Discriminative Evaluation of Sarcopenic Dysphagia Using Handgrip Strength or Calf Circumference in Patients with Dysphagia Using the Area under the Receiver Operating Characteristic Curve
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. SD Diagnosis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Logistic Regression Analysis
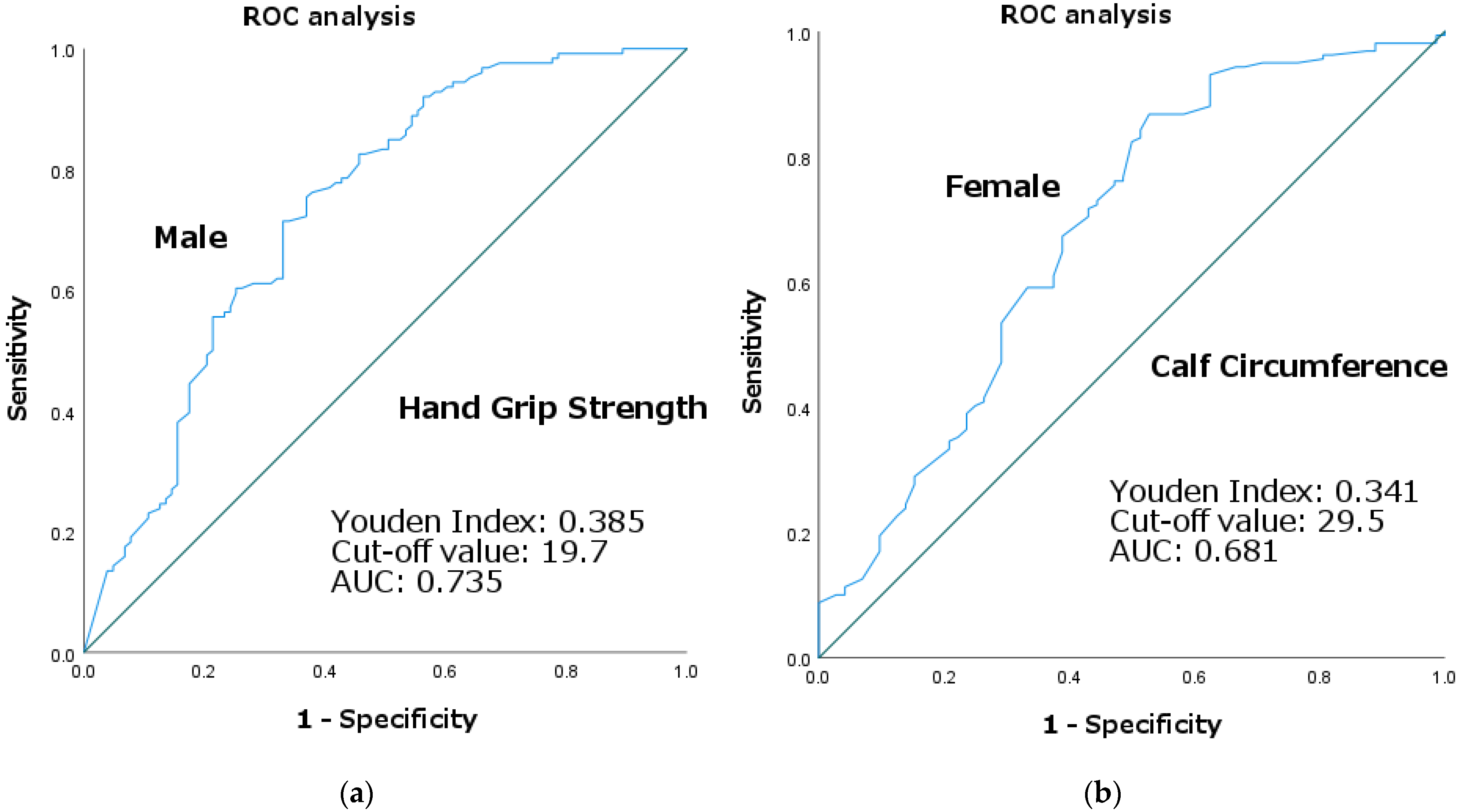
3.3. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Molfenter, S.M.; Lenell, C.; Lazarus, C.L. Volumetric changes to the pharynx in healthy aging: Consequence for pharyngeal swallow mechanics and function. Dysphagia 2019, 34, 129–137. [Google Scholar] [CrossRef]
- Wakabayashi, H. Presbyphagia and sarcopenic dysphagia: Association between aging, sarcopenia, and deglutition disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef]
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97. [Google Scholar] [CrossRef]
- Mori, T.; Fujishima, I.; Wakabayashi, H.; Oshima, F.; Itoda, M.; Kunieda, K.; Kayashita, J.; Nishioka, S.; Sonoda, A.; Kuroda, Y.; et al. Development, reliability, and validity of a diagnostic algorithm for sarcopenic dysphagia. JCSM Clin. Rep. 2017, 2, 1–10. [Google Scholar]
- Kawakami, R.; Murakami, H.; Sanada, K.; Tanaka, N.; Sawada, S.S.; Tabata, I.; Higuchi, M.; Miyachi, M. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr. Gerontol. Int. 2015, 15, 969–976. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Takahashi, R.; Murakami, T. The prevalence and prognosis of sarcopenic dysphagia in patients who require dysphagia rehabilitation. J. Nutr. Health Aging 2019, 23, 84–88. [Google Scholar] [CrossRef]
- Moncayo-Hernández, B.A.; Herrera-Guerrero, J.A.; Vinazco, S.; Ocampo-Chaparro, J.M.; Reyes-Ortiz, C.A. Sarcopenic dysphagia in institutionalised older adults. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2021, 68, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Wakabayashi, H.; Fujishima, I.; Kishima, M.; Itoda, M.; Yamakawa, M.; Wada, F.; Kato, R.; Furiya, Y.; Nishioka, S.; et al. Construction and quality evaluation of the Japanese sarcopenic dysphagia database. J. Nutr. Health Aging 2021, 25, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Nagano, A.; Maeda, K.; Shimizu, A.; Nagami, S.; Takigawa, N.; Ueshima, J.; Suenaga, M. Association of sarcopenic dysphagia with underlying sarcopenia following hip fracture surgery in older women. Nutrients 2020, 12, 1365. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takaki, M.; Akagi, J. Decreased skeletal muscle mass and risk factors of sarcopenic dysphagia: A prospective observational cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1290–1294. [Google Scholar] [CrossRef]
- Sakai, K.; Nakayama, E.; Tohara, H.; Maeda, T.; Sugimoto, M.; Takehisa, T.; Takehisa, Y.; Ueda, K. Tongue strength is associated with grip strength and nutritional status in older adult inpatients of a Rehabilitation Hospital. Dysphagia 2017, 32, 241–249. [Google Scholar] [CrossRef]
- Kurosawa, Y.; Hara, K.; Tohara, H.; Namiki, C.; Chantaramanee, A.; Nakane, A.; Nakagawa, K.; Yamaguchi, K.; Yoshimi, K.; Furuya, J.; et al. Calf circumference is a useful index for assessing dysphagia among community dwelling elderly recipients of long-term care. Tohoku J. Exp. Med. 2019, 248, 201–208. [Google Scholar] [CrossRef]
- Matsuo, H.; Yoshimura, Y. Calf circumference is associated with dysphagia in acute-care inpatients. Geriatr. Nurs. 2018, 39, 186–190. [Google Scholar] [CrossRef]
- Yuan, J.; Lin, Y.; Song, J.; Xia, R.; Jiang, Y.; Yang, X.; Li, Y.; Dong, B. Associations of sarcopenic parameters with dysphagia in older nursing home residents: A cross-sectional study. J. Nutr. Health Aging 2022, 26, 339–345. [Google Scholar] [CrossRef]
- Kimura, M.; Naganuma, A.; Ogawa, Y.; Inagawa, M.; Nishioka, S.; Momosaki, R.; Wakabayashi, H. Calf circumference and stroke are independent predictors for an improvement in the food intake level scale in the Japanese sarcopenic dysphagia database. Eur. Geriatr. Med. 2022, 13, 1211–1220. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Ribeiro, M.T.; Ferreira, R.C.; Vargas, A.M.; Ferreira e Ferreira, E. Validity and reproducibility of the revised oral assessment guide applied by community health workers. Gerodontology 2014, 31, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.M.; King, P.L.; Spencer, A.J.; Wright, F.A.; Carter, K.D. The oral health assessment tool—Validity and reliability. Aust. Dent. J. 2005, 50, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.B.; Laughlin, J.A.; Fiedler, R.C.; Granger, C.V. Interrater reliability of the 7-level functional independence measure (FIM). Scand. J. Rehabil. Med. 1994, 26, 115–119. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. MD State Med. J. 1965, 14, 61–65. [Google Scholar]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Nagai, T.; Wakabayashi, H.; Nishioka, S.; Momosaki, R. Functional prognosis in patients with sarcopenic dysphagia: An observational cohort study from the Japanese sarcopenic dysphagia database. Geriatr. Gerontol. Int. 2022, 22, 839–845. [Google Scholar] [CrossRef]
- Kassem, F.; Masalha, M.; Biadsee, A.; Nageris, B.; Kagan, R.; Nachmani, A. Analysis of sex-related differences in patients with dysphagia: Using a videofluoroscopy. Isr. Med. Assoc. J. 2022, 24, 464–469. [Google Scholar]
- Daniel, M.M.; Lorenzi, M.C.; da Costa Leite, C.; Lorenzi-Filho, G. Pharyngeal dimensions in healthy men and women. Clinics (Sao Paulo) 2007, 62, 5–10. [Google Scholar] [CrossRef]
- Clark, H.M.; Solomon, N.P. Age and sex differences in orofacial strength. Dysphagia 2012, 27, 2–9. [Google Scholar] [CrossRef]
- Auyeung, T.W.; Arai, H.; Chen, L.K.; Woo, J. Letter to the editor: Normative data of handgrip strength in 26344 older adults—A pooled dataset from eight cohorts in Asia. J. Nutr. Health Aging 2020, 24, 125–126. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Kishima, M.; Itoda, M.; Fujishima, I.; Kunieda, K.; Ohno, T.; Shigematsu, T.; Oshima, F.; Mori, T.; Ogawa, N.; et al. Japanese Working Group on Sarcopenic Dysphagia. Diagnosis and treatment of sarcopenic dysphagia: A scoping review. Dysphagia 2021, 36, 523–531. [Google Scholar] [CrossRef]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ohno, T.; Nomoto, A.; Kayashita, J.; Mori, N. The Japanese Working Group on Sarcopenic Dysphagia. Nutritional management enhances the recovery of swallowing ability in older patients with sarcopenic dysphagia. Nutrients 2021, 13, 596. [Google Scholar] [CrossRef]
- Miyauchi, N.; Nakamura, M.; Nakamura, I.; Momosaki, R. Effect of early versus delayed mobilization by physical therapists on oral intake in patients with sarcopenic dysphagia after pneumonia. Eur. Geriatr. Med. 2019, 10, 603–607. [Google Scholar] [CrossRef]
- Chen, K.C.; Jeng, Y.; Wu, W.T.; Wang, T.G.; Han, D.S.; Özçakar, L.; Chang, K.V. Sarcopenic dysphagia: A narrative review from diagnosis to intervention. Nutrients 2021, 13, 4043. [Google Scholar] [CrossRef]
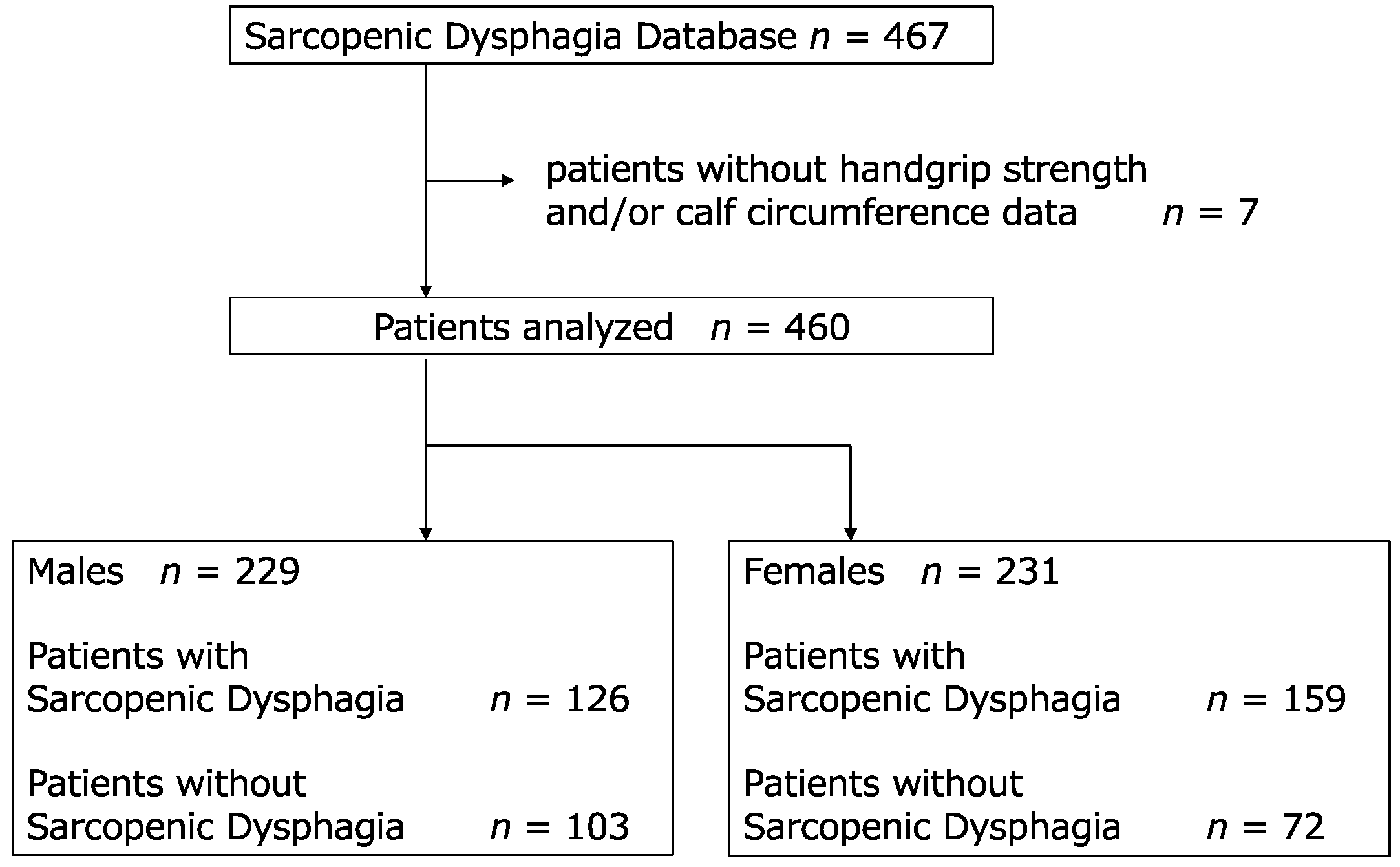
| Overall (n = 460) | Males (n = 229) | Females (n = 231) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | SD | Non-SD | p Value | Overall | SD | Non-SD | p Value | ||||
| SD, n, (%) | 285 (62.0) | 126 | 159 | 0.002 | ¶ | ||||||
| Non-SD, n, (%) | 175 (38.0) | 103 | 72 | ||||||||
| Age (years), median (IQR) | 83 (76–88) | 80 (71–87) | 83 (76–88) | 78 (69–83) | <0.001 | * | 85 (79–90) | 86 (82–90) | 81 (75–88) | <0.001 | * |
| BMI (kg/m2), median (IQR) | 19.9 (17.3–22.5) | 20.5 (17.7–22.8) | 19.9 (16.9–22.4) | 20.8 (18.6–23.6) | 0.019 | * | 19.5 (17.2–22.2) | 19.2 (17.1–21.3) | 20.8 (17.5–24.5) | 0.013 | * |
| HGS (kg), median (IQR) | 12.0 (6.3–18.8) | 17.9 (10.3–24.3) | 12.8 (6.9–19.5) | 22.2 (15.5–28.6) | <0.001 | * | 9.1 (5.4–13.0) | 9.2 (6.0–12.8) | 8.0 (5.0–13.6) | 0.796 | * |
| CC (cm), median (IQR) | 28.0 (25.1–31.0) | 29.4 (26.0–32.0) | 28 (24.8–30.8) | 31.0 (27.7–33.1) | <0.001 | * | 27.3 ± 3.6 | 26.5 ± 3.3 | 28.9 ± 3.8 | <0.001 | * |
| CCI, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (0–3.5) | 0.221 | * | 2 (1–4) | 2 (1–4) | 2 (0–3) | 0.487 | * |
| FILS, median (IQR) | 7 (3–8) | 7 (2–7) | 7 (3–7) | 6 (1–7) | 0.028 | * | 7 (6–8) | 7 (6–8) | 7 (1–7) | 0.001 | * |
| BI, median (IQR) | 25 (5–50) | 30 (10–50) | 25 (5–45) | 30 (10–55) | 0.088 | * | 20 (5–50) | 30 (5–50) | 17.5 (9–40) | 0.198 | * |
| Serum albumin (g/dL), mean ± SD | 3.3 (3.0–3.7) | 3.4 ± 0.6 | 3.2 ± 0.6 | 3.5 ± 0.6 | <0.001 | § | 3.3 (2.9–3.6) | 3.2 (2.9–3.6) | 3.4 (3.1–3.7) | 0.035 | * |
| CRP (mg/dL), median (IQR) | 0.7 (0.2–3.2) | 0.8 (0.15–3.7) | 1.1 (0.3–4.5) | 0.7 (0.1–2.5) | 0.051 | * | 0.6 (0.2–2.7) | 0.8 (0.3–3.0) | 0.3 (0.1–1.2) | 0.003 | * |
| B | Standard Error | Odds Ratio | 95% Confidence Interval of Odds Ratio | p Value | ||
|---|---|---|---|---|---|---|
| Age | 0.034 | 0.016 | 1.035 | 1.003 | 1.067 | 0.032 |
| Food Intake LEVEL Scale | 0.144 | 0.060 | 1.154 | 1.026 | 1.299 | 0.017 |
| Hand Grip Strength | −0.095 | 0.021 | 0.909 | 0.873 | 0.947 | <0.001 |
| Calf Circumference | −0.059 | 0.053 | 0.943 | 0.850 | 1.045 | 0.262 |
| Body Mass Index | 0.023 | 0.053 | 1.024 | 0.922 | 1.136 | 0.662 |
| Barthel Index | 0.009 | 0.007 | 1.009 | 0.996 | 1.022 | 0.179 |
| Charlson Comorbidity Index | −0.052 | 0.086 | 0.949 | 0.802 | 1.123 | 0.543 |
| B | Standard Error | Odds Ratio | 95% Confidence INTERVAL of Odds Ratio | p Value | ||
|---|---|---|---|---|---|---|
| Age | 0.028 | 0.018 | 1.028 | 0.993 | 1.065 | 0.116 |
| Food Intake LEVEL Scale | 0.14 | 0.07 | 1.151 | 1.003 | 1.320 | 0.045 |
| Hand Grip Strength | 0.015 | 0.032 | 1.015 | 0.953 | 1.080 | 0.647 |
| Calf Circumference | −0.266 | 0.07 | 0.767 | 0.668 | 0.880 | <0.001 |
| Body Mass Index | 0.054 | 0.061 | 1.055 | 0.937 | 1.188 | 0.377 |
| Barthel Index | 0.011 | 0.007 | 1.011 | 0.996 | 1.025 | 0.139 |
| Charlson Comorbidity Index | 0.022 | 0.092 | 1.022 | 0.853 | 1.224 | 0.814 |
| Variables | Age | Food Intake LEVEL Scale | Hand Grip Strength | Calf Circumference | Body Mass Index | Barthel Index | Charlson Comorbidity Index | |
|---|---|---|---|---|---|---|---|---|
| Age | Spearman’s ρ | — | ||||||
| p-value | — | |||||||
| Food Intake LEVEL Scale | Spearman’s ρ | 0.184 | — | |||||
| p-value | 0.005 | — | ||||||
| Hand Grip Strength | Spearman’s ρ | −0.277 | 0.091 | — | ||||
| p-value | <0.001 | 0.169 | — | |||||
| Calf Circumference | Spearman’s ρ | −0.286 | 0.119 | 0.539 | — | |||
| p-value | <0.001 | 0.073 | <0.001 | — | ||||
| Body Mass Index | Spearman’s ρ | −0.199 | −0.033 | 0.269 | 0.618 | — | ||
| p-value | 0.002 | 0.62 | <0.001 | <0.001 | — | |||
| Barthel Index | Spearman’s ρ | −0.022 | 0.191 | 0.507 | 0.279 | 0.044 | — | |
| p-value | 0.745 | 0.004 | <0.001 | <0.001 | 0.512 | — | ||
| Charlson Comorbidity Index | Pearson’s r | 0.177 | 0.113 | −0.175 | −0.196 | −0.129 | −0.157 | — |
| p-value | 0.007 | 0.089 | 0.008 | 0.003 | 0.052 | 0.017 | — |
| Variables | Age | Food Intake LEVEL Scale | Hand Grip Strength | Calf Circumference | Body Mass Index | Barthel Index | Charlson Comorbidity Index | |
|---|---|---|---|---|---|---|---|---|
| Age | Spearman’s ρ | — | ||||||
| p-value | — | |||||||
| Food Intake LEVEL Scale | Spearman’s ρ | 0.247 | — | |||||
| p-value | <0.001 | — | ||||||
| Hand Grip Strength | Spearman’s ρ | −0.104 | 0.174 | — | ||||
| p-value | 0.116 | 0.008 | — | |||||
| Calf Circumference | Spearman’s ρ | −0.177 | 0.037 | 0.319 | — | |||
| p-value | 0.007 | 0.58 | <0.001 | — | ||||
| Body Mass Index | Spearman’s ρ | 0.01 | −0.112 | 0.167 | 0.647 | — | ||
| p-value | 0.885 | 0.089 | 0.011 | <0.001 | — | |||
| Barthel Index | Spearman’s ρ | 0.102 | 0.296 | 0.466 | 0.266 | 0.11 | — | |
| p-value | 0.123 | <0.001 | <0.001 | <0.001 | 0.095 | — | ||
| Charlson Comorbidity Index | Pearson’s r | 0.149 | −0.072 | −0.15 | −0.11 | 0.003 | −0.068 | — |
| p-value | 0.024 | 0.278 | 0.022 | 0.096 | 0.964 | 0.303 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishimoto, H.; Wakabayashi, H.; Nishioka, S.; Momosaki, R. Discriminative Evaluation of Sarcopenic Dysphagia Using Handgrip Strength or Calf Circumference in Patients with Dysphagia Using the Area under the Receiver Operating Characteristic Curve. J. Clin. Med. 2023, 12, 118. https://doi.org/10.3390/jcm12010118
Kishimoto H, Wakabayashi H, Nishioka S, Momosaki R. Discriminative Evaluation of Sarcopenic Dysphagia Using Handgrip Strength or Calf Circumference in Patients with Dysphagia Using the Area under the Receiver Operating Characteristic Curve. Journal of Clinical Medicine. 2023; 12(1):118. https://doi.org/10.3390/jcm12010118
Chicago/Turabian StyleKishimoto, Hiroshi, Hidetaka Wakabayashi, Shinta Nishioka, and Ryo Momosaki. 2023. "Discriminative Evaluation of Sarcopenic Dysphagia Using Handgrip Strength or Calf Circumference in Patients with Dysphagia Using the Area under the Receiver Operating Characteristic Curve" Journal of Clinical Medicine 12, no. 1: 118. https://doi.org/10.3390/jcm12010118
APA StyleKishimoto, H., Wakabayashi, H., Nishioka, S., & Momosaki, R. (2023). Discriminative Evaluation of Sarcopenic Dysphagia Using Handgrip Strength or Calf Circumference in Patients with Dysphagia Using the Area under the Receiver Operating Characteristic Curve. Journal of Clinical Medicine, 12(1), 118. https://doi.org/10.3390/jcm12010118








