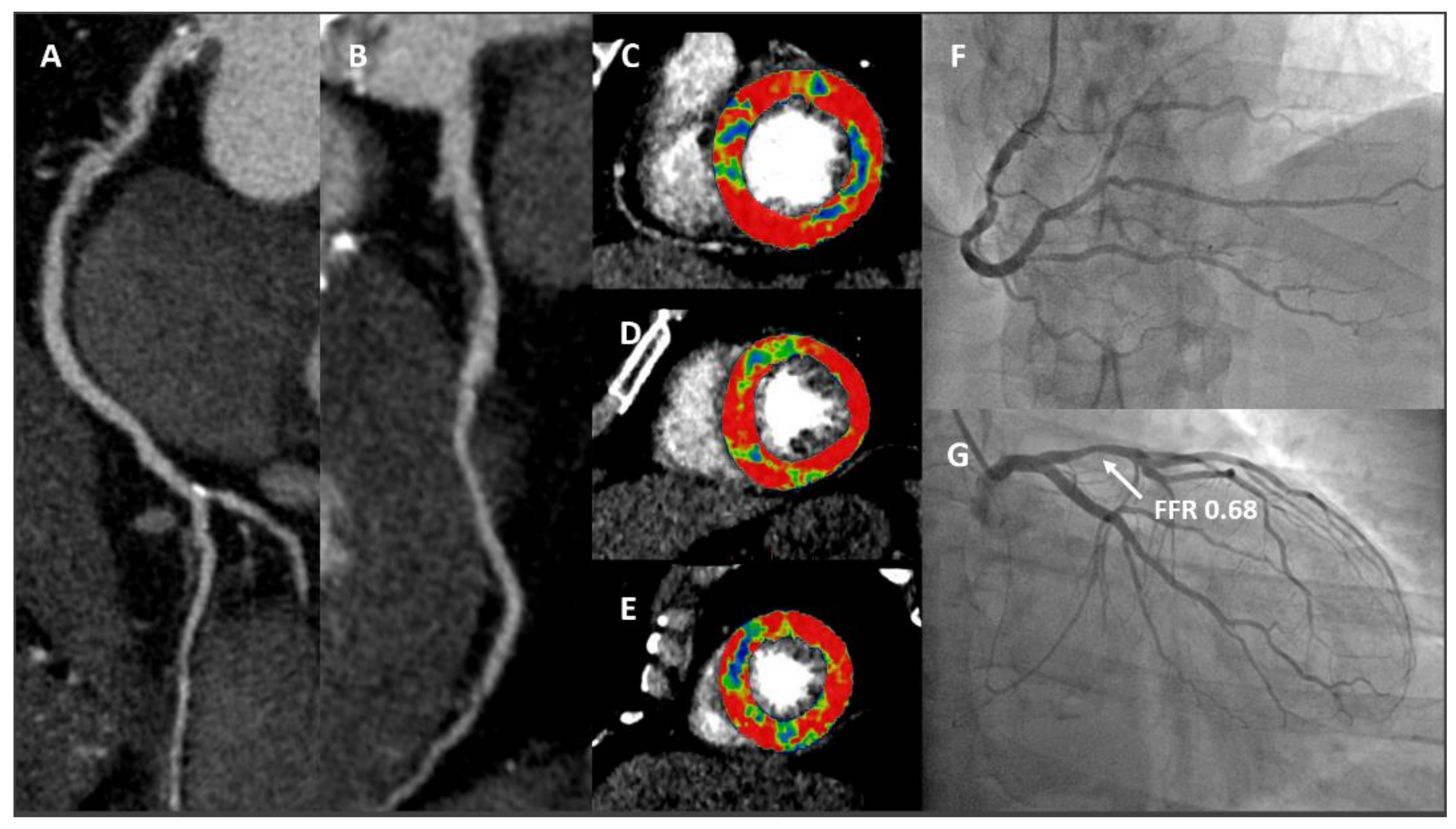
4. Function and Anatomy in a “One-Stop-Shop” Exam: CT-Derived Fractional Flow Reserve and CT Perfusion
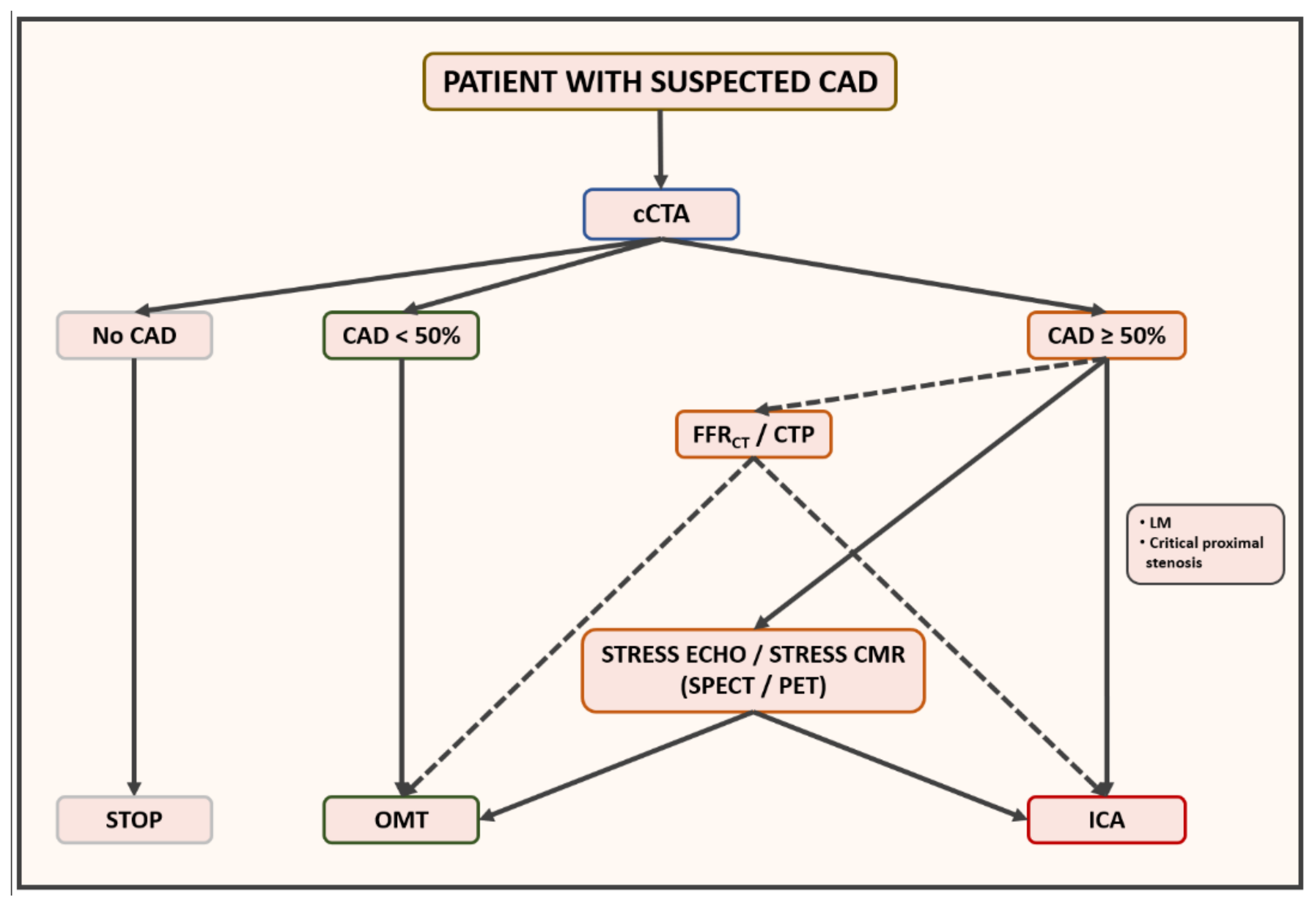
5. Proposed Management Algorithm for Patient with Suspected CAD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwartz, S.M.; deBlois, D.; O’Brien, E.R. The intima: Soil for atherosclerosis and restenosis. Circ. Res. 1995, 77, 445–465. [Google Scholar] [CrossRef]
- Lilly, L.S.; Braunwald, E. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012; Volume 2. [Google Scholar]
- Lee, S.E.; Chang, H.J.; Sung, J.M.; Park, H.B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J.; et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Stepinska, J.; Lettino, M.; Ahrens, I.; Bueno, H.; Garcia-Castrillo, L.; Khoury, A.; Lancellotti, P.; Mueller, C.; Muenzel, T.; Oleksiak, A.; et al. Diagnosis and risk stratification of chest pain patients in the emergency department: Focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 76–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chest Pain of Recent Onset: Assessment and Diagnosis. Clinical Guideline [CG95]. Published Date: March 2010; Last Updated: November 2016. Available online: https://www.nice.org.uk/guidance/cg95 (accessed on 16 October 2021).
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Foldyna, B.; Udelson, J.E.; Karady, J.; Banerji, D.; Lu, M.T.; Mayrhofer, T.; Bittner, D.O.; Meyersohn, N.M.; Emami, H.; Genders, T.S.S.; et al. Pretest probability for patients with suspected obstructive coronary artery disease: Re-evaluating Diamond-Forrester for the contemporary era and clinical implications: Insights from the PROMISE trial. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Cheng, V.Y.; Berman, D.S.; Rozanski, A.; Dunning, A.M.; Achenbach, S.; Al-Mallah, M.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H.J.; et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: Results from the multinational coronary CT angiography evaluation for clinical outcomes: An international multicenter registry (CONFIRM). Circulation 2011, 124, 2423–2432, 2421–2428. [Google Scholar] [CrossRef] [Green Version]
- Reeh, J.; Therming, C.B.; Heitmann, M.; Hojberg, S.; Sorum, C.; Bech, J.; Husum, D.; Dominguez, H.; Sehestedt, T.; Hermann, T.; et al. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur. Heart J. 2019, 40, 1426–1435. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Balady, G.J.; Bricker, J.T.; Chaitman, B.R.; Fletcher, G.F.; Froelicher, V.F.; Mark, D.B.; McCallister, B.D.; Mooss, A.N.; O’Reilly, M.G.; et al. ACC/AHA 2002 guideline update for exercise testing: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002, 106, 1883–1892. [Google Scholar] [CrossRef]
- Banerjee, A.; Newman, D.R.; Van den Bruel, A.; Heneghan, C. Diagnostic accuracy of exercise stress testing for coronary artery disease: A systematic review and meta-analysis of prospective studies. Int. J. Clin. Pract 2012, 66, 477–492. [Google Scholar] [CrossRef]
- Shaikh, K.; Budoff, M.J. Diagnostic Accuracy of Exercise Electrocardiogram in Women. J. Womens Health 2018, 27, 411–412. [Google Scholar] [CrossRef] [Green Version]
- Kwok, J.M.; Miller, T.D.; Christian, T.F.; Hodge, D.O.; Gibbons, R.J. Prognostic value of a treadmill exercise score in symptomatic patients with nonspecific ST-T abnormalities on resting ECG. JAMA 1999, 282, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Juni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.U.; Zamorano, J.L.; European Association of Echocardiography. Stress Echocardiography Expert Consensus Statement--Executive Summary: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur. Heart J. 2009, 30, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaibazzi, N.; Tuttolomondo, D.; Guaricci, A.I.; De Marco, F.; Pontone, G. Stress-echocardiography or coronary computed tomography in suspected chronic coronary syndrome after the 2019 European Guidelines? A practical guide. J. Cardiovasc. Med. 2021, 23, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Molinaro, S.; Pasanisi, E. The diagnostic accuracy of pharmacological stress echocardiography for the assessment of coronary artery disease: A meta-analysis. Cardiovasc. Ultrasound 2008, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Metz, L.D.; Beattie, M.; Hom, R.; Redberg, R.F.; Grady, D.; Fleischmann, K.E. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: A meta-analysis. J. Am. Coll. Cardiol. 2007, 49, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Sozzi, F.B.; Elhendy, A.; Rizzello, V.; Biagini, E.; van Domburg, R.T.; Schinkel, A.F.; Bax, J.J.; Vourvouri, E.; Danzi, G.B.; Poldermans, D. Prognostic significance of myocardial ischemia during dobutamine stress echocardiography in asymptomatic patients with diabetes mellitus and no prior history of coronary events. Am. J. Cardiol. 2007, 99, 1193–1195. [Google Scholar] [CrossRef]
- Sicari, R.; Cortigiani, L.; Bigi, R.; Landi, P.; Raciti, M.; Picano, E.; Echo-Dobutamine International Cooperative Study Groups. Prognostic value of pharmacological stress echocardiography is affected by concomitant antiischemic therapy at the time of testing. Circulation 2004, 109, 2428–2431. [Google Scholar] [CrossRef]
- Pontone, G.; Guaricci, A.I.; Palmer, S.C.; Andreini, D.; Verdecchia, M.; Fusini, L.; Lorenzoni, V.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; et al. Diagnostic performance of non-invasive imaging for stable coronary artery disease: A meta-analysis. Int. J. Cardiol. 2020, 300, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Ciampi, Q.; Wierzbowska-Drabik, K.; Urluescu, M.L.; Morrone, D.; Carpeggiani, C. The new clinical standard of integrated quadruple stress echocardiography with ABCD protocol. Cardiovasc. Ultrasound 2018, 16, 22. [Google Scholar] [CrossRef]
- Picano, E.; Scali, M.C. The lung water cascade in heart failure. Echocardiography 2017, 34, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Cortigiani, L.; Bombardini, T.; Corbisiero, A.; Mazzoni, A.; Bovenzi, F.; Picano, E. The additive prognostic value of end-systolic pressure-volume relation in patients with diabetes mellitus having negative dobutamine stress echocardiography by wall motion criteria. Heart 2009, 95, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Bombardini, T.; Gherardi, S.; Marraccini, P.; Schlueter, M.C.; Sicari, R.; Picano, E. The incremental diagnostic value of coronary flow reserve and left ventricular elastance during high-dose dipyridamole stress echocardiography in patients with normal wall motion at rest. Int. J. Cardiol. 2013, 168, 1683–1684. [Google Scholar] [CrossRef]
- Cortigiani, L.; Rigo, F.; Gherardi, S.; Bovenzi, F.; Molinaro, S.; Picano, E.; Sicari, R. Coronary flow reserve during dipyridamole stress echocardiography predicts mortality. JACC Cardiovasc. Imaging 2012, 5, 1079–1085. [Google Scholar] [CrossRef] [Green Version]
- Picano, E.; Zagatina, A.; Wierzbowska-Drabik, K.; Borguezan Daros, C.; D’Andrea, A.; Ciampi, Q. Sustainability and Versatility of the ABCDE Protocol for Stress Echocardiography. J. Clin. Med. 2020, 9, 3184. [Google Scholar] [CrossRef]
- Elhendy, A.; Mahoney, D.W.; Khandheria, B.K.; Burger, K.; Pellikka, P.A. Prognostic significance of impairment of heart rate response to exercise: Impact of left ventricular function and myocardial ischemia. J. Am. Coll. Cardiol. 2003, 42, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Ciampi, Q.; Zagatina, A.; Cortigiani, L.; Wierzbowska-Drabik, K.; Kasprzak, J.D.; Haberka, M.; Djordjevic-Dikic, A.; Beleslin, B.; Boshchenko, A.; Ryabova, T.; et al. Prognostic value of stress echocardiography assessed by the ABCDE protocol. Eur. Heart J. 2021, 42, 3869–3878. [Google Scholar] [CrossRef]
- Cantoni, V.; Green, R.; Acampa, W.; Zampella, E.; Assante, R.; Nappi, C.; Gaudieri, V.; Mannarino, T.; Cuocolo, R.; Di Vaia, E.; et al. Diagnostic performance of myocardial perfusion imaging with conventional and CZT single-photon emission computed tomography in detecting coronary artery disease: A meta-analysis. J. Nucl. Cardiol. 2021, 28, 698–715. [Google Scholar] [CrossRef]
- Green, R.; Cantoni, V.; Petretta, M.; Acampa, W.; Panico, M.; Buongiorno, P.; Punzo, G.; Salvatore, M.; Cuocolo, A. Negative predictive value of stress myocardial perfusion imaging and coronary computed tomography angiography: A meta-analysis. J. Nucl. Cardiol. 2018, 25, 1588–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Cai, F.; Geng, C.; Wang, Z.; Tang, X. Diagnostic Performance of CMR, SPECT, and PET Imaging for the Identification of Coronary Artery Disease: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 621389. [Google Scholar] [CrossRef]
- Slomka, P.J.; Betancur, J.; Liang, J.X.; Otaki, Y.; Hu, L.H.; Sharir, T.; Dorbala, S.; Di Carli, M.; Fish, M.B.; Ruddy, T.D.; et al. Rationale and design of the REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT). J. Nucl Cardiol. 2020, 27, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Imbert, L.; Marie, P.Y. CZT cameras: A technological jump for myocardial perfusion SPECT. J. Nucl. Cardiol. 2016, 23, 894–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panjer, M.; Dobrolinska, M.; Wagenaar, N.R.L.; Slart, R. Diagnostic accuracy of dynamic CZT-SPECT in coronary artery disease. A systematic review and meta-analysis. J. Nucl. Cardiol. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Roule, V.; Nganoa, C.; Roth, N.; Baavour, R.; Parienti, J.J.; Beygui, F.; Manrique, A. First validation of myocardial flow reserve assessed by dynamic (99m)Tc-sestamibi CZT-SPECT camera: Head to head comparison with (15)O-water PET and fractional flow reserve in patients with suspected coronary artery disease. The WATERDAY study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1079–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giubbini, R.; Bertoli, M.; Durmo, R.; Bonacina, M.; Peli, A.; Faggiano, I.; Albano, D.; Milan, E.; Stern, E.; Paghera, B.; et al. Comparison between N(13)NH3-PET and (99m)Tc-Tetrofosmin-CZT SPECT in the evaluation of absolute myocardial blood flow and flow reserve. J. Nucl. Cardiol. 2021, 28, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.M.; Poitrasson-Riviere, A.; Hagio, T.; Moody, J.B.; Arida-Moody, L.; Ficaro, E.P.; Murthy, V.L. Myocardial flow reserve estimation with contemporary CZT-SPECT and (99m)Tc-tracers lacks precision for routine clinical application. J. Nucl. Cardiol. 2021. [Google Scholar] [CrossRef]
- Zavadovsky, K.V.; Mochula, A.V.; Maltseva, A.N.; Shipulin, V.V.; Sazonova, S.I.; Gulya, M.O.; Liga, R.; Gimelli, A. The current status of CZT SPECT myocardial blood flow and reserve assessment: Tips and tricks. J. Nucl. Cardiol. 2021. [Google Scholar] [CrossRef]
- Nammas, W.; Maaniitty, T.; Knuuti, J.; Saraste, A. Cardiac perfusion by positron emission tomography. Clin. Physiol. Funct. Imaging 2021, 41, 385–400. [Google Scholar] [CrossRef]
- Kajander, S.; Joutsiniemi, E.; Saraste, M.; Pietila, M.; Ukkonen, H.; Saraste, A.; Sipila, H.T.; Teras, M.; Maki, M.; Airaksinen, J.; et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation 2010, 122, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Danad, I.; Uusitalo, V.; Kero, T.; Saraste, A.; Raijmakers, P.G.; Lammertsma, A.A.; Heymans, M.W.; Kajander, S.A.; Pietila, M.; James, S.; et al. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: Cutoff values and diagnostic accuracy of quantitative [(15)O]H2O PET imaging. J. Am. Coll. Cardiol. 2014, 64, 1464–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, S.; Dias, A.H.; Lauritsen, K.M.; Bouchelouche, K.; Tolbod, L.P.; Gormsen, L.C. Myocardial Viability Testing by Positron Emission Tomography: Basic Concepts, Mini-Review of the Literature and Experience From a Tertiary PET Center. Semin. Nucl. Med. 2020, 50, 248–259. [Google Scholar] [CrossRef]
- Khalaf, S.; Chamsi-Pasha, M.; Al-Mallah, M.H. Assessment of myocardial viability by PET. Curr. Opin. Cardiol. 2019, 34, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Al Moudi, M.; Sun, Z.H. Diagnostic value of (18)F-FDG PET in the assessment of myocardial viability in coronary artery disease: A comparative study with (99m)Tc SPECT and echocardiography. J. Geriatr. Cardiol. 2014, 11, 229–236. [Google Scholar] [CrossRef]
- van Waardhuizen, C.N.; Khanji, M.Y.; Genders, T.S.S.; Ferket, B.S.; Fleischmann, K.E.; Hunink, M.G.M.; Petersen, S.E. Comparative cost-effectiveness of non-invasive imaging tests in patients presenting with chronic stable chest pain with suspected coronary artery disease: A systematic review. Eur. Heart J. Qual Care Clin. Outcomes 2016, 2, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Filho, O.R.; Rickers, C.; Kwong, R.Y.; Jerosch-Herold, M. MR myocardial perfusion imaging. Radiology 2013, 266, 701–715. [Google Scholar] [CrossRef]
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N. Engl. J. Med. 2019, 380, 2418–2428. [Google Scholar] [CrossRef]
- Baritussio, A.; Scatteia, A.; Dellegrottaglie, S.; Bucciarelli-Ducci, C. Evidence and Applicability of Stress Cardiovascular Magnetic Resonance in Detecting Coronary Artery Disease: State of the Art. J. Clin. Med. 2021, 10, 3279. [Google Scholar] [CrossRef] [PubMed]
- Baessato, F.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; Fusini, L.; Scafuri, S.; Babbaro, M.; Mollace, R.; Collevecchio, A.; Guaricci, A.I.; et al. Stress CMR in Known or Suspected CAD: Diagnostic and Prognostic Role. Biomed. Res. Int. 2021, 2021, 6678029. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Bertella, E.; Loguercio, M.; Guglielmo, M.; Baggiano, A.; Aquaro, G.D.; Mushtaq, S.; Salerni, S.; Gripari, P.; et al. Prognostic value of dipyridamole stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: A mid-term follow-up study. Eur. Radiol. 2016, 26, 2155–2165. [Google Scholar] [CrossRef]
- Antiochos, P.; Ge, Y.; Heydari, B.; Steel, K.; Bingham, S.; Abdullah, S.M.; Mikolich, J.R.; Arai, A.E.; Bandettini, W.P.; Patel, A.R.; et al. Prognostic Value of Stress Cardiac Magnetic Resonance in Patients With Known Coronary Artery Disease. JACC Cardiovasc. Imaging 2021, 15, 60–71. [Google Scholar] [CrossRef]
- Pezel, T.; Hovasse, T.; Sanguineti, F.; Kinnel, M.; Garot, P.; Champagne, S.; Toupin, S.; Unterseeh, T.; Garot, J. Long-Term Prognostic Value of Stress CMR in Patients With Heart Failure and Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2021, 14, 2319–2333. [Google Scholar] [CrossRef]
- Heiberg, J.; Asschenfeldt, B.; Maagaard, M.; Ringgaard, S. Dynamic bicycle exercise to assess cardiac output at multiple exercise levels during magnetic resonance imaging. Clin. Imaging 2017, 46, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Craven, T.P.; Jex, N.; Chew, P.G.; Higgins, D.M.; Bissell, M.M.; Brown, L.A.E.; Saunderson, C.E.D.; Das, A.; Chowdhary, A.; Dall’Armellina, E.; et al. Exercise cardiovascular magnetic resonance: Feasibility and development of biventricular function and great vessel flow assessment, during continuous exercise accelerated by Compressed SENSE: Preliminary results in healthy volunteers. Int. J. Cardiovasc. Imaging 2021, 37, 685–698. [Google Scholar] [CrossRef]
- Heidary, S.; Patel, H.; Chung, J.; Yokota, H.; Gupta, S.N.; Bennett, M.V.; Katikireddy, C.; Nguyen, P.; Pauly, J.M.; Terashima, M.; et al. Quantitative tissue characterization of infarct core and border zone in patients with ischemic cardiomyopathy by magnetic resonance is associated with future cardiovascular events. J. Am. Coll. Cardiol. 2010, 55, 2762–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, R.Y.; Ge, Y.; Steel, K.; Bingham, S.; Abdullah, S.; Fujikura, K.; Wang, W.; Pandya, A.; Chen, Y.Y.; Mikolich, J.R.; et al. Cardiac Magnetic Resonance Stress Perfusion Imaging for Evaluation of Patients With Chest Pain. J. Am. Coll. Cardiol. 2019, 74, 1741–1755. [Google Scholar] [CrossRef]
- Kotecha, T.; Chacko, L.; Chehab, O.; O’Reilly, N.; Martinez-Naharro, A.; Lazari, J.; Knott, K.D.; Brown, J.; Knight, D.; Muthurangu, V.; et al. Assessment of Multivessel Coronary Artery Disease Using Cardiovascular Magnetic Resonance Pixelwise Quantitative Perfusion Mapping. JACC Cardiovasc. Imaging 2020, 13, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Monteagudo, J.M.; Martinez-Naharro, A.; Chacko, L.; Brown, J.; Knight, D.; Knott, K.D.; Hawkins, P.; Moon, J.C.; Plein, S.; et al. Quantitative cardiovascular magnetic resonance myocardial perfusion mapping to assess hyperaemic response to adenosine stress. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 273–281. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Quaglia, C.; Ballerini, G.; Nobili, E.; Pepi, M. Accuracy of multidetector spiral computed tomography in detecting significant coronary stenosis in patient populations with differing pre-test probabilities of disease. Clin. Radiol. 2007, 62, 978–985. [Google Scholar] [CrossRef]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Pontone, G.; Andreini, D.; Bartorelli, A.L.; Bertella, E.; Mushtaq, S.; Foti, C.; Formenti, A.; Chiappa, L.; Annoni, A.; Cortinovis, S.; et al. Feasibility and diagnostic accuracy of a low radiation exposure protocol for prospective ECG-triggering coronary MDCT angiography. Clin. Radiol. 2012, 67, 207–215. [Google Scholar] [CrossRef]
- Haase, R.; Schlattmann, P.; Gueret, P.; Andreini, D.; Pontone, G.; Alkadhi, H.; Hausleiter, J.; Garcia, M.J.; Leschka, S.; Meijboom, W.B.; et al. Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: Meta-analysis of individual patient data. BMJ 2019, 365, l1945. [Google Scholar] [CrossRef] [Green Version]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- Maurovich-Horvat, P.; Hoffmann, U.; Vorpahl, M.; Nakano, M.; Virmani, R.; Alkadhi, H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc. Imaging 2010, 3, 440–444. [Google Scholar] [CrossRef] [Green Version]
- Motoyama, S.; Sarai, M.; Harigaya, H.; Anno, H.; Inoue, K.; Hara, T.; Naruse, H.; Ishii, J.; Hishida, H.; Wong, N.D.; et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 2009, 54, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narula, J.; Nakano, M.; Virmani, R.; Kolodgie, F.D.; Petersen, R.; Newcomb, R.; Malik, S.; Fuster, V.; Finn, A.V. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J. Am. Coll. Cardiol. 2013, 61, 1041–1051. [Google Scholar] [CrossRef] [Green Version]
- Creager, M.D.; Hohl, T.; Hutcheson, J.D.; Moss, A.J.; Schlotter, F.; Blaser, M.C.; Park, M.A.; Lee, L.H.; Singh, S.A.; Alcaide-Corral, C.J.; et al. (18)F-Fluoride Signal Amplification Identifies Microcalcifications Associated With Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ. Cardiovasc. Imaging 2019, 12, e007835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, U.; Moselewski, F.; Nieman, K.; Jang, I.K.; Ferencik, M.; Rahman, A.M.; Cury, R.C.; Abbara, S.; Joneidi-Jafari, H.; Achenbach, S.; et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J. Am. Coll Cardiol. 2006, 47, 1655–1662. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.J.; Lin, F.Y.; Lee, S.E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.W.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, U.; Ferencik, M.; Udelson, J.E.; Picard, M.H.; Truong, Q.A.; Patel, M.R.; Huang, M.; Pencina, M.; Mark, D.B.; Heitner, J.F.; et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017, 135, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.V.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Choi, G.; Koo, B.K.; Hwang, D.; Park, J.; Zhang, J.; Kim, K.J.; Tong, Y.; Kim, H.J.; Grady, L.; et al. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc. Imaging 2019, 12, 1032–1043. [Google Scholar] [CrossRef]
- van Rosendael, A.R.; van den Hoogen, I.J.; Gianni, U.; Ma, X.; Tantawy, S.W.; Bax, A.M.; Lu, Y.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; et al. Association of Statin Treatment With Progression of Coronary Atherosclerotic Plaque Composition. JAMA Cardiol. 2021, 6, 1257–1266. [Google Scholar] [CrossRef]
- van Rosendael, A.R.; Narula, J.; Lin, F.Y.; van den Hoogen, I.J.; Gianni, U.; Al Hussein Alawamlh, O.; Dunham, P.C.; Pena, J.M.; Lee, S.E.; Andreini, D.; et al. Association of High-Density Calcified 1K Plaque With Risk of Acute Coronary Syndrome. JAMA Cardiol. 2020, 5, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Madaj, P.; Li, D.; Nakanishi, R.; Andreini, D.; Pontone, G.; Conte, E.; O’Rourke Franzcr, R.; Hamilton-Craig, C.; Nimmagadda, M.; Kim, N.; et al. Lower Radiation Dosing in Cardiac CT Angiography: The CONVERGE Registry. J. Nucl. Med. Technol. 2020, 48, 58–62. [Google Scholar] [CrossRef]
- Pontone, G.; Bertella, E.; Mushtaq, S.; Loguercio, M.; Cortinovis, S.; Baggiano, A.; Conte, E.; Annoni, A.; Formenti, A.; Beltrama, V.; et al. Coronary artery disease: Diagnostic accuracy of CT coronary angiography--a comparison of high and standard spatial resolution scanning. Radiology 2014, 271, 688–694. [Google Scholar] [CrossRef]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguade-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef] [Green Version]
- Neglia, D.; Liga, R.; Caselli, C.; Carpeggiani, C.; Lorenzoni, V.; Sicari, R.; Lombardi, M.; Gaemperli, O.; Kaufmann, P.A.; Scholte, A.; et al. Anatomical and functional coronary imaging to predict long-term outcome in patients with suspected coronary artery disease: The EVINCI-outcome study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1273–1282. [Google Scholar] [CrossRef]
- Lorenzoni, V.; Bellelli, S.; Caselli, C.; Knuuti, J.; Underwood, S.R.; Neglia, D.; Turchetti, G.; Giuseppe Turchetti For the EVINCI Investigators. Cost-effectiveness analysis of stand-alone or combined non-invasive imaging tests for the diagnosis of stable coronary artery disease: Results from the EVINCI study. Eur. J. Health Econ. 2019, 20, 1437–1449. [Google Scholar] [CrossRef] [Green Version]
- The SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): An open-label, parallel-group, multicentre trial. Lancet 2015, 385, 2383–2391. [Google Scholar] [CrossRef] [Green Version]
- The SCOT-HEART Investigators; Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.D.; Williams, M.C.; Dweck, M.R.; Mills, N.L.; Boon, N.A.; Daghem, M.; Bing, R.; Moss, A.J.; Mangion, K.; Flather, M.; et al. Guiding Therapy by Coronary CT Angiography Improves Outcomes in Patients With Stable Chest Pain. J. Am. Coll. Cardiol. 2019, 74, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; Lopez-Sendon, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Shaw, L.J.; Min, J.K.; Page, C.B.; Berman, D.S.; Chaitman, B.R.; Picard, M.H.; Kwong, R.Y.; O’Brien, S.M.; Huang, Z.; et al. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation 2021, 144, 1024–1038. [Google Scholar] [CrossRef]
- van Rosendael, A.R.; Shaw, L.J.; Xie, J.X.; Dimitriu-Leen, A.C.; Smit, J.M.; Scholte, A.J.; van Werkhoven, J.M.; Callister, T.Q.; DeLago, A.; Berman, D.S.; et al. Superior Risk Stratification With Coronary Computed Tomography Angiography Using a Comprehensive Atherosclerotic Risk Score. JACC Cardiovasc. Imaging 2019, 12, 1987–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne-Grinter, M.; Kwiecinski, J.; Doris, M.; McElhinney, P.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; Shah, A.S.V.; et al. Association of coronary artery calcium score with qualitatively and quantitatively assessed adverse plaque on coronary CT angiography in the SCOT-HEART trial. Eur. Heart J. Cardiovasc. Imaging 2021. [Google Scholar] [CrossRef]
- Yang, S.; Koo, B.K.; Hwang, D.; Zhang, J.; Hoshino, M.; Lee, J.M.; Murai, T.; Park, J.; Shin, E.S.; Doh, J.H.; et al. High-Risk Morphological and Physiological Coronary Disease Attributes as Outcome Markers After Medical Treatment and Revascularization. JACC Cardiovasc. Imaging 2021, 14, 1977–1989. [Google Scholar] [CrossRef]
- Taylor, C.A.; Fonte, T.A.; Min, J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: Scientific basis. J. Am. Coll. Cardiol. 2013, 61, 2233–2241. [Google Scholar] [CrossRef] [Green Version]
- Ball, C.; Pontone, G.; Rabbat, M. Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Datasets: The Next Frontier in Noninvasive Assessment of Coronary Artery Disease. Biomed. Res. Int. 2018, 2018, 2680430. [Google Scholar] [CrossRef]
- Pontone, G.; Muscogiuri, G.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Mushtaq, S.; Baggiano, A.; Conte, E.; Beltrama, V.; Annoni, A.; et al. The New Frontier of Cardiac Computed Tomography Angiography: Fractional Flow Reserve and Stress Myocardial Perfusion. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norgaard, B.L.; Mortensen, M.B.; Parner, E.; Leipsic, J.; Steffensen, F.H.; Grove, E.L.; Mathiassen, O.N.; Sand, N.P.; Pedersen, K.; Riedl, K.A.; et al. Clinical outcomes following real-world computed tomography angiography-derived fractional flow reserve testing in chronic coronary syndrome patients with calcification. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1182–1189. [Google Scholar] [CrossRef]
- Douglas, P.S.; De Bruyne, B.; Pontone, G.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. 1-Year Outcomes of FFRCT-Guided Care in Patients With Suspected Coronary Disease: The PLATFORM Study. J. Am. Coll. Cardiol. 2016, 68, 435–445. [Google Scholar] [CrossRef]
- Rabbat, M.; Leipsic, J.; Bax, J.; Kauh, B.; Verma, R.; Doukas, D.; Allen, S.; Pontone, G.; Wilber, D.; Mathew, V.; et al. Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography Safely Defers Invasive Coronary Angiography in Patients with Stable Coronary Artery Disease. J. Clin. Med. 2020, 9, 604. [Google Scholar] [CrossRef] [Green Version]
- Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Norgaard, B.L.; Berman, D.S.; Raff, G.; Hurwitz-Koweek, L.M.; Pontone, G.; Kawasaki, T.; Sand, N.P.; et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: Lessons from the ADVANCE Registry. Eur. Heart J. 2018, 39, 3701–3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Merkus, D.; Klotz, E.; Mollet, N.; de Feyter, P.J.; Krestin, G.P. Stress myocardial perfusion: Imaging with multidetector CT. Radiology 2014, 270, 25–46. [Google Scholar] [CrossRef] [Green Version]
- Pontone, G.; Andreini, D.; Baggiano, A.; Bertella, E.; Mushtaq, S.; Conte, E.; Beltrama, V.; Guaricci, A.I.; Pepi, M. Functional relevance of coronary artery disease by cardiac magnetic resonance and cardiac computed tomography: Myocardial perfusion and fractional flow reserve. Biomed. Res. Int. 2015, 2015, 297696. [Google Scholar] [CrossRef]
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Del Torto, A.; Mushtaq, S.; et al. Diagnostic accuracy of simultaneous evaluation of coronary arteries and myocardial perfusion with single stress cardiac computed tomography acquisition compared to invasive coronary angiography plus invasive fractional flow reserve. Int. J. Cardiol. 2018, 273, 263–268. [Google Scholar] [CrossRef]
- Cury, R.C.; Magalhaes, T.A.; Paladino, A.T.; Shiozaki, A.A.; Perini, M.; Senra, T.; Lemos, P.A.; Cury, R.C.; Rochitte, C.E. Dipyridamole stress and rest transmural myocardial perfusion ratio evaluation by 64 detector-row computed tomography. J. Cardiovasc. Comput. Tomogr. 2011, 5, 443–448. [Google Scholar] [CrossRef]
- Bamberg, F.; Marcus, R.P.; Becker, A.; Hildebrandt, K.; Bauner, K.; Schwarz, F.; Greif, M.; von Ziegler, F.; Bischoff, B.; Becker, H.C.; et al. Dynamic myocardial CT perfusion imaging for evaluation of myocardial ischemia as determined by MR imaging. JACC Cardiovasc. Imaging 2014, 7, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Baggiano, A.; Fazzari, F.; Guglielmo, M.; Muscogiuri, G.; Berzovini, C.M.; Pasquini, A.; Mushtaq, S.; et al. Incremental Diagnostic Value of Stress Computed Tomography Myocardial Perfusion With Whole-Heart Coverage CT Scanner in Intermediate- to High-Risk Symptomatic Patients Suspected of Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 338–349. [Google Scholar] [CrossRef]
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Del Torto, A.; Mushtaq, S.; et al. Dynamic Stress Computed Tomography Perfusion With a Whole-Heart Coverage Scanner in Addition to Coronary Computed Tomography Angiography and Fractional Flow Reserve Computed Tomography Derived. JACC Cardiovasc. Imaging 2019, 12, 2460–2471. [Google Scholar] [CrossRef]
- Lubbers, M.; Coenen, A.; Kofflard, M.; Bruning, T.; Kietselaer, B.; Galema, T.; Kock, M.; Niezen, A.; Das, M.; van Gent, M.; et al. Comprehensive Cardiac CT With Myocardial Perfusion Imaging Versus Functional Testing in Suspected Coronary Artery Disease: The Multicenter, Randomized CRESCENT-II Trial. JACC Cardiovasc. Imaging 2018, 11, 1625–1636. [Google Scholar] [CrossRef]
- Baggiano, A.; Fusini, L.; Del Torto, A.; Vivona, P.; Guglielmo, M.; Muscogiuri, G.; Soldi, M.; Martini, C.; Fraschini, E.; Rabbat, M.G.; et al. Sequential Strategy Including FFRCT Plus Stress-CTP Impacts on Management of Patients with Stable Chest Pain: The Stress-CTP RIPCORD Study. J. Clin. Med. 2020, 9, 2147. [Google Scholar] [CrossRef]
- Pontone, G.; De Cecco, C.; Baggiano, A.; Guaricci, A.I.; Guglielmo, M.; Leiner, T.; Lima, J.; Maurovich-Horvat, P.; Muscogiuri, G.; Nance, J.W.; et al. Design of CTP-PRO study (impact of stress Cardiac computed Tomography myocardial Perfusion on downstream resources and PROgnosis in patients with suspected or known coronary artery disease: A multicenter international study). Int. J. Cardiol. 2019, 292, 253–257. [Google Scholar] [CrossRef]
- Pontone, G.; Moharem-Elgamal, S.; Maurovich-Horvat, P.; Gaemperli, O.; Pugliese, F.; Document, r.; Westwood, M.; Stefanidis, A.; Fox, K.F.; Popescu, B.A. Training in cardiac computed tomography: EACVI certification process. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Baessato, F.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; Fusini, L.; Scafuri, S.; Babbaro, M.; Mollace, R.; Collevecchio, A.; Guaricci, A.I.; et al. The Incremental Role of Coronary Computed Tomography in Chronic Coronary Syndromes. J. Clin. Med. 2020, 9, 3925. [Google Scholar] [CrossRef]
- Hadamitzky, M.; Taubert, S.; Deseive, S.; Byrne, R.A.; Martinoff, S.; Schomig, A.; Hausleiter, J. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur. Heart J. 2013, 34, 3277–3285. [Google Scholar] [CrossRef] [Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; Pontone, G.; Hlatky, M.A.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: The prospective longitudinal trial of FFR(CT): Outcome and resource impacts study. Eur. Heart J. 2015, 36, 3359–3367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baggiano, A.; Italiano, G.; Guglielmo, M.; Fusini, L.; Guaricci, A.I.; Maragna, R.; Giacari, C.M.; Mushtaq, S.; Conte, E.; Annoni, A.D.; et al. Changing Paradigms in the Diagnosis of Ischemic Heart Disease by Multimodality Imaging. J. Clin. Med. 2022, 11, 477. https://doi.org/10.3390/jcm11030477
Baggiano A, Italiano G, Guglielmo M, Fusini L, Guaricci AI, Maragna R, Giacari CM, Mushtaq S, Conte E, Annoni AD, et al. Changing Paradigms in the Diagnosis of Ischemic Heart Disease by Multimodality Imaging. Journal of Clinical Medicine. 2022; 11(3):477. https://doi.org/10.3390/jcm11030477
Chicago/Turabian StyleBaggiano, Andrea, Gianpiero Italiano, Marco Guglielmo, Laura Fusini, Andrea Igoren Guaricci, Riccardo Maragna, Carlo Maria Giacari, Saima Mushtaq, Edoardo Conte, Andrea Daniele Annoni, and et al. 2022. "Changing Paradigms in the Diagnosis of Ischemic Heart Disease by Multimodality Imaging" Journal of Clinical Medicine 11, no. 3: 477. https://doi.org/10.3390/jcm11030477
APA StyleBaggiano, A., Italiano, G., Guglielmo, M., Fusini, L., Guaricci, A. I., Maragna, R., Giacari, C. M., Mushtaq, S., Conte, E., Annoni, A. D., Formenti, A., Mancini, M. E., Andreini, D., Rabbat, M., Pepi, M., & Pontone, G. (2022). Changing Paradigms in the Diagnosis of Ischemic Heart Disease by Multimodality Imaging. Journal of Clinical Medicine, 11(3), 477. https://doi.org/10.3390/jcm11030477













