Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis
Abstract
:1. Introduction
2. Hypertrophic Cardiomyopathy
3. Dilated Cardiomyopathy
4. Restrictive Cardiomyopathies
4.1. Idiopathic Restrictive Cardiomyopathy
4.2. Cardiac Amyloidosis
4.3. Fabry Disease
4.4. Iron Overload Cardiomyopathy
4.5. Cardiac Sarcoidosis
4.6. Endomyocardial Fibrosis
5. Arrhythmogenic Cardiomyopathy
6. Left Ventricular Noncompaction
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ACM | arrhythmogenic cardiomyopathy |
| AL | primary amyloidosis |
| ATTR | transthyretin amyloidosis |
| C/CL | cardiac compared to the contralateral chest |
| CA | cardiac amyloidosis |
| CMR | cardiovascular magnetic resonance |
| CRT | cardiac resynchronization therapy |
| CS | cardiac sarcoidosis |
| CT | computed tomography |
| DCM | dilated cardiomyopathy |
| ECG | electrocardiogram |
| ECV | extracellular volume |
| EMF | endomyocardial fibrosis |
| ESC | European Society of Cardiology |
| FD | Anderson–Fabry disease |
| FDG | 18F-fluorodeoxyglucose |
| GCS | global circumferential strain |
| GLS | global longitudinal strain |
| HCM | hypertrophic cardiomyopathy |
| ICD | implantable cardioverter-defibrillator |
| IOC | iron overload cardiomyopathy |
| LGE | late gadolinium enhancement |
| LV | left ventricle |
| LVEF | left ventricular ejection fraction |
| LVH | left ventricular hypertrophy |
| LVNC | left ventricular noncompaction |
| LVOTO | left ventricular outflow tract obstruction |
| MIBG | 123I-meta-iodobenzylguanidine |
| MW | myocardial work |
| PET | positron emission tomography |
| RCM | restrictive cardiomyopathies |
| RV | right ventricle |
| RVEF | right ventricle ejection fraction |
| SAM | systolic anterior motion |
| SCD | sudden cardiac death |
| TAPSE | tricuspid annular plane systolic excursion |
| TTE | transthoracic echocardiography |
References
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [PubMed]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2020, 76, e159–e240. [Google Scholar] [PubMed]
- Cardim, N.; Galderisi, M.; Edvardsen, T.; Plein, S.; Popescu, B.A.; D’Andrea, A.; Bruder, O.; Cosyns, B.; Davin, L.; Donal, E.; et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: An expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur. Hear. J.-Cardiovasc. Imaging 2015, 16, 280. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Moral, J.A.; Gutierrez-Garcia-Moreno, L.; Matabuena-Gomez-Limon, J.; Niella, N.; Maldonado, G.; Valle-Racero, J.I.; Niella, M.; Teixido-Tura, G.; Garcia-Dorado, D.; Ferrazzi, P.; et al. Structural abnormalities in hypertrophic cardiomyopathy beyond left ventricular hypertrophy by multimodality imaging evaluation. Echocardiography 2019, 36, 1241–1252. [Google Scholar] [CrossRef]
- Greulich, S.; Seitz, A.; Herter, D.; Günther, F.; Probst, S.; Bekeredjian, R.; Gawaz, M.; Sechtem, U.; Mahrholdt, H. Long-term risk of sudden cardiac death in hypertrophic cardiomyopathy: A cardiac magnetic resonance outcome study. Eur. Hear. J. Cardiovasc. Imaging 2021, 22, 732–741. [Google Scholar] [CrossRef]
- Harris, K.M.; Spirito, P.; Maron, M.S.; Zenovich, A.G.; Formisano, F.; Lesser, J.R.; Mackey-Bojack, S.; Manning, W.J.; Udelson, J.E.; Maron, B.J. Prevalence, Clinical Profile, and Significance of Left Ventricular Remodeling in the End-Stage Phase of Hypertrophic Cardiomyopathy. Circulation 2006, 114, 216–225. [Google Scholar] [CrossRef]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur. Heart J. 2013, 35, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Tower-Rader, A.; Mohananey, D.; To, A.; Lever, H.M.; Popovic, Z.B.; Desai, M.Y. Prognostic Value of Global Longitudinal Strain in Hypertrophic Cardiomyopathy: A Systematic Review of Existing Literature. JACC Cardiovasc. Imaging 2019, 12, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.B.; Davies, R.H.; Bhuva, A.N.; Knott, K.D.; Seraphim, A.; Alfarih, M.; Lau, C.; Hughes, R.K.; Lopes, L.R.; Shiwani, H.; et al. Diagnosis and risk stratification in hypertrophic cardiomyopathy using machine learning wall thickness measurement: A comparison with human test-retest performance. Lancet Digit. Health 2021, 3, e20–e28. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentias, A.; Raeisi-Giglou, P.; Smedira, N.G.; Feng, K.; Sato, K.; Wazni, O.; Kanj, M.; Flamm, S.D.; Thamilarasan, M.; Popovic, Z.B.; et al. Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Preserved Systolic Function. J. Am. Coll. Cardiol. 2018, 72, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.; Ferreira, A.M.; Arteaga-Fernández, E.; Antunes, M.; Mesquita, J.; Abecasis, J.; Marques, H.; Saraiva, C.; Matos, D.N.; Rodrigues, R.; et al. The amount of late gadolinium enhancement outperforms current guideline-recommended criteria in the identification of patients with hypertrophic cardiomyopathy at risk of sudden cardiac death. J. Cardiovasc. Magn. Reson. 2019, 21, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.; Reed, E.; Sheppard, M.; Elkington, A.G.; Ho, S.; Burke, M.; Petrou, M.; Pennell, D.J. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2004, 43, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Bruder, O.; Wagner, A.; Jensen, C.J.; Schneider, S.; Ong, P.; Kispert, E.-M.; Nassenstein, K.; Schlosser, T.; Sabin, G.V.; Sechtem, U.; et al. Myocardial Scar Visualized by Cardiovascular Magnetic Resonance Imaging Predicts Major Adverse Events in Patients With Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of Techniques for the Quantification of Myocardial Scar of Differing Etiology Using Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Raman, B.; Ariga, R.; Spartera, M.; Sivalokanathan, S.; Chan, K.; Dass, S.; Petersen, E.S.; Daniels, M.J.; Francis, J.; Smillie, R.; et al. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: Mechanisms and clinical implications. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Green, J.J.; Berger, J.S.; Kramer, C.M.; Salerno, M. Prognostic Value of Late Gadolinium Enhancement in Clinical Outcomes for Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2012, 5, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Dass, S.; Suttie, J.J.; Piechnik, S.K.; Ferreira, V.M.; Holloway, C.J.; Banerjee, R.; Mahmod, M.; Cochlin, L.; Karamitsos, T.D.; Robson, M.D.; et al. Myocardial Tissue Characterization Using Magnetic Resonance Noncontrast T1 Mapping in Hypertrophic and Dilated Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Arcari, L.; Hinojar, R.; Engel, J.; Freiwald, T.; Platschek, S.; Zainal, H.; Zhou, H.; Vasquez, M.; Keller, T.; Rolf, A.; et al. Native T1 and T2 provide distinctive signatures in hypertrophic cardiac conditions—Comparison of uremic, hypertensive and hypertrophic cardiomyopathy. Int. J. Cardiol. 2020, 306, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Min, J.; Chen, C.; Zhu, L.; Gu, S.; Zhou, M.; Yang, W.; Yan, F. Incremental Values of T1 Mapping in the Prediction of Sudden Cardiac Death Risk in Hypertrophic Cardiomyopathy: A Comparison With Two Guidelines. Front. Cardiovasc. Med. 2021, 8, 661673. [Google Scholar] [CrossRef]
- Avanesov, M.; Münch, J.; Weinrich, J.; Well, L.; Säring, D.; Stehning, C.; Tahir, E.; Bohnen, S.; Radunski, U.K.; Muellerleile, K.; et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur. Radiol. 2017, 27, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Hear. J.-Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [Green Version]
- Smiseth, A.O.; Morris, A.D.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, A.F.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2021. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, X.; Delano, M.C.; Jiang, T.; Zhang, C.; Liu, Y.; Zhang, Z. Assessment of myocardial fibrosis and coronary arteries in hypertrophic cardiomyopathy using combined arterial and delayed enhanced CT: Comparison with MR and coronary angiography. Eur. Radiol. 2012, 23, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; De Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation 2013, 128, 240–327. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.-Y.; Marwick, T.H.; Kim, H.-S.; Kim, M.-K.; Hong, K.-S.; Oh, D.-J. Global 2-Dimensional Strain as a New Prognosticator in Patients With Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 618–624. [Google Scholar] [CrossRef] [Green Version]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-L.; Chan, Y.-H.; Wu, V.C.; Lee, H.-F.; Hsiao, F.C.; Chu, P.H. Incremental prognostic value of global myocardial work over ejection fraction and global longitudinal strain in patients with heart failure and reduced ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Delgado, V.; Bucciarelli-Ducci, C.; Galli, E.; Haugaa, K.; Charron, P.; Voigt, J.-U.; Cardim, N.; Masci, P.G.; Galderisi, M.; et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: An expert consensus document from the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 1075–1093. [Google Scholar] [CrossRef] [Green Version]
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular Magnetic Resonance, Fibrosis, and Prognosis in Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.C.; Weiss, R.G.; Thiemann, D.R.; Kitagawa, K.; Schmidt, A.; Dalal, D.; Lai, S.; Bluemke, D.; Gerstenblith, G.; Marbán, E.; et al. Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance Heralds an Adverse Prognosis in Nonischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2414–2421. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.; Cornel, J.; van de Ven, P.M.; van Rossum, A.C.; Allaart, C.P.; Germans, T. The Prognostic Value of Late Gadolinium-Enhanced Cardiac Magnetic Resonance Imaging in Nonischemic Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 1274–1284. [Google Scholar] [CrossRef]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Mckenna, P.; et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Coelho-Filho, O.; Danik, S.B.; Shah, R.V.; Dodson, J.A.; Verdini, D.J.; Tokuda, M.; Daly, C.; Tedrow, U.B.; Stevenson, W.G.; et al. CMR Quantification of Myocardial Scar Provides Additive Prognostic Information in Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2013, 6, 944–954. [Google Scholar] [CrossRef] [Green Version]
- Halliday, B.P.; Gulati, A.; Ali, A.; Guha, K.; Newsome, S.J.; Arzanauskaite, M.; Vassiliou, V.; Lota, A.S.; Izgi, C.; Tayal, U.; et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 2017, 135, 2106–2115. [Google Scholar] [CrossRef] [Green Version]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.; et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2019, 12, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Brown, P.F.; Bradley, J.; Nucifora, G.; Claver, E.; de Frutos, F.; Dallaglio, P.D.; Comin-Colet, J.; Anguera, I.; Miller, C.A.; et al. Improved Risk Stratification for Ventricular Arrhythmias and Sudden Death in Patients With Nonischemic Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 77, 2890–2905. [Google Scholar] [CrossRef]
- Mandawat, A.; Chattranukulchai, P.; Mandawat, A.; Blood, A.J.; Ambati, S.; Hayes, B.; Rehwald, W.; Kim, H.W.; Heitner, J.F.; Shah, D.J.; et al. Progression of Myocardial Fibrosis in Nonischemic DCM and Association with Mortality and Heart Failure Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1338–1350. [Google Scholar] [CrossRef]
- Romano, S.; Judd, R.M.; Kim, R.J.; Kim, H.W.; Klem, I.; Heitner, J.F.; Shah, D.J.; Jue, J.; White, B.E.; Indorkar, R.; et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2018, 11, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.Y.; Gebker, R.; Kelle, S.; Hinojar, R.; Doltra, A.; Varma, N.; Child, N.; et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy All-Cause Mortality and Heart Failure. JACC Cardiovasc. Imaging 2016, 9, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thavendiranathan, P.; Walls, M.; Giri, S.; Verhaert, D.; Rajagopalan, S.; Moore, S.; Simonetti, O.P.; Raman, S.V. Improved Detection of Myocardial Involvement in Acute Inflammatory Cardiomyopathies Using T2 Mapping. Circ. Cardiovasc. Imaging 2012, 5, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Gulati, A.; Ismail, T.F.; Jabbour, A.; Alpendurada, F.; Guha, K.; Ismail, N.A.; Raza, S.; Khwaja, J.; Brown, T.D.; Morarji, K.; et al. The Prevalence and Prognostic Significance of Right Ventricular Systolic Dysfunction in Nonischemic Dilated Cardiomyopathy. Circulation 2013, 128, 1623–1633. [Google Scholar] [CrossRef] [Green Version]
- Merlo, M.; Gobbo, M.; Stolfo, D.; Losurdo, P.; Ramani, F.; Barbati, G.; Pivetta, A.; Di Lenarda, A.; Anzini, M.; Gigli, M.; et al. The Prognostic Impact of the Evolution of RV Function in Idiopathic DCM. JACC Cardiovasc. Imaging 2016, 9, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Coiro, S.; Riccini, C.; Mengoni, A.; D’Antonio, A.; Ambrosio, G. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: Superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ. Cardiovasc. Imaging 2018, 11, e006894. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Dini, F.L.; Faggiano, P.; Agricola, E.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680. [Google Scholar] [CrossRef]
- Risum, N.; Tayal, B.; Hansen, T.F.; Bruun, N.E.; Jensen, M.T.; Lauridsen, T.K.; Saba, S.; Kisslo, J.; Gorcsan, J.; Sogaard, P. Identification of Typical Left Bundle Branch Block Contraction by Strain Echocardiography Is Additive to Electrocardiography in Prediction of Long-Term Outcome After Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2015, 66, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Feneon, D.; Behaghel, A.; Bernard, A.; Fournet, M.; Mabo, P.; Daubert, J.-C.; Leclercq, C.; Donal, E. Left atrial function, a new predictor of response to cardiac resynchronization therapy? Heart Rhythm. 2015, 12, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Leclercq, C.; Hubert, A.; Bernard, A.; Smiseth, O.A.; Mabo, P.; Samset, E.; Hernandez, A.; Donal, E. Role ofmyocardial constructive work in the identification of responders to CRT. Eur. Heart J. Cardiovasc. Imaging. 2018, 19, 1010–1018. [Google Scholar] [CrossRef]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging predictors of response to cardiac resynchronization therapy: Left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur. Heart J. 2020, 41, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Ferrick, K.J.; Nakata, T.; Kaplan, R.C.; Rozengarten, M.; Latif, F.; Ng, K.; Marcano, V.; Heller, S.; Fisher, J.D.; et al. I-123 MIBG imaging and heart rate variability analysis to predict the need for an implantable cardioverter defribillator. J. Nucl. Cardiol. 2003, 10, 121–131. [Google Scholar] [CrossRef]
- Lee, H.-J.; Im, D.J.; Youn, J.-C.; Chang, S.; Suh, Y.J.; Hong, Y.; Kim, Y.J.; Hur, J.; Choi, B.W. Assessment of myocardial delayed enhancement with cardiac computed tomography in cardiomyopathies: A prospective comparison with delayed enhancement cardiac magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2016, 33, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Bucciarelli-Ducci, C.; Caforio, A.L.P.; Cardim, N.; Charron, P.; Cosyns, B.; Dehaene, A.; Derumeaux, G.; Donal, E.; Dweck, M.R.; et al. Multimodality Imaging in Restrictive Cardiomyopathies: An EACVI expert consensus document In collaboration with the “Working Group on myocardial and pericardial diseases” of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiogr. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1090–1091. [Google Scholar] [CrossRef] [Green Version]
- Anderson, H.N.; Cetta, F.; Driscoll, D.J.; Olson, T.M.; Ackerman, M.J.; Johnson, J.N. Idiopathic Restrictive Cardiomyopathy in Children and Young Adults. Am. J. Cardiol. 2018, 121, 1266–1270. [Google Scholar] [CrossRef]
- González-López, E.; López-Sainz, Á.; Garcia-Pavia, P. Diagnóstico y tratamiento de la amiloidosis cardiaca por transtiretina. Progreso y esperanza. Rev. Española Cardiol. 2017, 70, 991–1004. [Google Scholar] [CrossRef]
- Dorbala, S.; Cuddy, S.; Falk, R.H. How to Image Cardiac Amyloidosis: A Practical Approach. JACC Cardiovasc. Imaging 2020, 13, 1368–1383. [Google Scholar] [CrossRef]
- Barros-Gomes, S.; Williams, B.; Nhola, L.F.; Grogan, M.; Maalouf, J.F.; Dispenzieri, A.; Pellikka, P.A.; Villarraga, H.R. Prognosis of Light Chain Amyloidosis With Preserved LVEF: Added Value of 2D Speckle-Tracking Echocardiography to the Current Prognostic Staging System. JACC Cardiovasc. Imaging 2017, 10, 398–407. [Google Scholar]
- Castaño, A.; Narotsky, D.L.; Hamid, N.; Khalique, O.K.; Morgenstern, R.; DeLuca, A.; Rubin, J.; Chiuzan, C.; Nazif, T.; Vahl, T.; et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. 2017, 38, 2879–2887. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.S.; Bokhari, S.; Damy, T.; Dorbala, S.; Drachman, B.M.; Fontana, M.; Grogan, M.; Kristen, A.V.; Lousada, I.; Nativi-Nicolau, J.; et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Heart Fail. 2019, 12, e006075. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Wen, I.; Bhat, A.; Chen, H.H.L.; Gan, G.C.H.; Pathan, F.; Tan, T.C. The Role of Multi-modality Imaging in the Diagnosis of Cardiac Amyloidosis: A Focused Update. Front. Cardiovasc. Med. 2020, 7, 590557. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015, 132, 1570–1579. [Google Scholar]
- Pan, J.A.; Kerwin, M.J.; Salerno, M. Native T1 Mapping, Extracellular Volume Mapping, and Late Gadolinium Enhancement in Cardiac Amyloidosis: A Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13, 1299–1310. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Treibel, T.; Abdel-Gadir, A.; Bulluck, H.; Zumbo, G.; Knight, D.S.; Kotecha, T.; Francis, R.; Hutt, D.F.; Rezk, T.; et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2017, 70, 466–477. [Google Scholar] [CrossRef]
- Brownrigg, J.; Lorenzini, M.; Lumley, M.; Elliott, P. Diagnostic performance of imaging investigations in detecting and differentiating cardiac amyloidosis: A systematic review and meta-analysis. ESC Heart Fail. 2019, 6, 1041–1051. [Google Scholar] [CrossRef]
- Hanna, M.; Ruberg, F.L.; Maurer, M.S.; Dispenzieri, A.; Dorbala, S.; Falk, R.H.; Hoffman, J.; Jaber, W.; Soman, P.; Witteles, R.M.; et al. Cardiac Scintigraphy With Technetium-99m-Labeled Bone-Seeking Tracers for Suspected Amyloidosis. J. Am. Coll. Cardiol. 2020, 75, 2851–2862. [Google Scholar] [CrossRef]
- Hutt, D.F.; Fontana, M.; Burniston, M.; Quigley, A.-M.; Petrie, A.; Ross, J.C.; Page, J.; Martinez-Naharro, A.; Wechalekar, A.D.; Lachmann, H.J.; et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1344–1350. [Google Scholar] [CrossRef]
- Castano, A.; Haq, M.; Narotsky, D.L.; Goldsmith, J.; Weinberg, R.L.; Morgenstern, R.; Pozniakoff, T.; Ruberg, F.L.; Miller, E.; Berk, J.L.; et al. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging. JAMA Cardiol. 2016, 1, 880–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperry, B.W.; Vranian, M.N.; Tower-Rader, A.; Hachamovitch, R.; Hanna, M.; Brunken, R.; Phelan, D.; Cerqueira, M.D.; Jaber, W.A. Regional Variation in Technetium Pyrophosphate Uptake in Transthyretin Cardiac Amyloidosis and Impact on Mortality. JACC Cardiovasc. Imaging 2018, 11, 234–242. [Google Scholar] [CrossRef]
- Deva, D.P.; Hanneman, K.; Li, Q.; Ng, M.Y.; Wasim, S.; Morel, C.; Iwanochko, R.M.; Thavendiranathan, P.; Crean, A.M. Cardiovascular magnetic resonance demonstration of the spectrum of morphological phenotypes and patterns of myocardial scarring in Anderson-Fabry disease. J. Cardiovasc. Magn. Reson. 2016, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- Krämer, J.; Niemann, M.; Liu, D.; Hu, K.; Machann, W.; Beer, M.; Wanner, C.; Ertl, G.; Weidemann, F. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur. Heart J. 2013, 34, 1587–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.; Sachdev, B.; Elkington, A.G.; McKenna, W.J.; Mehta, A.; Pennell, D.; Leed, P.J.; Elliott, P. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease Evidence for a disease specific abnormality of the myocardial interstitium. Eur. Heart J. 2003, 24, 2151–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biegstraaten, M.; Arngrímsson, R.; Barbey, F.; Boks, L.; Cecchi, F.; Deegan, P.B.; Feldt-Rasmussen, U.; Geberhiwot, T.; Germain, D.P.; Hendriksz, C.; et al. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: The European Fabry Working Group consensus document. Orphanet, J. Rare Dis. 2015, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Nordin, S.; Kozor, R.; Baig, S.; Abdel-Gadir, A.; Medina-Menacho, K.; Rosmini, S.; Captur, G.; Tchan, M.; Geberhiwot, T.; Murphy, E.; et al. Cardiac Phenotype of Prehypertrophic Fabry Disease. Circ. Cardiovasc. Imaging 2018, 11, e007168. [Google Scholar] [CrossRef] [Green Version]
- Nappi, C.; Altiero, M.; Imbriaco, M.; Nicolai, E.; Giudice, C.A.; Aiello, M.; Diomiaiuti, C.T.; Pisani, A.; Spinelli, L.; Cuocolo, A. First experience of simultaneous PET/MRI for the early detection of cardiac involvement in patients with Anderson-Fabry disease. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1025–1031. [Google Scholar] [CrossRef]
- Imbriaco, M.; Pellegrino, T.; Piscopo, V.; Petretta, M.; Ponsiglione, A.; Nappi, C.; Puglia, M.; Dell’Aversana, S.; Riccio, E.; Spinelli, L.; et al. Cardiac sympathetic neuronal damage precedes myocardial fibrosis in patients with Anderson-Fabry disease. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2266–2273. [Google Scholar] [CrossRef] [Green Version]
- Gujja, P.; Rosing, D.R.; Tripodi, D.J.; Shizukuda, Y. Iron Overload Cardiomyopathy: Better Understanding of an Increasing Disorder. J. Am. Coll. Cardiol. 2010, 56, 1001–1012. [Google Scholar] [CrossRef] [Green Version]
- Kirk, P.; Roughton, M.; Porter, J.B.; Walker, J.M.; Tanner, M.A.; Patel, J.; Wu, D.; Taylor, J.; Westwood, M.A.; Anderson, L.J.; et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in Thalassemia Major. Circulation 2009, 120, 1961–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghugre, N.R.; Enriquez, C.M.; Gonzalez, I., Jr.; Coates, T.D.; Wood, J.C. MRI detects myocardial iron in the human heart. Magn. Reson. Med. 2006, 56, 681–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skold, C.M.; Larsen, F.F.; Rasmussen, E.; Pehrsson, S.K.; Eklund, A.G. Determination of cardiac involvement in sarcoidosis by magnetic resonance imaging and Doppler echocardiography. J. Intern. Med. 2002, 252, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Vita, T.; Okada, D.R.; Veillet-Chowdhury, M.; Bravo, P.E.; Mullins, E.; Hulten, E.; Agrawal, M.; Madan, R.; Taqueti, V.R.; Steigner, M.; et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ. Cardiovasc. Imaging 2018, 11, e007030. [Google Scholar] [CrossRef]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; DaSilva, J.; Birnie, D.; et al. The Use of 18F-FDG PET in the Diagnosis of Cardiac Sarcoidosis: A Systematic Review and Metaanalysis Including the Ontario Experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Blankstein, R.; Osborne, M.; Naya, M.; Waller, A.; Kim, C.K.; Murthy, V.; Kazemian, P.; Kwong, R.Y.; Tokuda, M.; Skali, H.; et al. Cardiac Positron Emission Tomography Enhances Prognostic Assessments of Patients With Suspected Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2014, 63, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Dweck, M.; Abgral, R.; Trivieri, M.G.; Robson, P.M.; Karakatsanis, N.; Mani, V.; Palmisano, A.; Miller, M.A.; Lala, A.; Chang, H.L.; et al. Hybrid Magnetic Resonance Imaging and Positron Emission Tomography With Fluorodeoxyglucose to Diagnose Active Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2018, 11, 94–107. [Google Scholar] [CrossRef]
- Mocumbi, A.O.; Carrilho, C.; Sarathchandra, P.; Ferreira, M.B.; Yacoub, M.; Burke, M. Echocardiography accurately assesses the pathological abnormalities of chronic endomyocardial fibrosis. Int. J. Cardiovasc. Imaging 2010, 27, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Mocumbi, A.; Ferreira, M.B.; Sidi, D.; Yacoub, M.H. A Population Study of Endomyocardial Fibrosis in a Rural Area of Mozambique. N. Engl. J. Med. 2008, 359, 43–49. [Google Scholar] [CrossRef]
- Salemi, V.M.; Rochitte, C.E.; Shiozaki, A.A.; Andrade, J.M.; Parga, J.R.; de Ávila, L.F.; Benvenuti, L.A.; Cestari, I.N.; Picard, M.H.; Kim, R.J.; et al. Late Gadolinium Enhancement Magnetic Resonance Imaging in the Diagnosis and Prognosis of Endomyocardial Fibrosis Patients. Circ. Cardiovasc. Imaging 2011, 4, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed Modification of the Task Force Criteria. Eur. Heart J. 2010, 121, 1533–1541. [Google Scholar] [CrossRef]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C. Arrhythmogenic left ventricular cardiomyopathy. Heart 2021, 120, 2613–2614. [Google Scholar] [CrossRef]
- Aquaro, G.D.; De Luca, A.; Cappelletto, C.; Raimondi, F.; Bianco, F.; Botto, N.; Lesizza, P.; Grigoratos, C.; Minati, M.; Dell’Omodarme, M.; et al. Prognostic Value of Magnetic Resonance Phenotype in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 2753–2765. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.J.; Cox, M.G.; Riele, A.T.; De Boeck, B.W.; Doevendans, P.A.; Hauer, R.N.; Cramer, M.J. Early Detection of Regional Functional Abnormalities in Asymptomatic ARVD/C Gene Carriers. J. Am. Soc. Echocardiogr. 2012, 25, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Saguner, A.M.; Vecchiati, A.; Baldinger, S.H.; Rüeger, S.; Medeiros-Domingo, A.; Mueller-Burri, A.S.; Haegeli, L.M.; Biaggi, P.; Manka, R.; Lüscher, T.F.; et al. Different prognostic value of functional right ventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ. Cardiovasc. Imaging 2014, 7, 230–239. [Google Scholar] [CrossRef]
- Sarvari, S.I.; Haugaa, K.H.; Anfinsen, O.-G.; Leren, T.P.; Smiseth, O.A.; Kongsgaard, E.; Amlie, J.P.; Edvardsen, T. Right ventricular mechanical dispersion is related to malignant arrhythmias: A study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur. Heart J. 2011, 32, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Pinamonti, B.; Dragos, A.M.; Pyxaras, S.A.; Merlo, M.; Pivetta, A.; Barbati, G.; Di Lenarda, A.; Morgera, T.; Mestroni, L.; Sinagra, G. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: Results from a 10-year registry. Eur. Heart J. 2011, 32, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.; Marchlinski, F.; Anastasakis, A. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: An international task force consensus statement. Eur. Heart J. 2015, 36, 3227–3237. [Google Scholar] [CrossRef]
- Cadrin-Tourigny, J.; Bosman, L.P.; Nozza, A.; Wang, W.; Tadros, R.; Bhonsale, A.; Bourfiss, M.; Fortier, A.; Lie, Ø.H.; Saguner, A.M.; et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2019, 40, 1850–1858. [Google Scholar] [CrossRef] [Green Version]
- Towbin, J.A.; Lorts, A.; Lynn, J. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 22, 813–825. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Oechslin, E.N.; Attenhofer Jost, C.H.; Rojas, J.R.; Kaufmann, P.A.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left Ventricular Non-Compaction: Insights From Cardiovascular Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.-Y.; Vidal, V.; Bartoli, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Captur, G.; Muthurangu, V.; Cook, C.; Flett, A.S.; Wilson, R.; Barison, A.; Sado, D.M.; Anderson, S.; McKenna, W.J.; Mohun, T.J.; et al. Quantification of left ventricular trabeculae using fractal analysis. J. Cardiovasc. Magn. Reson. 2013, 15, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemrak, F.; Ahlman, M.A.; Captur, G.; Mohiddin, S.; Kawel-Boehm, N.; Prince, M.; Moon, J.; Hundley, W.G.; Lima, J.A.; Bluemke, D.; et al. The Relationship of Left Ventricular Trabeculation to Ventricular Function and Structure Over a 9.5-Year Follow-Up. J. Am. Coll. Cardiol. 2014, 64, 1971–1980. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.; Dabiesingh, D.S.; Bhumireddy, G.P.; Mohamed, A.; Asfour, A.; Briggs, W.M.; Ho, J.; Khan, S.A.; Grossman, A.; Klem, I.; et al. Prevalence and Prognostic Significance of Left Ventricular Noncompaction in Patients Referred for Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, V.R.; Lyle, M.; Miranda, W.R.; Farwati, M.; Isath, A.; Patlolla, S.H.; Hodge, D.O.; Asirvatham, S.J.; Kapa, S.; Deshmukh, A.J.; et al. Long-Term Survival of Patients With Left Ventricular Noncompaction. J. Am. Hear. Assoc. 2021, 10, e015563. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Pontone, G.; Bogaert, J.; Roghi, A.; Barison, A.; Schwitter, J.; Mushtaq, S.; Vovas, G.; Sormani, P.; Aquaro, G.D.; et al. Long-Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction: A Prospective Multicenter Study. J. Am. Coll. Cardiol. 2016, 68, 2166–2181. [Google Scholar] [CrossRef]
- Casas, G.; Limeres, J.; Oristrell, G.; Gutierrez-Garcia, L.; Andreini, D.; Borregan, M.; Larrañaga-Moreira, J.M.; Lopez-Sainz, A.; Codina-Solà, M.; Teixido-Tura, G.; et al. Clinical Risk Prediction in Patients With Left Ventricular Myocardial Noncompaction. J. Am. Coll. Cardiol. 2021, 78, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.I.; Michelena, H.; Martinez, M.; Pellikka, P.; Bruce, C.J.; Connolly, H.M.; Villarraga, H.R.; Veress, G.; Oh, J.K.; Miller, F. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart 2009, 96, 440–447. [Google Scholar] [CrossRef]
- Tarando, F.; Coisne, D.; Galli, E.; Rousseau, C.; Viera, F.; Bosseau, C.; Habib, G.; Lederlin, M.; Schnell, F.; Donal, E. Left ventricular non-compaction and idiopathic dilated cardiomyopathy: The significant diagnostic value of longitudinal strain. Int. J. Cardiovasc. Imaging 2016, 33, 83–95. [Google Scholar] [CrossRef]
- Ramchand, J.; Podugu, P.; Obuchowski, N.; Harb, S.C.; Chetrit, M.; Milinovich, A.; Griffin, B.; Burrell, L.M.; Tang, W.H.W.; Kwon, D.H.; et al. Novel Approach to Risk Stratification in Left Ventricular Non-Compaction Using A Combined Cardiac Imaging and Plasma Biomarker Approach. J. Am. Hear. Assoc. 2021, 10, e019209. [Google Scholar] [CrossRef]
- Grigoratos, C.; Barison, A.; Ivanov, A.; Andreini, D.; Amzulescu, M.S.; Mazurkiewicz, L.; De Luca, A.; Grzybowski, J.; Masci, P.G.; Marczak, M.; et al. Meta-Analysis of the Prognostic Role of Late Gadolinium Enhancement and Global Systolic Impairment in Left Ventricular Noncompaction. JACC Cardiovasc. Imaging 2019, 12, 2141–2151. [Google Scholar] [CrossRef]
- Araujo-Filho, J.A.B.; Assuncao, A.N.; De Melo, M.D.T.; Bière, L.; Lima, C.R.; Dantas, R.N.; Nomura, C.H.; Salemi, V.M.C.; Jerosch-Herold, M.; Parga, J. Myocardial T1 mapping and extracellular volume quantification in patients with left ventricular non-compaction cardiomyopathy. Eur. Hear. J.-Cardiovasc. Imaging 2018, 19, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreisbach, J.G.; Mathur, S.; Houbois, C.P.; Oechslin, E.; Ross, H.; Hanneman, K.; Wintersperger, B.J. Cardiovascular magnetic resonance based diagnosis of left ventricular non-compaction cardiomyopathy: Impact of cine bSSFP strain analysis. J. Cardiovasc. Magn. Reson. 2020, 22, 9. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Ma, X.; Li, S.; Ueda, T.; Wang, Z.; Lu, A.; Zhou, W.; Zou, H.; Zhao, L.; Gong, L. Value of Cardiac Magnetic Resonance Fractal Analysis Combined With Myocardial Strain in Discriminating Isolated Left Ventricular Noncompaction and Dilated Cardiomyopathy. J. Magn. Reson. Imaging 2019, 50, 153–163. [Google Scholar] [CrossRef]
- Sigvardsen, E.P.; Fuchs, A.; Kühl, J.T.; Afzal, S.; Køber, L.; Nordestgaard, B.G.; Kofoed, K.F. Left ventricular trabeculation and major adverse cardiovascular events: The Copenhagen General Population Study. Eur. Hear. J.-Cardiovasc. Imaging 2021, 22, 67–74. [Google Scholar] [CrossRef] [PubMed]
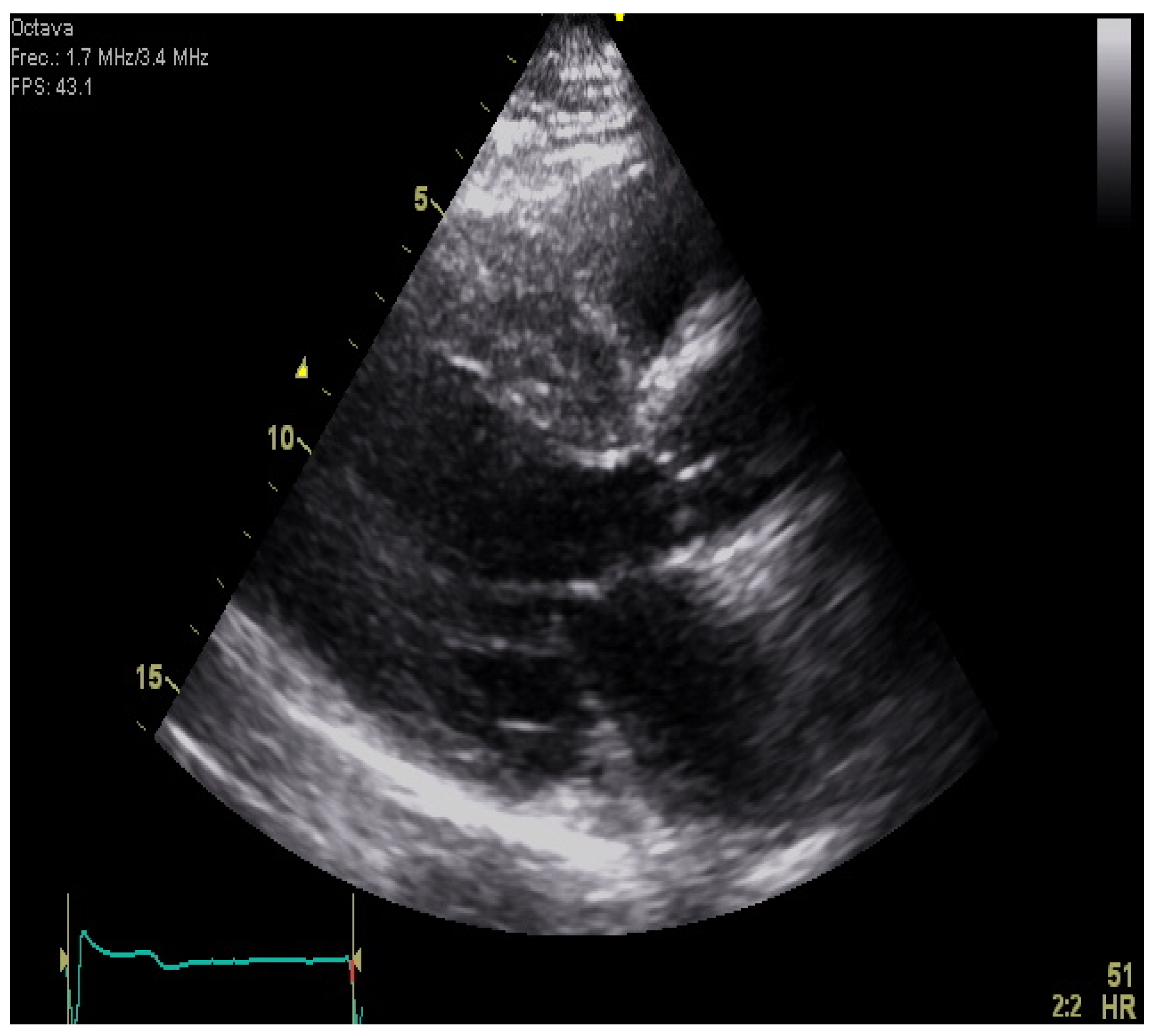
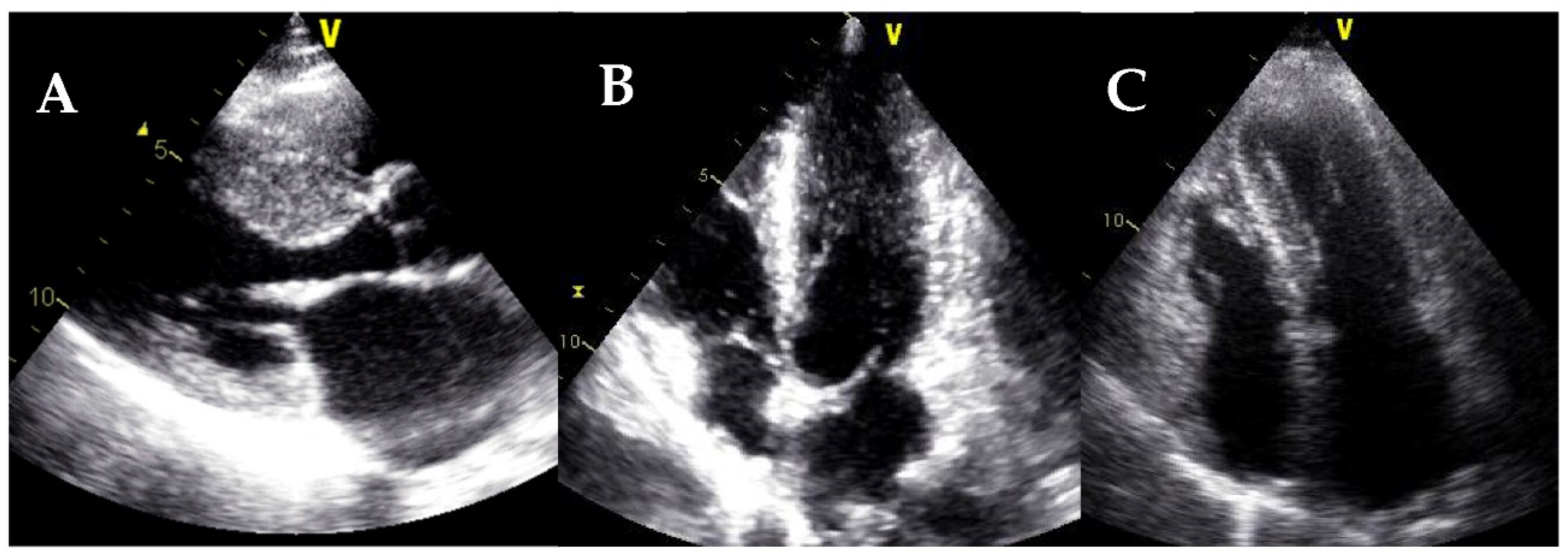



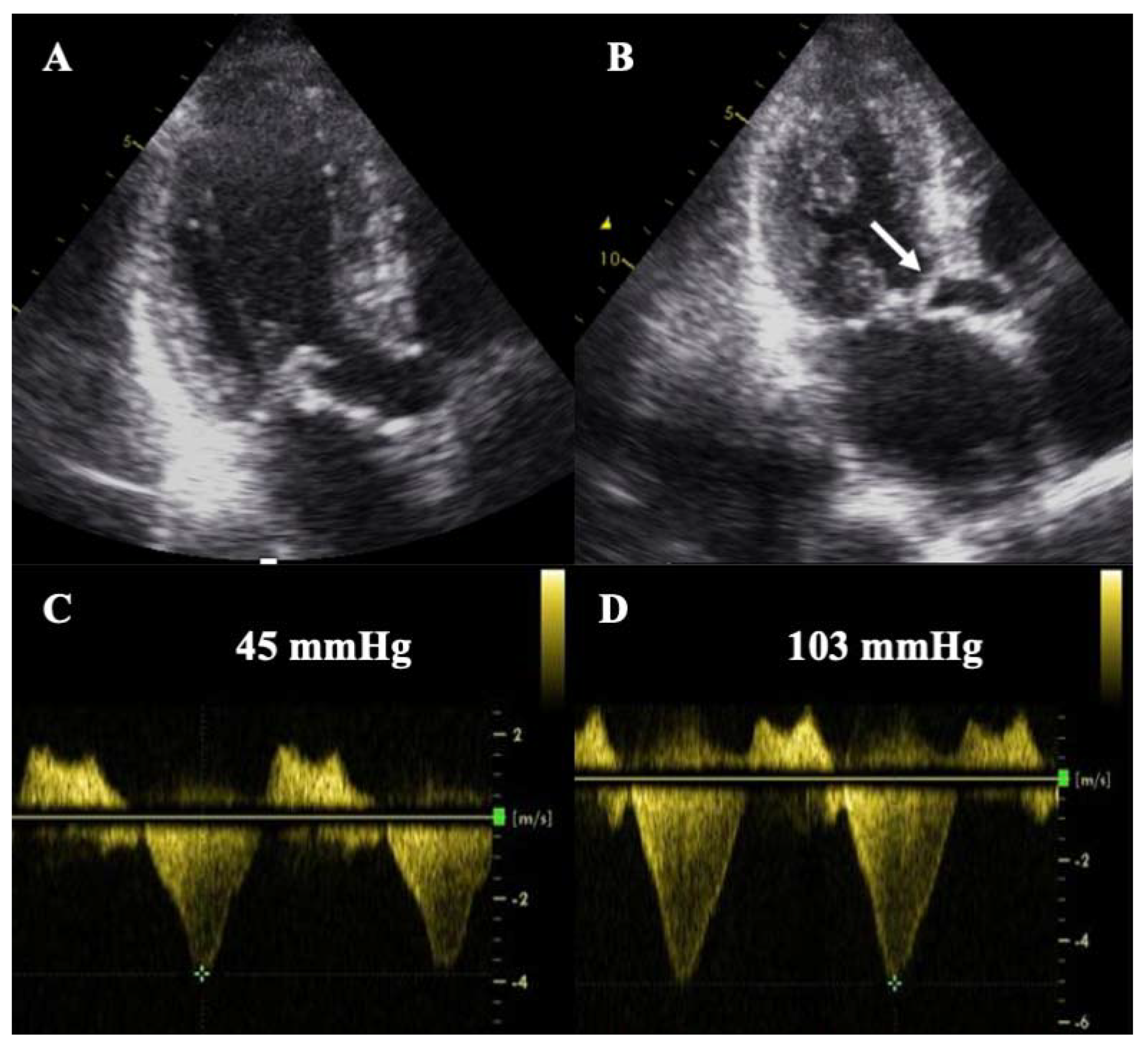
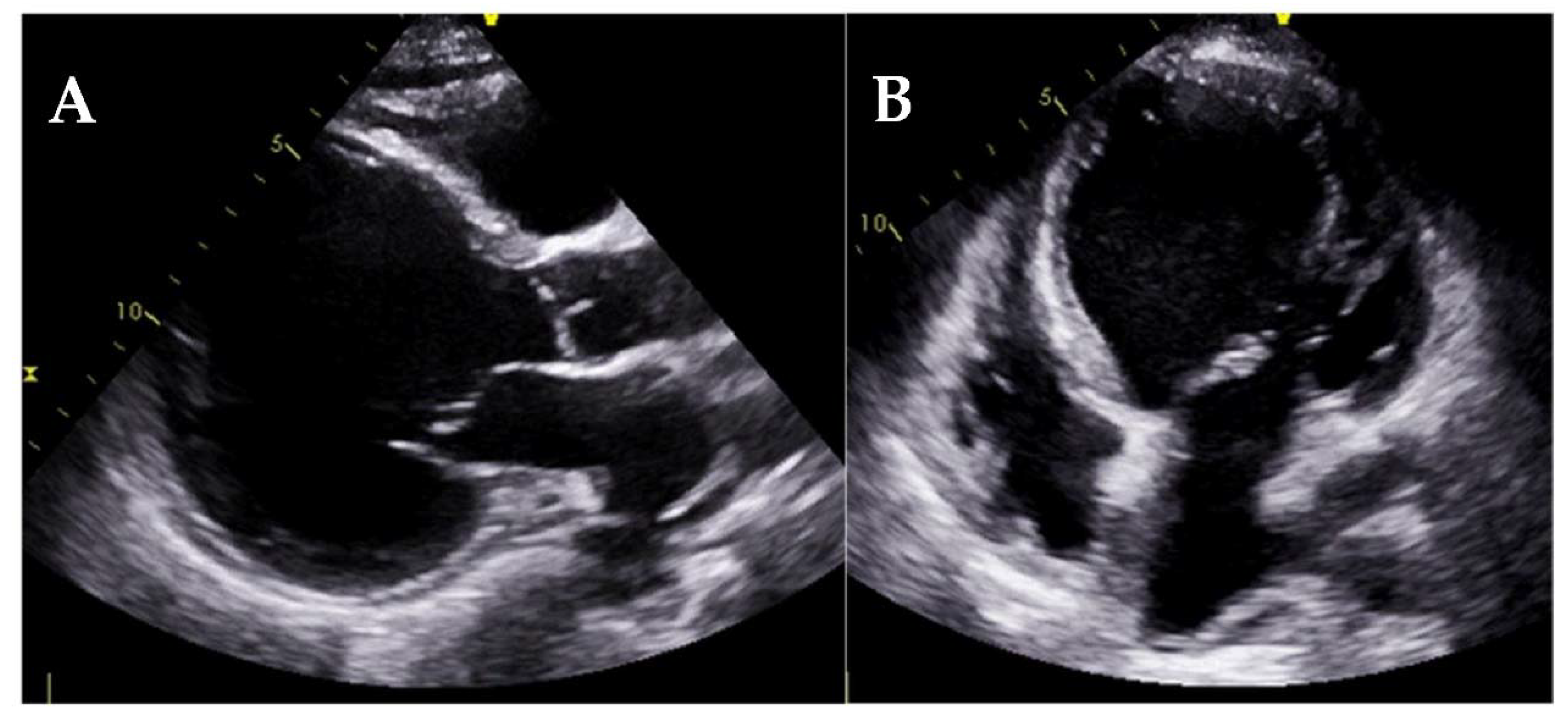
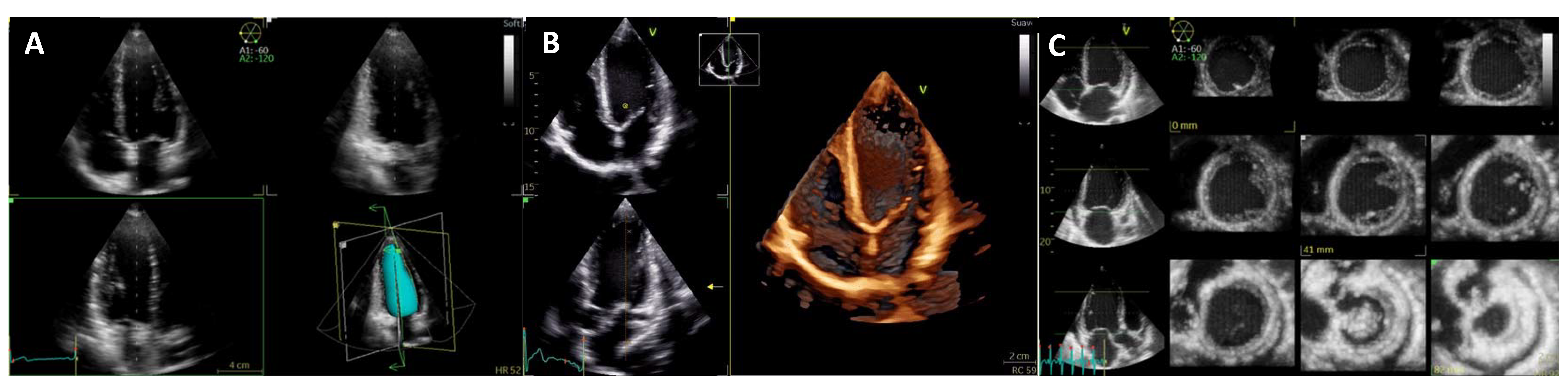
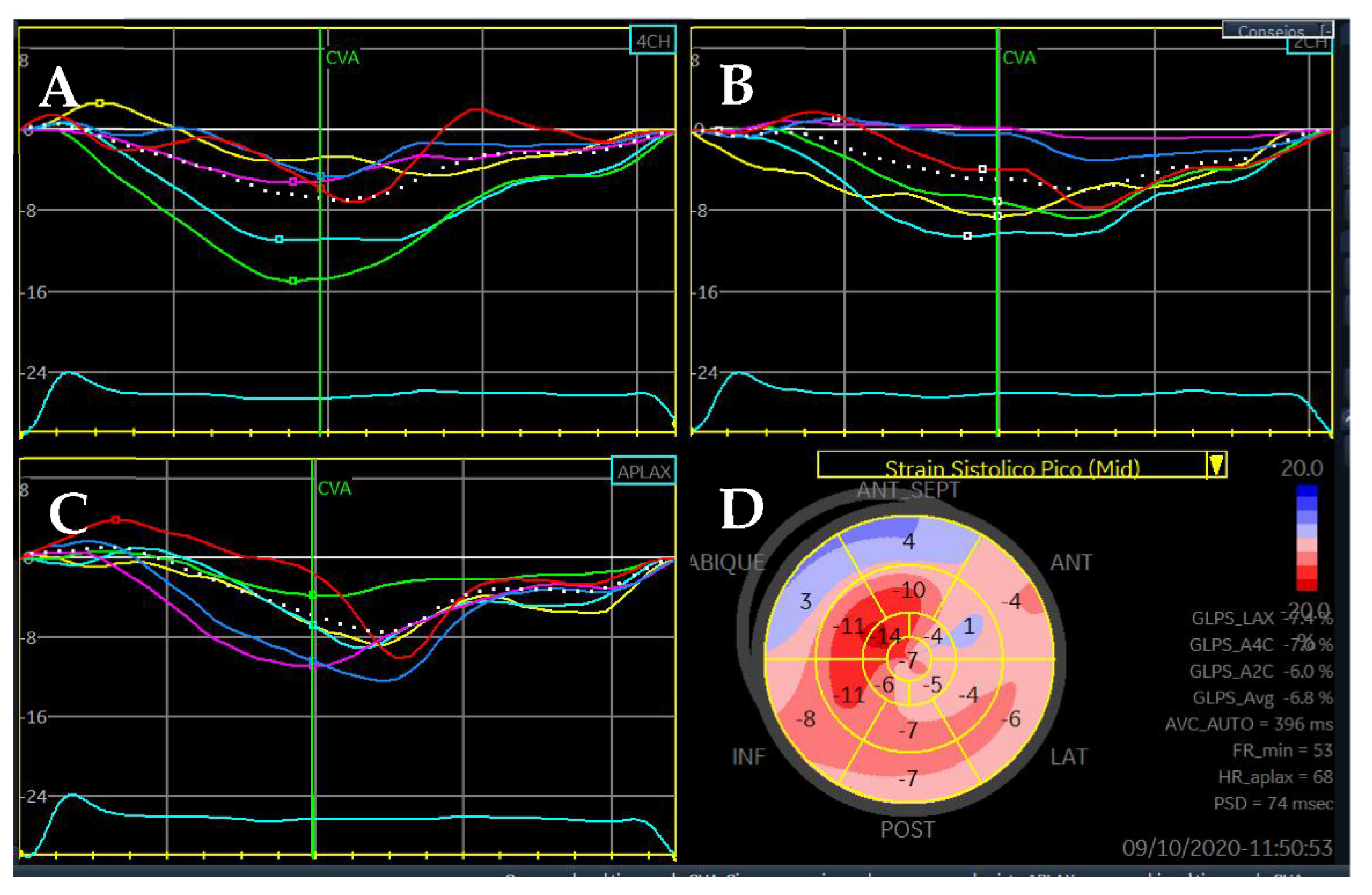
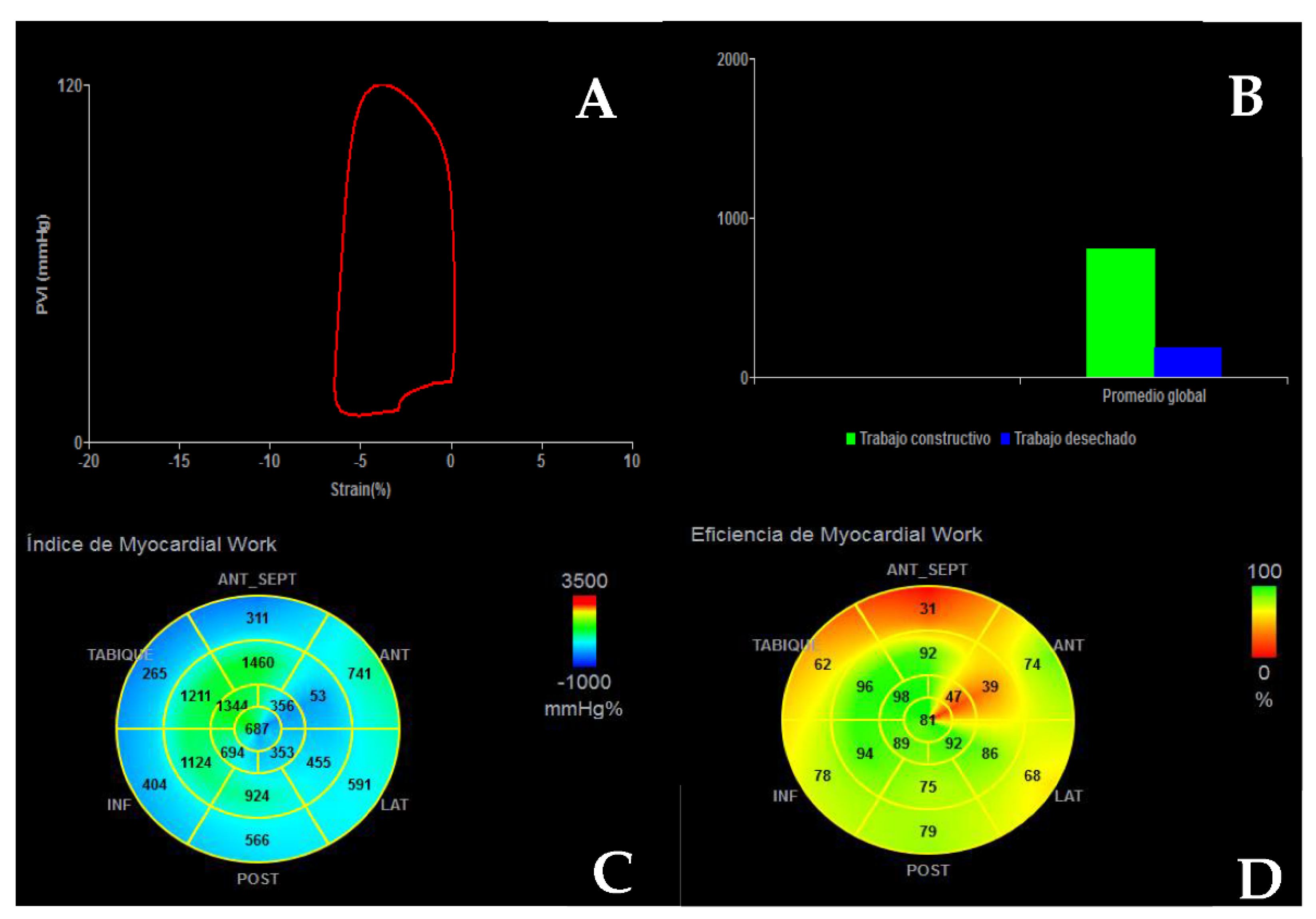

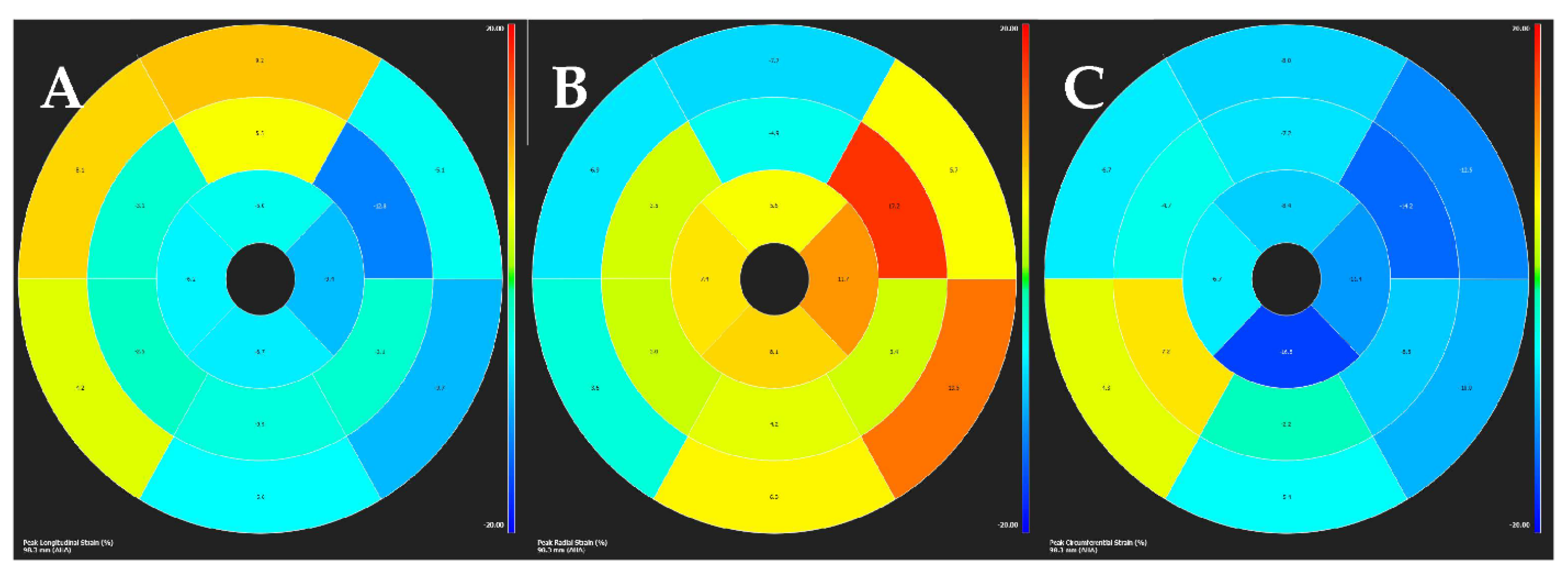
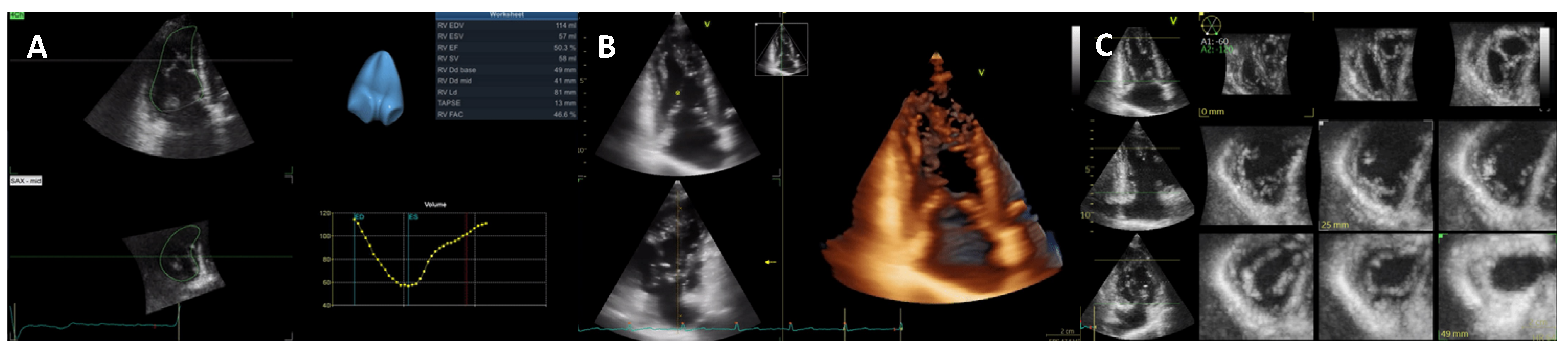
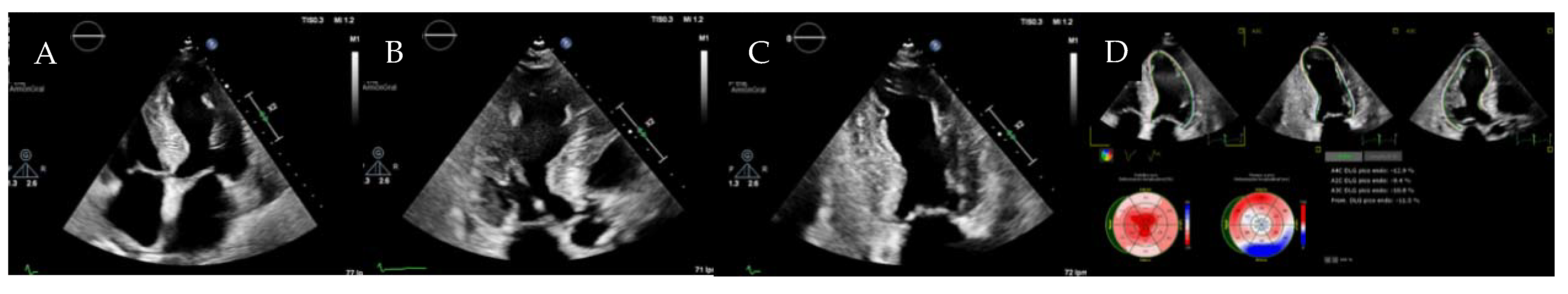
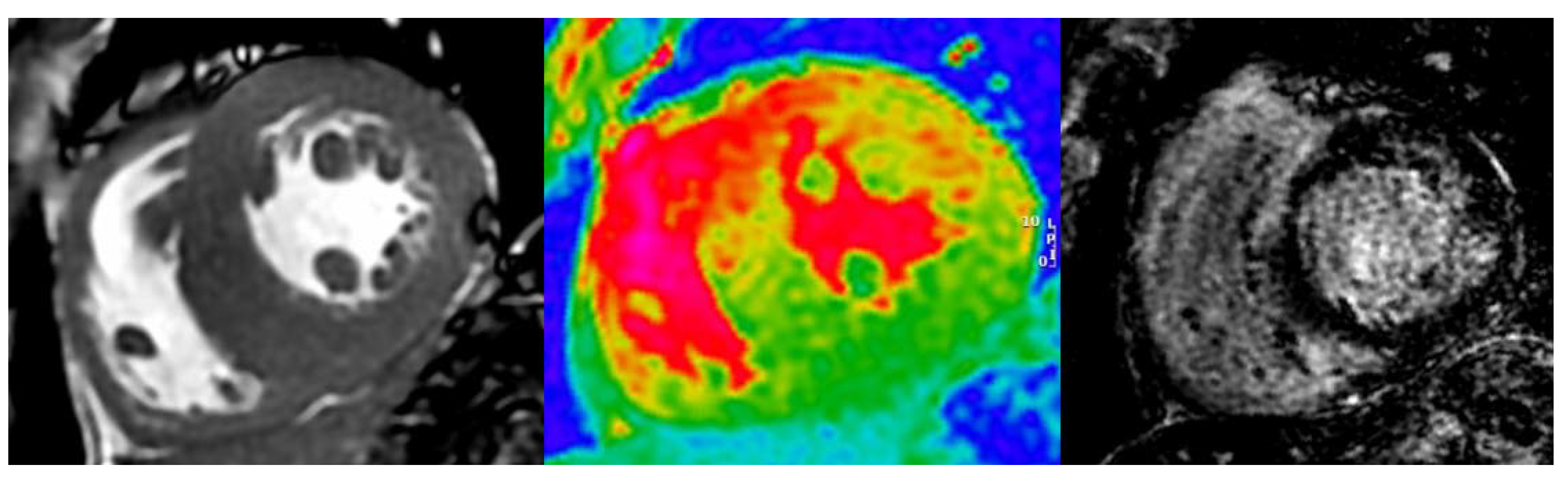
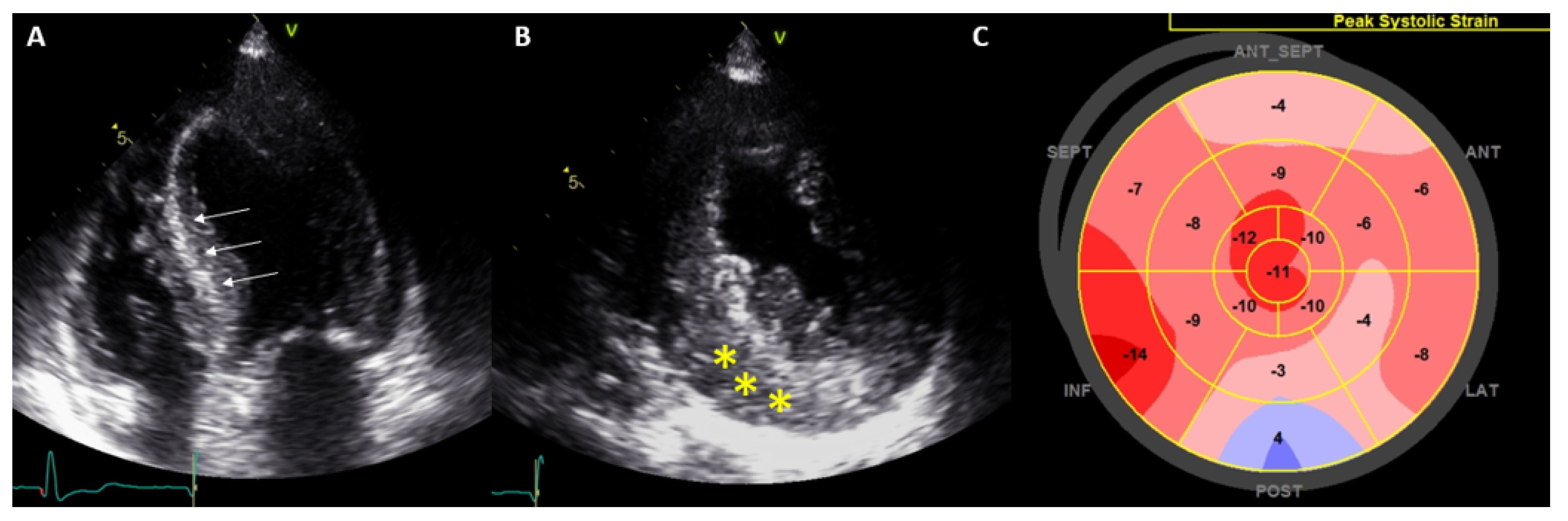
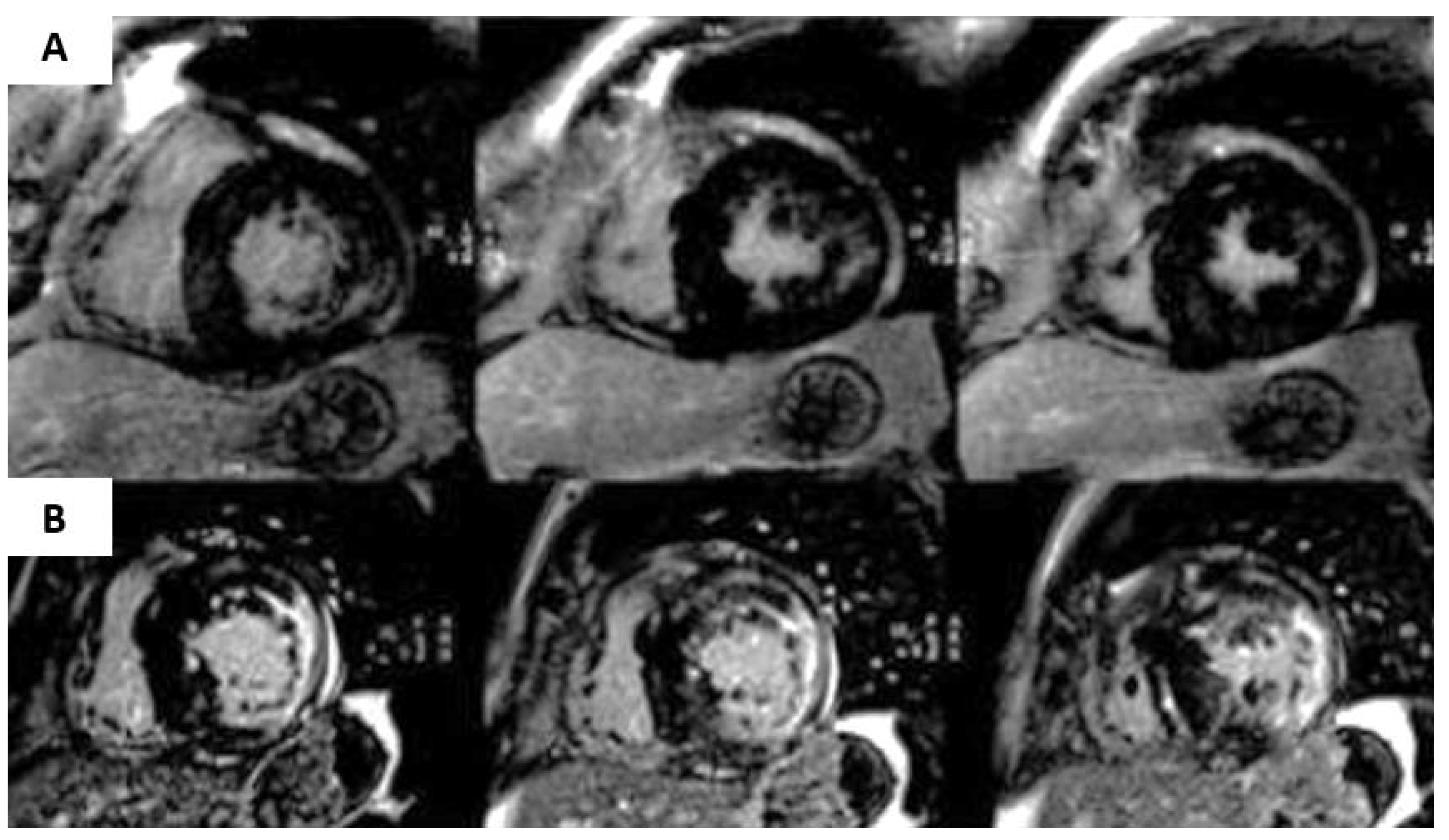
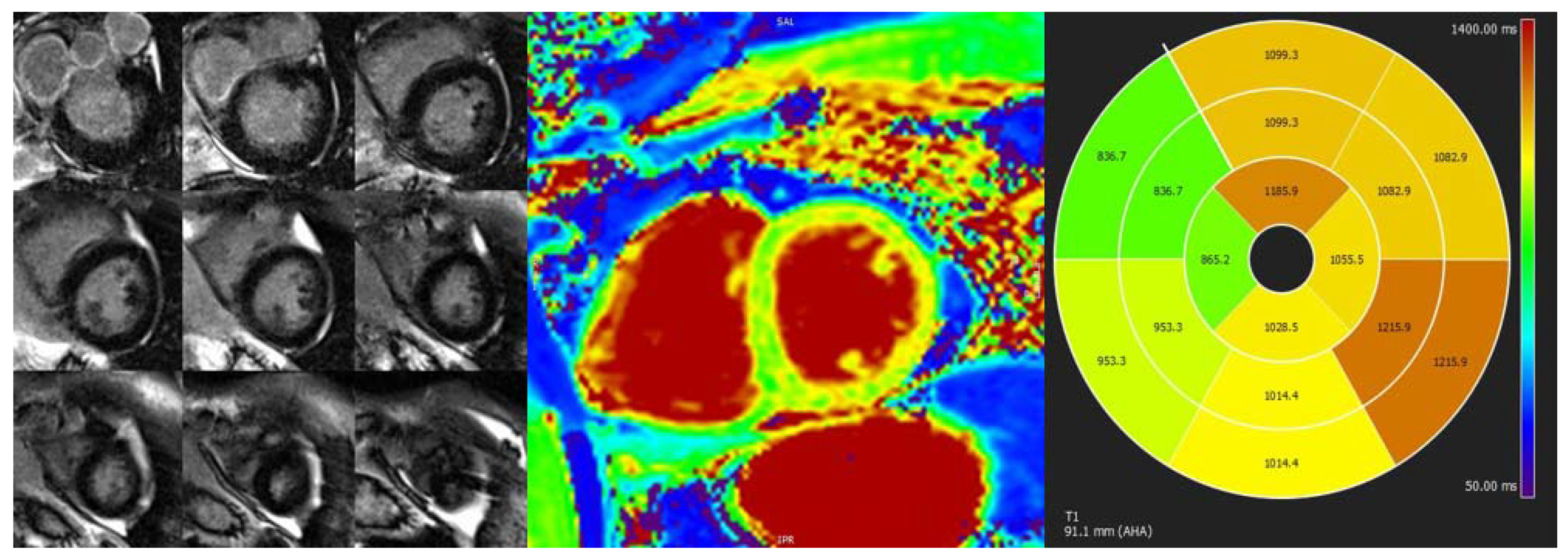
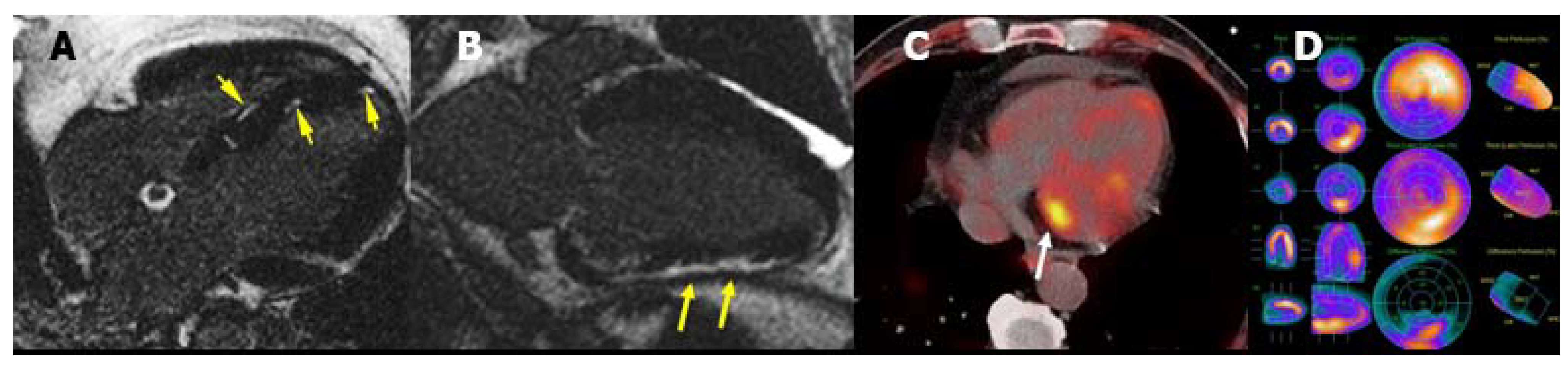

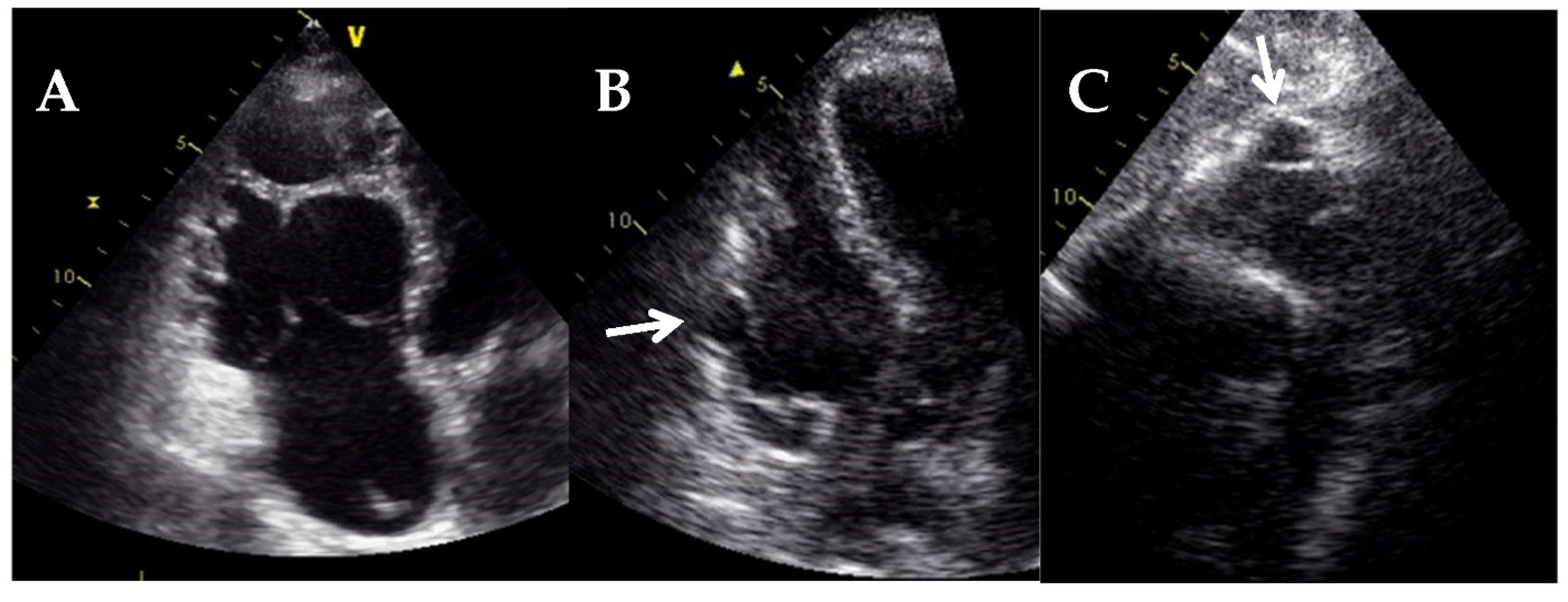
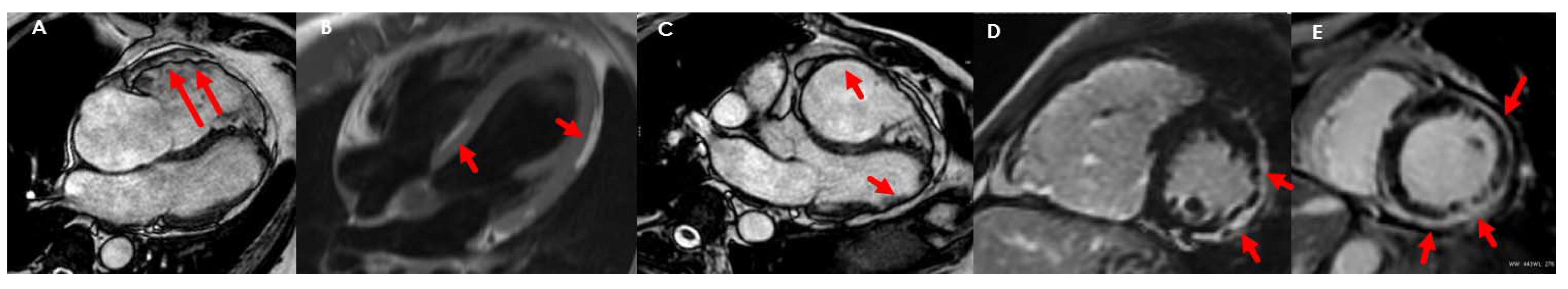
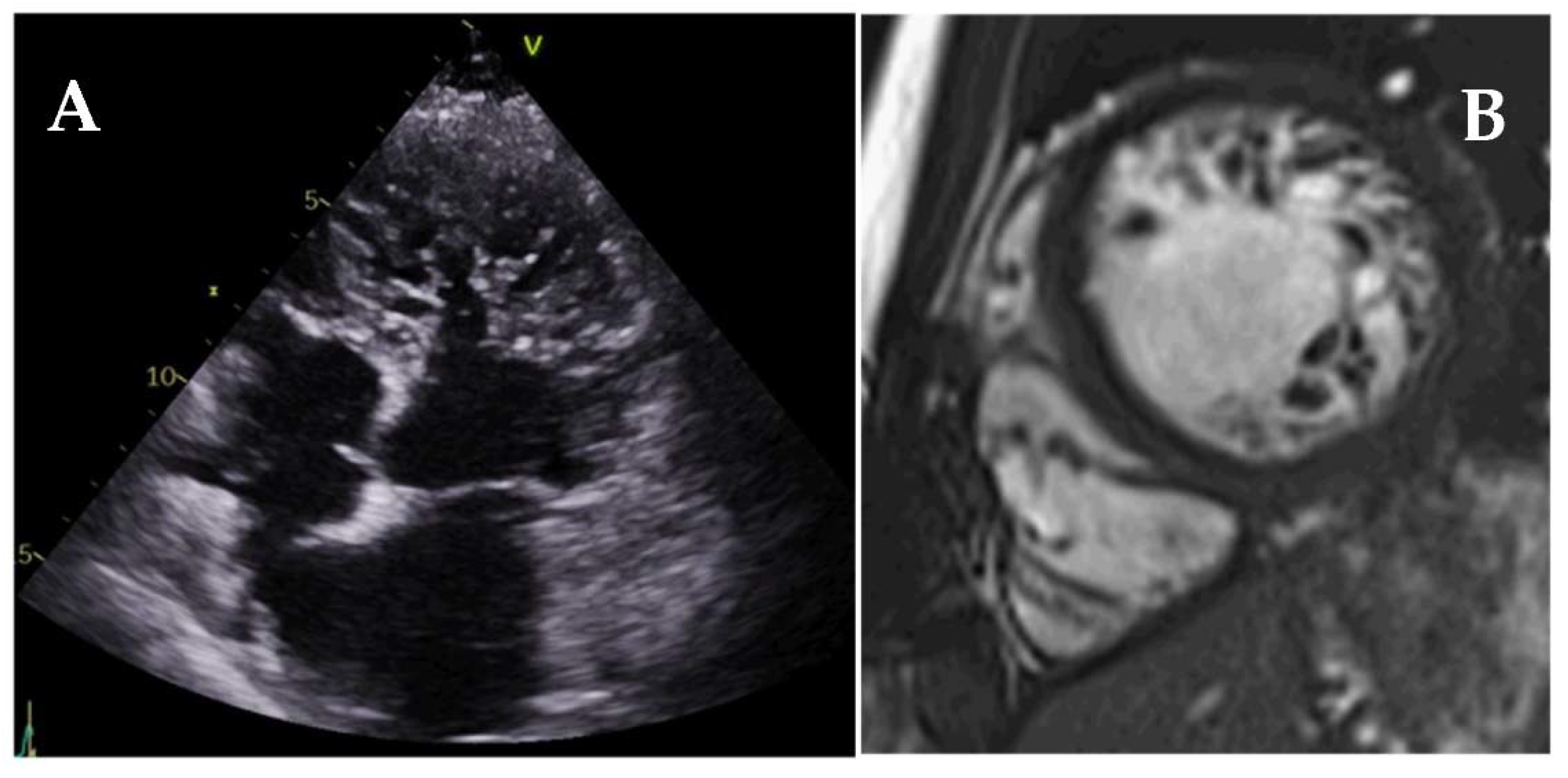

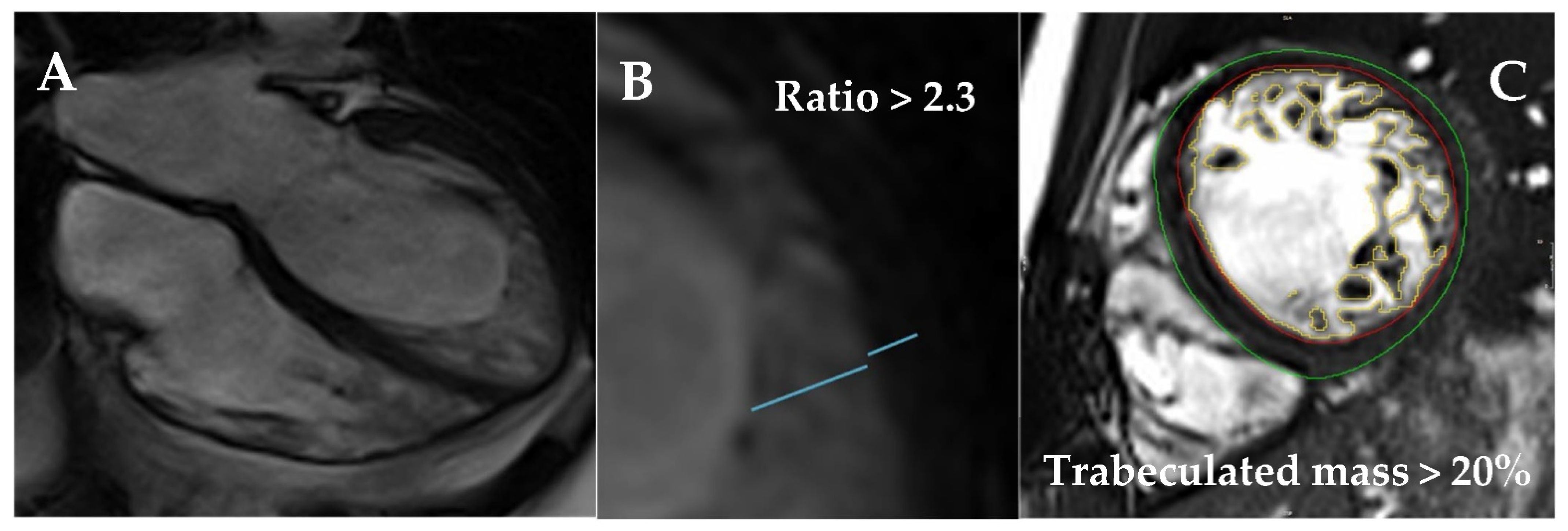
| Diagnostic Criteria | Ancillary Signs | Prognostic Markers |
|---|---|---|
| Maximal wall thickness >15 mm (>13 mm in relatives of HCM patients) | Papillary muscle abnormalities | Maximal wall thickness (>30 mm) |
| Asymmetric septal hypertrophy (ratio septal/posterior wall thickness >1.3 or >1.5 in hypertensive patients) | Mitral valve and subvalvular structure abnormalities | Left atrial size (anteroposterior diameter) |
| Myocardial clefts/crypts | Maximum LVOT gradient (at rest or induced by exercise) | |
| Aneurysms | LVEF < 50% | |
| Abnormal GLS | ||
| Presence, extension, and progression of LGE | ||
| Increased T1/ECV values |
| LVEF ≤ 35% |
| RV systolic dysfunction (RVEF < 45%) |
| Significant (secondary) mitral regurgitation |
| Advanced diastolic dysfunction |
| Abnormal strain and myocardial work values |
| Presence, extension, pattern, and progression of LGE |
| Increased T1 and ECV values |
| Restrictive Cardiomyopathy | |||
|---|---|---|---|
| Non-Infiltrative Disorders | Infiltrative Disorders | Storage Diseases | Endomyocardial Diseases |
|
|
|
|
| ATTR | AL | |
|---|---|---|
| Left ventricular wall thickness and LV mass | ++++ | ++ |
| Asymmetrical septal hypertrophy | 79% | 14% |
| Transmural LGE | 63% | 27% |
| Subendocardial LGE | 24% | 39% |
| Native T1 elevation | ++ | ++++ |
| Native T2 relaxation time | ++ | ++++ |
| ECV | ++++ | ++ |
| Echocardiography | CMR | Scintigraphy |
|---|---|---|
|
|
|
| CARDIAC STRUCTURE | FINDINGS |
|---|---|
| Left ventricle | Concentric left ventricular hypertrophy Binary septum (low sensitivity and specificity for FD) Prominent papillary muscles Preserved LV function until end-stages Diastolic dysfunction Abnormal global longitudinal or radial strain, even in the absence of LVH |
| Right ventricle | Right ventricular hypertrophy Preserved RV function until end-stages Abnormal global longitudinal strain even with preserved EF |
| Atrium | Biauricular dilation Increased end-diastolic pressure Reduced auricular strain |
| Valves | Increase in mitral and aortic valve thickness Valvular regurgitation (generally, mild) |
| Aorta | Dilation of the aortic root and the ascending aorta (not descending aorta) |
| Global and/or Regional Dysfunction and Structural Alterations | |
|---|---|
| Major | By 2D TTE: regional RV akinesia, dyskinesia, or aneurysm and 1 of the following (end diastole):
|
By CMR: regional RV akinesia or dyskinesia or dyssynchronous RV contraction and 1 of the following:
| |
| By RV angiography: regional RV akinesia, dyskinesia, or aneurysm | |
| Minor | By 2D-TTE: regional RV akinesia or dyskinesia and 1 of the following (end-diastole):
|
By CMR: regional RV akinesia or dyskinesia or dyssynchronous RV contraction and 1 of the following
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas, G.; Rodríguez-Palomares, J.F. Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis. J. Clin. Med. 2022, 11, 578. https://doi.org/10.3390/jcm11030578
Casas G, Rodríguez-Palomares JF. Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis. Journal of Clinical Medicine. 2022; 11(3):578. https://doi.org/10.3390/jcm11030578
Chicago/Turabian StyleCasas, Guillem, and José F. Rodríguez-Palomares. 2022. "Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis" Journal of Clinical Medicine 11, no. 3: 578. https://doi.org/10.3390/jcm11030578
APA StyleCasas, G., & Rodríguez-Palomares, J. F. (2022). Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis. Journal of Clinical Medicine, 11(3), 578. https://doi.org/10.3390/jcm11030578





