Abstract
Background: There is clinical interest in determining the effects of low-load blood flow restriction (LL-BFR) resistance training on muscle strength and hypertrophy compared with traditional high- and low-load (HL and LL) resistance training in healthy older adults and the influence of LL-BFR training cuff-pressure on these outcomes. Methods: A search was performed on the MEDLINE, PEDro, CINHAL, Web of Science, Science Direct, Scopus, and CENTRAL databases. Results: The analysis included 14 studies. HL resistance training produces a small increase in muscle strength (eight studies; SMD, −0.23 [−0.41; −0.05]) but not in muscle hypertrophy (six studies; (SMD, 0.08 [−0.22; 0.38]) when compared with LL-BFR resistance training. Compared with traditional LL resistance training, LL-BFR resistance training produces small–moderate increases in muscle strength (seven studies; SMD, 0.44 [0.28; 0.60]) and hypertrophy (two studies; SMD, 0.51 [0.06; 0.96]). There were greater improvements in muscle strength when higher cuff pressures were applied versus traditional LL resistance training but not versus HL resistance training. Conclusions: LL-BFR resistance training results in lower muscle strength gains than HL resistance training and greater than traditional LL resistance training in healthy adults older than 60 years. LL-BFR resistance training promotes a similar muscle hypertrophy to HL resistance training but is greater than that of traditional LL resistance training. Applying cuff pressures above the limb occlusion pressure could enhance the increases in muscle strength compared with traditional LL resistance training.
1. Introduction
Life expectancy has been increasing significantly worldwide in recent years, with an expected doubling of the population older than 60 years predicted to happen by 2050 [1]. The aging process tends to reduce muscle strength and mass, which profoundly affects the functionality and disability [2], resulting in a higher number of falls, hospital admissions, frailty, and mortality [3]. Furthermore, the sedentary behavior that predominates in older age results in the premature onset of ill health and frailty [4] and has detrimental effects on the cardiometabolic markers associated with cardiovascular disease [5].
According to the most recent systematic reviews, resistance training should be considered as the primary non-pharmacological therapy to manage the loss of muscle mass and strength in older adults [6,7]. Resistance training delays or reverses sarcopenia increases the skeletal muscle mass, strength and power, enhances mobility, physical functioning, performance in activities of daily living and psychosocial well-being, preserves independence, and reduces the risk of falls [7,8]. The intensity of the training appears to be a critical variable, with greater effects on the strength gains in older adults from high-load (HL) resistance training compared with moderate- and low-load (LL) resistance training [9,10,11], even in frail older adults [12]. However, HL exercises might be contraindicated for older adults with specific pathological conditions and those unable to lift a sufficient weight to induce hypertrophy [13].
In recent years, a promising new resistance training modality has emerged: blood flow restriction (BFR), which uses a pneumatic cuff to partially or totally occlude the arterial and venous blood flow during exercise [14]. Among the advantages of this type of training is the use of low-intensity training (20–30% of one repetition maximum [1RM]), called LL resistance training with BFR (LL-BFR), which generates physiological stress and an activation of the anabolic pathways that increase muscle size and strength similar to that of traditional HL resistance training (≥70% 1RM) [14,15,16].
Several systematic reviews [17,18,19] have already explored the muscle strength and mass benefits of LL-BFR compared with traditional HL and LL resistance training in older adults; however, the reviews have several limitations that need to be carefully addressed. The reviews included studies with highly heterogeneous samples that did not distinguish between healthy participants and older adult participants with the disease [18,19] and included individuals younger than 60 years (50–60 years) [17,18], who should not be considered as older adults. The reviews’ conclusions might therefore be suspect and require a careful interpretation before extrapolating them to older adults without pathological conditions.
Previous reviews have highlighted the large variability in BFR training protocols, especially regarding the cuff pressure, requiring further study. Several studies on healthy young adults have suggested that high cuff pressures are uncomfortable [20,21] and do not appear to result in enhanced muscle adaptations [22]. Therefore, there is a gap in the knowledge concerning the optimal cuff pressure for the maximum adaptation of aging human skeletal muscle. Identifying the resistance training strategies that offer the same benefits as high-intensity training could counteract the functional decline occurring with progressive age. LL-BFR training might therefore be a more feasible approach to improving the muscle’s strength and function over an individual’s lifetime.
The present systematic review and meta-analysis aimed to provide an update on the effects of LL-BFR resistance training on muscle strength and hypertrophy compared with traditional HL and LL resistance training in healthy adults older than 60 years. As a secondary objective, we analyzed the influence of the cuff pressure of LL-BFR training on muscle strength and hypertrophy in this population.
2. Materials and Methods
This systematic review and meta-analysis followed the criteria of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [23] and was registered in PROSPERO (CRD42022323396).
2.1. Study Selection Criteria
The inclusion and exclusion of the reviewed studies relied on the clinical and methodological aspects based on the PICO (Population-Intervention-Comparison-Outcome of interest) strategy [24].
- Population: The study participants had to be healthy and older than 60 years, in accordance with the currently accepted thresholds for senescence [25], with no gender limitation. No participant was included who had engaged in structured training in the previous 3 months. We excluded the data from participants who had any disorders.
- Intervention and comparison: The included studies had to compare a BFR resistance training against (1) an active HL control group in which resistance training was performed without BFR at high intensities (≥70% of 1RM) or (2) an active LL control group in which resistance training was performed at 20–40% of 1RM without BFR. The minimum training period was 3 weeks, given that this is the minimum period for physiological adaptations to occur with BFR training [26]. BFR interventions on the lower and/or upper limb strength were included.
- Outcomes: The outcomes of interest were the muscle strength and hypertrophy; thus, all the included studies had to assess at least one of these factors. Muscle strength was assessed using maximal dynamic strength by 1RM tests (found directly or reliably estimated from 10RM [27]), measuring the maximal isometric and isokinetic strength. These measures have been found to be valid and reliable in the evaluation of muscle strength in older adults [28,29,30]. The muscle hypertrophy was evaluated by the muscle cross-sectional area, estimated muscle mass, muscle thickness, and body perimeters, all having proven to be valid and reliable methods [31,32,33,34]. These measurements could be performed anywhere on both the upper and lower limb musculature.
- Study design: Only randomized controlled trials and crossover trials were included. Articles were included if they were published in English, Spanish, or Portuguese.
2.2. Search Strategy
The search strategy was performed following the guidelines of Russell-Rose et al. [35]. The searches were conducted in the MEDLINE, PEDro, CINHAL, Web of Science, Science Direct, Scopus, and CENTRAL electronic databases, with no date restrictions, up to 15 March 2022. The search string was created with three sections: the first encompassed synonyms for the MeSH term “aged” (e.g., elderly, older adults, or senior); the second was composed of synonyms for the MeSH term “blood flow restriction therapy” (e.g., restriction training, vascular occlusion, or KAATSU); and the third included a high-quality filter of a randomized controlled trail. To ensure the inclusion of a study with at least one search term within a section, all the synonyms were connected with the “OR” boolean operator, while the sections were connected with the “AND” boolean operator. The search string was adapted to each database, according to the data in File S1. To detect the additional relevant studies, the references of previously published systematic reviews in this field were reviewed. If additional information from the studies was needed, the authors were contacted by e-mail. Two independent reviewers conducted the search using the same methodology (RFG and MGV), and any discrepancies were resolved with the intervention of a third reviewer (ILUV).
2.3. Selection Criteria and Data Extraction
In the first phase, two independent reviewers (RFG and MGV) screened the titles, abstracts, and keywords of the studies following the Cochrane recommendations [36]. In the second phase, full-text copies of peer-reviewed relevant papers were reviewed and checked as to whether they met the inclusion criteria and to identify and record the reasons for excluding ineligible studies. A third reviewer (ILUV) was consulted in case of a disagreement. The relevant data were extracted for each included study (RFG and MGV).
2.4. Methodological Quality and Risk of Bias Assessment
The PEDro scale was employed to assess the quality of the included trials because it is a reliable method for assessing the quality of randomized controlled trials [37,38]. The PEDro scale consists of 11 items, with a maximum score of 10 points. The total score for each study was stratified as follows: poor (<4 points), fair (4–5 points), good (6–8 points), and excellent (9–10 points) [38]. The risk of bias for each included study was assessed in accordance with the Cochrane recommendations using 6 criteria that were individually rated [36]. For each domain, the risk of bias was categorized as high, low, or uncertain, and the reasons were recorded along with a descriptive justification for the judgement. In the “other bias” category, we clarified the specific criteria that could have affected the results.
Two independent trained assessors (RFG and MGV) examined the quality and risk of bias of the selected studies using the same methods, and disagreements were resolved by a consensus or by consulting the third reviewer (ILUV). The inter-rater reliability was determined using the Kappa coefficient: (1) >0.81–1.00 indicated an excellent agreement between the assessors; (2) 0.61–0.80 indicated a good agreement; (3) 0.41–0.60 indicated a moderate agreement; and (4) 0.21–0.40 indicated a poor agreement [39].
2.5. Qualitative Analysis
The qualitative analysis was performed according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE), following the recommendations of Andrews et al. [40].
2.6. Data Analysis
The statistical analysis was performed with RStudio 3.0 software using the ‘metaphor’ and ‘esc’ packages. All the significance tests were conducted at a level of 5%. A meta-analysis was performed only when the data for the analyzed variables were represented in at least 3 studies/comparisons. The data synthesis was categorized by groups according to the degree of limb cuff pressure: the cuff pressure near the limb occlusion pressure versus being greater than the limb occlusion pressure [41].
To increase the accuracy and thus the generalizability of our analyses, multiple comparisons from several studies (e.g., isotonic and isometric force measures) were included in all the analyses [42]. In the pre- and post-intervention, the mean difference and standard deviation (SD) values for the muscle strength and hypertrophy in each study/comparison were used to calculate the standardized mean difference (SMD). The change in the SD was calculated in accordance with the Cochrane recommendations, using the most conservative model (r = 0) [36]. When necessary, the mean scores and SDs were estimated from the graphs.
The summary statistics for all the analyses are presented using forest plots. A random-effects model was employed to determine the overall effect size (SMD). The effect size of the statistical significance of the overall SMD was examined using Hedges’ g and interpreted as follows: (1) a small effect (g = 0.20–0.49); (2) a moderate effect (g = 0.50–0.79); and (3) a large effect (g ≥ 0.80) [43].
The degree of heterogeneity among the studies was estimated using Cochran’s Q statistic test and the inconsistency index (I2) [44]. Heterogeneity was considered when the Cochran’s Q statistic test was significant (p < 0.1) and/or the I2 was >50% [45].
Influential or outlier studies were investigated according to the recommendations of Viechtbauer and Cheung [46]. The robustness of the results obtained in the meta-analysis was assessed with a leave-one-out sensitivity analysis [47].
To detect any publication bias, the funnel plots were visually assessed, with an asymmetric graph considered to indicate the presence of bias. Egger’s regression test was employed to quantitatively assess the publication bias [48,49].
3. Results
3.1. Study Selection
The search strategy yielded a total of 664 citations. After excluding the articles not meeting the inclusion criteria and after including supplementary articles by cross-references, a total of 14 studies were considered for the final analysis. Figure 1 displays the flowchart of the search strategy.
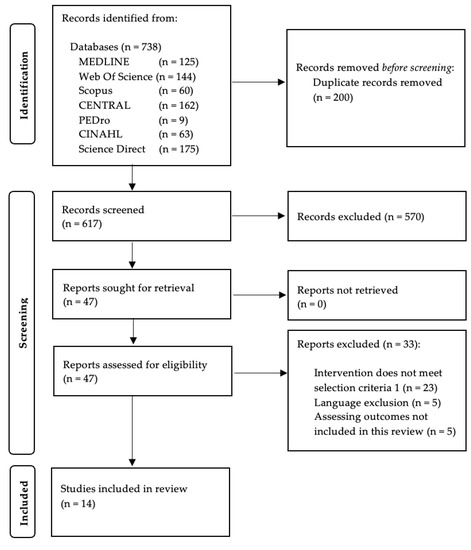
Figure 1.
PRISMA flow diagram.
3.2. Characteristics of the Included Studies
A total of 340 healthy older adults were included (mean age 69.42 years, 220 women and 120 men) (Table 1). Eight studies examined the effects of LL-BFR training versus traditional HL resistance training [28,50,51,52,53,54,55,56], and seven studies evaluated the effects of LL-BFR training versus traditional LL resistance training [28,57,58,59,60,61,62]. The LL-BFR training workload was set at 20–40% 1RM, whereas the traditional HL resistance training workload was 70–80% 1RM, and the traditional LL resistance training workload was 20–30% 1RM. The prescribed cuff pressures varied widely, ranging from 67 mm Hg [56] to 270 mm Hg [60,61]. Seven studies employed cuff pressures greater than the limb occlusion pressure [28,50,53,54,55,60,61], and eight studies employed cuff pressures near the limb occlusion pressures [28,50,51,53,56,57,60,61]. Only Letieri et al. [28] included two BFR groups, one employing high cuff pressures and the other employing low cuff pressures. Most of the program lasted 12 weeks [50,51,54,55,56,60,61]; the shorter interventions were 4 weeks long [53,58,59] and the longest lasted 16 weeks [28]. The training was implemented two [50,51,52,54,55,60,61,62] and four [56] times per week. Eight studies included lower limb training [28,50,51,54,55,56,58,62], three studies included upper limb training [53,60,61], and three studies included upper and lower limb training [52,57,59] (Table 1). None of the studies reported serious adverse events.

Table 1.
Methodological characteristics and results of included studies.
3.3. Methodological Quality and Risk of Bias of the Included Studies
The mean PEDro score of the included studies was 5.6 (range 4–7) (Table 2). The level of inter-evaluator agreement was high for the inter-rater reliability (к = 0.86).

Table 2.
PEDro scores for included studies (n = 14).
The risk of bias assessment of the included trials is summarized in File S2. In general, the risk of bias of the included trials in the current meta-analysis was low. The highest risk of bias was found in the reporting bias and adequate stopping rules. However, 11 studies had an unclear risk of concealment of random allocation and the blinding of the outcome assessors. Due to the nature of the groups selected for this meta-analysis, all the participants underwent prescribed physical exercise (LL, HL, or LL-BFR resistance training); therefore, we judged all the studies to be at low risk of performance bias. The totality of the assessed studies adhered to strict protocols, a necessary requirement in exercise interventions in order to reduce the risk of differential behavior by the personnel delivering the intervention [63].
3.4. Low-Load Blood Flow Restriction Training versus High-Load Resistance Training
There was moderate-quality evidence from 8 studies [28,50,51,52,53,54,55,56] (23 comparisons; n = 189) that HL resistance training produces a small and statistically significant increase in muscle strength compared with LL-BFR training (SMD = −0.23 [−0.41; −0.05]; Z = −2.52; p = 0.012) (Figure 2 and Table 3). There was low-quality evidence from six studies [50,51,53,54,55,56] (9 comparisons; n = 114), given that there was no statistically significant difference between the two types of training for the muscle hypertrophy (SMD = 0.08 [−0.22; 0.38]; Z = 0.52; p = 0.60) (Figure 2 and Table 3). The heterogeneity was not significant, with an I2 of 0% for both the muscle strength and hypertrophy analysis (strength: Q = 10.48; p = 0.98; hypertrophy: Q = 0.28; p = 1.00). For both the muscle strength and hypertrophy analyses, no single study significantly affected the overall SMD, and no evidence of publication bias was detected (symmetrical shape of the funnel plot; Egger’s test, p > 0.05) (Files S3 and S4).
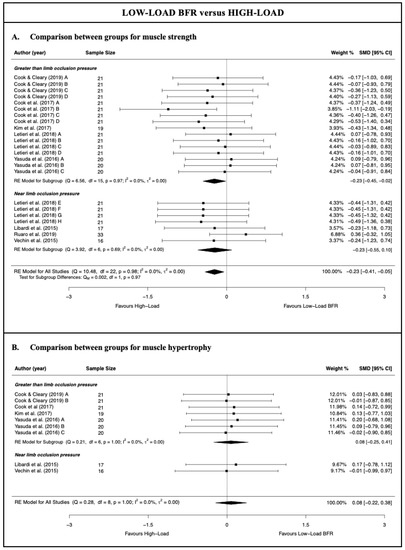
Figure 2.
Synthesis forest plot for muscle strength and hypertrophy for low-load blood flow restriction training versus high-load resistance training [28,50,51,52,53,54,55,56].

Table 3.
GRADE evidence profile for the effects of low-load blood flow restriction training.
With respect to the subgroup meta-analysis according to the degree of limb cuff pressure applied to perform the training, the results showed no statistically significant differences in terms of the muscle strength between the overall SMD obtained by applying pressure greater than or near that which was required to occlude the limb and that obtained with the HL resistance training (p = 0.97). In contrast to the meta-analysis results from the subgroup with that greater than the limb occlusion pressure, the subgroup with the near limb occlusion pressure showed a similar increase in muscle strength to that obtained with HL resistance training (moderate-quality evidence; four studies [28,51,52,56] and seven comparisons; n = 87; SMD = −0.23 [−0.55; 0.10]; Z = −1.37; p = 0.17). The Ruaro et al. [52] study likely had a strong influence on the results of the meta-analysis; however, that study could be considered as an outlier. In fact, the leave-one-out analysis suggested that when removing the Ruaro et al. [52] study, HL resistance training produced a greater increase in muscle strength than LL-BFR training at the near limb occlusion pressure.
For the muscle hypertrophy, a subgroup meta-analysis could be performed only for the studies which applied that greater than the limb occlusion pressure, given that only two studies/comparisons applied a near limb occlusion pressure. The results of the meta-analysis for this subgroup were very similar to those of the meta-analysis for all the studies, reporting no statistically significant differences between the application of HL resistance training and LL-BFR resistance training for the muscle hypertrophy (low-quality evidence; four studies [50,53,54,55] and seven comparisons; n = 81; SMD = 0.08 [−0.25; 0.41]; Z = 0.46; p = 0.65). No single study significantly affected the overall SMD obtained for this subgroup.
3.5. Low-Load Blood Flow Restriction versus Traditional Low-Load Resistance Training
The meta-analysis results showed that LL-BFR training produces a small–moderate and statistically significant increase in the muscle strength (moderate-quality evidence; 7 studies [28,57,58,59,60,61,62] and 25 comparisons; n = 201; SMD = 0.44 [0.28; 0.60]; Z = 5.45; p < 0.001) and a moderate and statistically significant increase in muscle hypertrophy (low-quality evidence; two studies [60,61] and five comparisons; n = 31; SMD = 0.51 [0.06; 0.96]; Z = 2.22; p = 0.026) compared with traditional LL resistance training (Figure 3 and Table 3). The heterogeneity was not significant, with an I2 of 0% for both the muscle strength and hypertrophy analysis (strength: Q = 19.51; p = 0.72; hypertrophy: Q = 0.94; p = 0.92). For both the muscle strength and hypertrophy analyses, no outlier studies and no evidence of publication bias were detected (symmetrical shape of the funnel plot; Egger’s test, p > 0.05) (Files S5 and S6). However, eliminating the Yasuda et al. 2015a [60] study would imply an absence of statistically significant differences in muscle hypertrophy between the two training modalities.
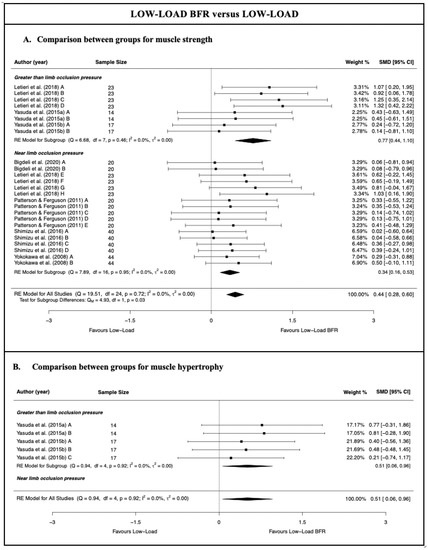
Figure 3.
Synthesis forest plot for muscle strength and hypertrophy for low-load blood flow restriction training versus traditional low-load resistance training [28,57,58,59,60,61,62].
For muscle strength, the results of the subgroup meta-analysis showed that, compared with traditional LL resistance training, LL-BFR training applied with a greater than limb occlusion pressure produced a greater increase in muscle strength than LL-BFR training applied with near limb occlusion pressure (p = 0.026). Although there is moderate-quality evidence that both LL-BFR subgroups obtained statistically significant differences compared with traditional LL resistance training, LL-BFR applied with a greater than limb occlusion pressure obtained a large overall effect size (three studies [28,60,61] and eight comparisons; n = 54; SMD = 0.77 [0.44; 1.10]; Z = 4.57; p < 0.001), whereas LL-BFR applied with the near limb occlusion pressure obtained a small overall effect size (5 studies [28,57,58,59,62] and 17 comparisons; n = 147; SMD = 0.34 [0.16; 0.53]; Z = 3.72; p < 0.001). No single study significantly affected the overall SMD obtained for these subgroups.
No subgroup meta-analysis by the degree of the limb cuff pressure could be performed for muscle hypertrophy, given that no study had applied near limb occlusion pressure.
4. Discussion
This is the first study to systematically assess the effect of LL-BFR resistance training compared with traditional resistance training on the muscle strength and hypertrophy in a healthy population older than 60 years, achieving the lowest heterogeneity compared with previous reviews [17,18,19] and allowing for a better interpretation of the results. The review provided moderate-quality evidence suggesting that, compared with traditional HL resistance training, LL-BFR resistance training promotes lower muscle strength gains, as well as low-quality evidence showing similar improvements in muscle hypertrophy in older adults. However, LL-BFR resistance training offered greater increases in terms of muscle strength (moderate-quality evidence) and hypertrophy (low-quality evidence) than traditional LL resistance training in this population. This study is also a pioneering review of the influence of BFR cuff pressure on muscle strength and hypertrophy in adults older than 60 years. There was no difference in either the muscle strength or hypertrophy when applying a pressure greater than or near the pressure required to occlude the limb blood flow compared with traditional HL resistance training. Nevertheless, greater improvements occurred in the muscle strength when higher cuff pressures were applied versus traditional LL resistance training.
The pooled data suggest that traditional HL resistance training is more effective than LL-BFR resistance training for increasing the muscle’s strength but not the hypertrophy (although it is correlated, an increased muscle strength is not always reflected in an increased muscle size [64]). However, the low-effect sizes observed are still too small to make this assertion with certainty. These results are consistent with those found in healthy younger and older adults [17,18,65]. HL resistance training has been reported to cause greater mechanical strain on the trained musculature than LL-BFR resistance training [66]. This increased strain can occur by mechanotransduction, enhancing the release of localized hormones [67] and the recruitment of fast twitch fibers [68], mechanisms that promote increased muscle strength levels. In healthy young adults, the strength gains attributable to neural adaptations are approximately 60% [69]; in older adults, however, the contribution of neural adaptations is even greater [70]. Studies have reported that there is greater electromyographic activity in HL resistance training (approximately 80% of maximum voluntary isometric contraction [MVIC]) compared with LL-BFR resistance training (approximately 30% MVIC) [68,71], inducing neuronal plasticity that leads to a greater recruitment of motor units in the medium term and thus a greater adaptation in terms of muscle strength.
Based on the results of this review, LL-BFR resistance training offers superior increases in the muscle strength and hypertrophy than traditional LL resistance training. The effect sizes observed indicate a greater robustness in the results. These findings agree with those in the literature regarding older adults [17,18], younger adults [65], and those with musculoskeletal injuries [72]. Metabolic stress appears to play a major role in the physical effects of LL-BFR resistance training, given that a similar mechanical strain has been suggested between traditional LL resistance training and LL-BFR resistance training [66,68]. In contrast to traditional LL resistance training, there is a lower oxygen bioavailability during BFR training [73], which accelerates the onset of anaerobic glycolysis in muscles [21], and consequently the accumulation of fatigue metabolites such as lactate [21,59,73,74] and hydrogen ions [21]. These mechanisms correlate with local hormone secretion [62,73,74], cell swelling [75], muscle damage [76], and the increased production of reactive oxygen species [77], all of which are related to the anabolic muscle phenomena.
Our study’s second aim was to determine the effect of LL resistance training with the effect that varying degrees of BFR cuff pressure has on the muscle strength and hypertrophy in this population. Not even the high pressures applied to the cuff during LL-BFR resistance training mimicked the benefits of HL resistance training, suggesting that the additional metabolic stress of BFR is not comparable to the mechanical and metabolic stress provided by HL resistance training. However, when comparing LL-BFR resistance training versus traditional LL resistance training, higher muscle strength increases were observed when higher cuff pressures were applied. These findings differ from those obtained in a previous meta-analysis, which indicated that the cuff pressure in BFR resistance training did not influence muscle strength and hypertrophy in older adults [18]. Nevertheless, these results should be interpreted with caution, given that the review included studies with individuals younger than 60 years with various conditions that could influence the results. In addition, that meta-analysis considered the cuff pressure value in mmHg, whereas our meta-analysis dichotomized this variable according to whether the cuff pressure was greater than the limb occlusion pressure. Using the absolute value of the cuff pressure may be slightly more inaccurate, as the pressure required to occlude a limb depends on other variables such as the limb circumference. Hence, it might be better to analyze whether a pressure greater than the limb occlusion pressure was applied to the limb than the absolute value. Physiologically, a higher restriction pressure could imply a lower blood supply and consequently higher metabolic stress during exercise, which could cause a greater increase in muscle strength and hypertrophy. This hypothesis is supported by the results of the study by Letieri et al. [28] with two groups that performed BFR resistance training using high (185.75 ± 5.45 mm Hg) and low (105.45 ± 6.5 mm Hg) cuff pressures with the same methodology. The authors observed greater increases in the muscle strength and hypertrophy in the high-pressure group after 6 weeks of training. In contrast, other studies have observed no differences between the groups with different cuff pressures in only one session [78,79]. The discrepancy between the results could partly be due to the duration of the interventions. Therefore, there is a need to investigate the middle-term effects of the cuff pressure on muscle strength and hypertrophy.
Based on the results of this review, the pressure applied to the cuff during LL-BFR resistance training could affect exercise adaptations. However, given that the differences are small and based on few studies, these conclusions have been interpreted with caution. In fact, the Ruaro et al. [52] study could be considered to be an outlier among studies employing pressures near the limb occlusion pressure because BFR resistance training was only applied to a wrist flexion exercise, which was a movement not included in the HL resistance training group. Given that no improvements were expected in the HL resistance training group, the benefits of the LL-BFR intervention were therefore exaggerated. The leave-one-out analysis suggested that, when removing the Ruaro et al. [52] study, HL resistance training produced a greater increase in muscle strength than LL-BFR resistance training with a near limb occlusion pressure. More research is needed to investigate the clinical and physiological effects of high or low pressures applied to the cuff during LL-BFR resistance training.
Within the clinical implications of this review, the most effective intervention to counteract the progressive physiological deterioration that occurs with age appears to be HL resistance training. However, the use of LL-BFR resistance training could represent an alternative training method for individuals intolerant to higher-intensity training protocols and in cases where its application is contraindicated. Thus, for healthy adults older than 60 years without contraindications, LL-BFR resistance training may be prescribed in combination with HL resistance training to aim for optimal muscular strength and hypertrophy responses. It is well known that LL-BFR resistance training is more uncomfortable than traditional LL resistance training [57,80]. Therefore, motivated older adults could start with LL-BFR resistance training while the less motivated could start with traditional LL resistance training and progressively integrate BFR resistance training. After adapting to the cuff, higher pressures could be introduced, given that higher pressures are presumed to be more effective in improving muscle strength in older adults.
This systematic review and meta-analysis presents certain limitations. The training protocols used in the included studies are widely heterogeneous in terms of their duration, type of exercise (i.e., static or dynamic contractions), and BFR application parameters. Optimal protocols for BFR resistance training are still to be established and are needed to standardize the trial interventions. The studies included in certain sub-analyses are scarce, which could lead to possible changes in the conclusions if new studies are added. No subgroup meta-analysis according to the degree of the limb cuff pressure could be performed for muscle hypertrophy. The small sample size and the high risk of bias in the treatment effect estimates for the selected studies could also have influenced the results of this systematic review. More clinical trials with a high methodological quality are therefore needed to overcome these limitations.
5. Conclusions
There was moderate-quality evidence that, compared with HL resistance training, LL-BFR resistance training promotes lower muscle strength gains but similar improvements in the muscle hypertrophy (low-quality evidence) in a healthy population older than 60 years. The benefits in terms of the muscle strength (moderate-quality evidence) and hypertrophy (low-quality evidence) were greater for LL-BFR resistance training than with traditional LL resistance training in older adults. There was no difference in either the muscle strength or hypertrophy when applying pressure greater than or near the pressure required to occlude the limb blood flow compared with HL resistance training. However, it appears that applying cuff pressures above the limb occlusion pressure could enhance increases in the muscle strength compared with traditional LL resistance training (moderate-quality evidence). In this systematic review and meta-analysis update, we found evidence of an increased interest in the efficacy of LL-BFR resistance training on the muscle strength and hypertrophy in healthy older adults; however, the certainty of the evidence using the GRADE methodology is still low to moderate.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11247389/s1, File S1: Search strategy; File S2: Risk of bias summary and graph; File S3: Sensitivity and Publication Bias Funnel Plots for the Comparison in Muscle strength be-tween Low-Load Blood Flow Restriction Resistance Training and High-Load Resistance Training; File S4: Sensitivity and Publication Bias Funnel Plots for the Comparison in Muscle Hypertrophy between Low-Load Blood Flow Restriction Resistance Training and High-Load Resistance Training; File S5: Sensitivity and Publication Bias Funnel Plots for the Comparison in Muscle Strength be-tween Low-Load Blood Flow Restriction Resistance Training and Traditional Low-Load Resistance Training; File S6: Sensitivity and Publication Bias Funnel Plots for the Comparison in Muscle Hypertrophy between Low-Load Blood Flow Restriction Resistance Training and Traditional Low-Load Resistance Training.
Author Contributions
I.L.-d.-U.-V. devised the project, the main conceptual ideas, investigation, methodology, and the formal analysis. R.F.-G. and M.G.-V. performed the data curation and wrote the original draft; J.I.-G., G.P.-M. and T.d.C. supervised the manuscript writing and contributed to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This systematic review and meta-analysis followed the criteria of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines and was registered in PROSPERO (CRD42022323396).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Ageing and Health; World Health Organization: Geneva, Switzerland, 2021.
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology 2016, 17, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.J.; Stebbings, G.K.; Onambele, G.L. The emergence of sedentary behaviour physiology and its effects on the cardiometabolic profile in young and older adults. Age 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Grgic, J.; Garofolini, A.; Orazem, J.; Sabol, F.; Schoenfeld, B.J.; Pedisic, Z. Effects of Resistance Training on Muscle Size and Strength in Very Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sport Med. 2020, 50, 1983–1999. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Papa, E.V.; Dong, X.; Hassan, M. Resistance training for activity limitations in older adults with skeletal muscle function deficits: A systematic review. Clin. Interv. Aging 2017, 12, 955–961. [Google Scholar] [CrossRef]
- Csapo, R.; Alegre, L.M. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: A meta-analysis. Scand. J. Med. Sci. Sports 2016, 26, 995–1006. [Google Scholar] [CrossRef]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef]
- Steib, S.; Schoene, D.; Pfeifer, K. Dose-response relationship of resistance training in older adults: A meta-analysis. Med. Sci. Sports Exerc. 2010, 42, 902–914. [Google Scholar] [CrossRef]
- Valenzuela, T. Efficacy of progressive resistance training interventions in older adults in nursing homes: A systematic review. J. Am. Med. Dir. Assoc. 2012, 13, 418–428. [Google Scholar] [CrossRef]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.S.; Bailey, L.; Wilk, K.E.; Mangine, R.E.; Head, P.; Grindstaff, T.L.; Morrison, D.S. Blood Flow Restriction Training. J. Athl. Train. 2021, 56, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Yasuda, T.; Midorikawa, T.; Sato, Y.; Kearns, C.F.; Inoue, K.; Koizumi, K.; Ishii, N. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int. J. KAATSU Train. Res. 2005, 1, 6–12. [Google Scholar] [CrossRef]
- Takarada, Y.; Tsuruta, T.; Ishii, N. Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn. J. Physiol. 2004, 54, 585–592. [Google Scholar] [CrossRef]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D.; Koenig, D.; König, D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 95–108. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Wang, J. Effect of blood flow restriction combined with low-intensity training on the lower limbs muscle strength and function in older adults: A meta-analysis. Exp. Gerontol. 2022, 164, 111827. [Google Scholar] [CrossRef]
- Rodrigo-Mallorca, D.; Loaiza-Betancur, A.F.; Monteagudo, P.; Blasco-Lafarga, C.; Chulvi-Medrano, I. Resistance Training with Blood Flow Restriction Compared to Traditional Resistance Training on Strength and Muscle Mass in Non-Active Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Heal. 2021, 18, 11441. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Kim, D.; Fahs, C.A.; Thiebaud, R.S.; Abe, T.; Larson, R.D.; Bemben, D.A.; Bemben, M.G. The effects of resistance exercise with and without different degrees of blood-flow restriction on perceptual responses. J. Sports Sci. 2015, 33, 1472–1479. [Google Scholar] [CrossRef]
- Yasuda, T.; Abe, T.; Brechue, W.F.; Iida, H.; Takano, H.; Meguro, K.; Kurano, M.; Fujita, S.; Nakajima, T. Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. Metabolism 2010, 59, 1510–1519. [Google Scholar] [CrossRef]
- Kim, D.; Loenneke, J.; Thiebaud, R.; Abe, T.; Bemben, M. The acute muscular effects of cycling with and without different degrees of blood flow restriction. Acta Physiol. Hung. 2015, 102, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Scherbov, S.; Sanderson, W. New Measures of Population Ageing; United Nations: Rome, Italy, 2020; pp. 1–90. [Google Scholar]
- Hill, E.C.; Housh, T.J.; Keller, J.L.; Smith, C.M.; Anders, J.V.; Schmidt, R.J.; Johnson, G.O.; Cramer, J.T. Patterns of responses and time-course of changes in muscle size and strength during low-load blood flow restriction resistance training in women. Eur. J. Appl. Physiol. 2021, 121, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Brzycki, M. Strength Testing—Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Educ. Recreat. Dance 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Letieri, R.V.; Teixeira, A.M.; Furtado, G.E.; Lamboglia, C.G.; Rees, J.L.; Gomes, B.B. Effect of 16 weeks of resistance exercise and detraining comparing two methods of blood flow restriction in muscle strength of healthy older women: A randomized controlled trial. Exp. Gerontol. 2018, 114, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Capranica, L.; Battenti, M.; Demarie, S.; Figura, F. Reliability of isokinetic knee extension and flexion strength testing in elderly women. J. Sports Med. Phys. Fit. 1998, 38, 169–176. [Google Scholar]
- Katoh, M.; Isozaki, K. Reliability of Isometric Knee Extension Muscle Strength Measurements of Healthy Elderly Subjects Made with a Hand-held Dynamometer and a Belt. J. Phys. Ther. Sci. 2014, 26, 1855–1859. [Google Scholar] [CrossRef]
- Wu, Y.F.; Zhang, X.Y.; Ren, S.; Yu, Y.X.; Chang, C.Q. Measurement and evaluation of the quadriceps muscle mass in young men based on magnetic resonance imaging. J. Peking Univ. Health Sci. 2021, 53, 843–849. [Google Scholar] [CrossRef]
- Sanada, K.; Kearns, C.F.; Midorikawa, T.; Abe, T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur. J. Appl. Physiol. 2006, 96, 24–31. [Google Scholar] [CrossRef]
- Meza-Valderrama, D.; Sánchez- Rodríguez, D.; Perkisas, S.; Duran, X.; Bastijns, S.; Dávalos-Yerovi, V.; Da Costa, E.; Marco, E. The feasibility and reliability of measuring forearm muscle thickness by ultrasound in a geriatric inpatient setting: A cross-sectional pilot study. BMC Geriatr. 2022, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Spenst, L.; Drinkwater, D.; Clarys, J. Anthropometric estimation of muscle mass in men. Med. Sci. Sports Exerc. 1990, 22, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Russell-Rose, T.; Chamberlain, J. Expert Search Strategies: The Information Retrieval Practices of Healthcare Information Professionals. JMIR Med. Inform. 2017, 5, e33. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Sally, G. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0; Cochrane Collaboration: Melbourne, Australia, 2012; pp. 199–255. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.K.; Teasell, R.W.; Foley, N.C.; Speechley, M.R. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. J. Clin. Epidemiol. 2005, 58, 668–673. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Andrews, J.; Guyatt, G.; Oxman, A.D.; Alderson, P.; Dahm, P.; Falck-Ytter, Y.; Nasser, M.; Meerpohl, J.; Post, P.N.; Kunz, R.; et al. GRADE guidelines: 14. Going from evidence to recommendations: The significance and presentation of recommendations. J. Clin. Epidemiol. 2013, 66, 719–725. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, N.; Pang, F.; Chen, T. Resistance Training with Blood Flow Restriction on Vascular Function: A Meta-analysis. Int. J. Sports Med. 2021, 42, 577–587. [Google Scholar] [CrossRef]
- Hagger, M.S. Meta-analysis in sport and exercise research: Review, recent developments, and recommendations. Eur. J. Sport Sci. 2006, 6, 103–115. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Bown, M.J.; Sutton, A.J. Quality Control in Systematic Reviews and Meta-analyses. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Sutton, A.J.; Abrams, K.R.; Lambert, P.C. Sensitivity analyses allowed more appropriate and reliable meta-analysis conclusions for multiple outcomes when missing data was present. J. Clin. Epidemiol. 2004, 57, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Tomaru, T.; Nakajima, T. Thigh muscle size and vascular function after blood flow-restricted elastic band training in older women. Oncotarget 2016, 7, 33595–33607. [Google Scholar] [CrossRef]
- Vechin, F.C.; Libardi, C.A.; Conceição, M.S.; Damas, F.R.; Lixandrão, M.E.; Berton, R.P.B.; Tricoli, V.A.; Roschel, H.A.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T.; et al. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J. Strength Cond. Res. 2015, 29, 1071–1076. [Google Scholar] [CrossRef]
- Ruaro, M.F.; Santana, J.O.; Gusmao, N.; De Franca, E.; Carvalho, B.N.; Farinazo, K.B.; Bonorino, S.L.; Corralo, V.; Antonio de Sá, C.; Caperuto, E.C. Effects of strength training with and without blood flow restriction on quality of life in elderly women. J. Phys. Educ. Sport 2019, 19, 531–539. [Google Scholar]
- Kim, J.; Lang, J.A.; Pilania, N.; Franke, W.D. Effects of blood flow restricted exercise training on muscular strength and blood flow in older adults. Exp. Gerontol. 2017, 99, 127–132. [Google Scholar] [CrossRef]
- Cook, S.B.; LaRoche, D.P.; Villa, M.R.; Barile, H.; Manini, T.M. Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp. Gerontol. 2017, 99, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B.; Cleary, C.J. Progression of blood flow restricted resistance training in older adults at risk of mobility limitations. Front. Physiol. 2019, 10, 738. [Google Scholar] [CrossRef] [PubMed]
- Libardi, C.A.; Chacon-Mikahil, M.P.T.; Cavaglieri, C.R.; Tricoli, V.; Roschel, H.; Vechin, F.C.; Conceição, M.S.; Ugrinowitsch, C. Effect of concurrent training with blood flow restriction in the elderly. Front. Physiol. 2015, 36, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, S.; Dehghaniyan, M.H.; Amani-Shalamzari, S.; Rajabi, H.; Gahreman, D.E. Functional training with blood occlusion influences muscle quality indices in older adults. Arch. Gerontol. Geriatr. 2020, 90, 104110. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Ferguson, R.A. Enhancing strength and postocclusive calf blood flow in older people with training with blood-flow restriction. J. Aging Phys. Act. 2011, 19, 201–213. [Google Scholar] [CrossRef]
- Shimizu, R.; Hotta, K.; Yamamoto, S.; Matsumoto, T.; Kamiya, K.; Kato, M.; Hamazaki, N.; Kamekawa, D.; Akiyama, A.; Kamada, Y.; et al. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur. J. Appl. Physiol. 2016, 116, 749–757. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Iida, H.; Nakajima, T. Effects of detraining after blood flow-restricted low-load elastic band training on muscle size and arterial stiffness in older women. Springerplus 2015, 4, 348. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Uchida, Y.; Koshi, H.; Iida, H.; Masamune, K.; Yamasoba, T.; Sato, Y.; Nakajima, T. Effects of Low-Load, Elastic Band Resistance Training Combined With Blood Flow Restriction on Muscle Size and Arterial Stiffness in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 950–958. [Google Scholar] [CrossRef]
- Yokokawa, Y.; Hongo, M.; Urayama, H.; Nishimura, T.; Kai, I. Effects of low-intensity resistance exercise with vascular occlusion on physical function in healthy elderly people. PubMed Biosci. Trends 2008, 2, 117–123. [Google Scholar]
- Barcot, O.; Boric, M.; Dosenovic, S.; Poklepovic Pericic, T.; Cavar, M.; Puljak, L. Risk of bias assessments for blinding of participants and personnel in Cochrane reviews were frequently inadequate. J. Clin. Epidemiol. 2019, 113, 104–113. [Google Scholar] [CrossRef]
- Buckner, S.L.; Dankel, S.J.; Mattocks, K.T.; Jessee, M.B.; Mouser, J.G.; Counts, B.R.; Loenneke, J.P. The problem Of muscle hypertrophy: Revisited. Muscle Nerve 2016, 54, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Hussain, S.R. A Review on the Mechanisms of Blood-Flow Restriction Resistance Training-Induced Muscle Hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef]
- Adams, G.R. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J. Appl. Physiol. 2002, 93, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B.; Murphy, B.G.; Labarbera, K.E. Neuromuscular Function after a Bout of Low-Load Blood Flow–Restricted Exercise. Med. Sci. Sports Exerc. 2013, 45, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Roi, G.S.; Landoni, L.; Minetti, A.E.; Cerretelli, P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 59, 310–319. [Google Scholar] [CrossRef]
- Häkkinen, K.; Alen, M.; Kallinen, M.; Newton, R.U.; Kraemer, W.J. Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur. J. Appl. Physiol. 2000, 83, 51–62. [Google Scholar] [CrossRef]
- Bordessa, J.M.; Hearn, M.C.; Reinfeldt, A.E.; Smith, T.A.; Baweja, H.S.; Levy, S.S.; Rosenthal, M.D. Comparison of blood flow restriction devices and their effect on quadriceps muscle activation. Phys. Ther. Sport 2021, 49, 90–97. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Fry, C.S.; Glynn, E.L.; Drummond, M.J.; Timmerman, K.L.; Fujita, S.; Abe, T.; Dhanani, S.; Volpi, E.; Rasmussen, B. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiol. 2010, 108, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Abe, T.; Bemben, M.G. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypotheses 2012, 78, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. Does exercise-induced muscle damage play a role in skeletal muscle hypertrophy? J. Strength Cond. Res. 2012, 26, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Barili, A.; da Corralo, V.S.; Cardoso, A.M.; Mânica, A.; da Bonadiman, B.S.R.; Bagatini, M.D.; Da Silva-Grigoletto, M.E.; de Oliveira, G.G.; De Sá, C.A. Acute responses of hemodynamic and oxidative stress parameters to aerobic exercise with blood flow restriction in hypertensive elderly women. Mol. Biol. Rep. 2018, 45, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-H.; Sohn, S.-M.; You, H.-J.; Yoon, E.-S.; Lee, B.-I.; Park, S.-H.; Kim, D.-W. Use of a biopsy punch for end-to-side anastomosis in free-tissue transfer. J. Plast. Surg. Hand Surg. 2020, 54, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Kim, D.; Fahs, C.A.; Thiebaud, R.S.; Abe, T.; Larson, R.D.; Bemben, D.A.; Bemben, M.G. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve 2015, 51, 713–721. [Google Scholar] [CrossRef]
- Ozaki, H.; Loenneke, J.P.; Abe, T. Blood flow-restricted walking in older women: Does the acute hormonal response associate with muscle hypertrophy? Clin. Physiol. Funct. Imaging 2017, 37, 379–383. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).