A Comprehensive Analysis of the Impact of Inorganic Matter on Membrane Organic Fouling: A Mini Review
Abstract
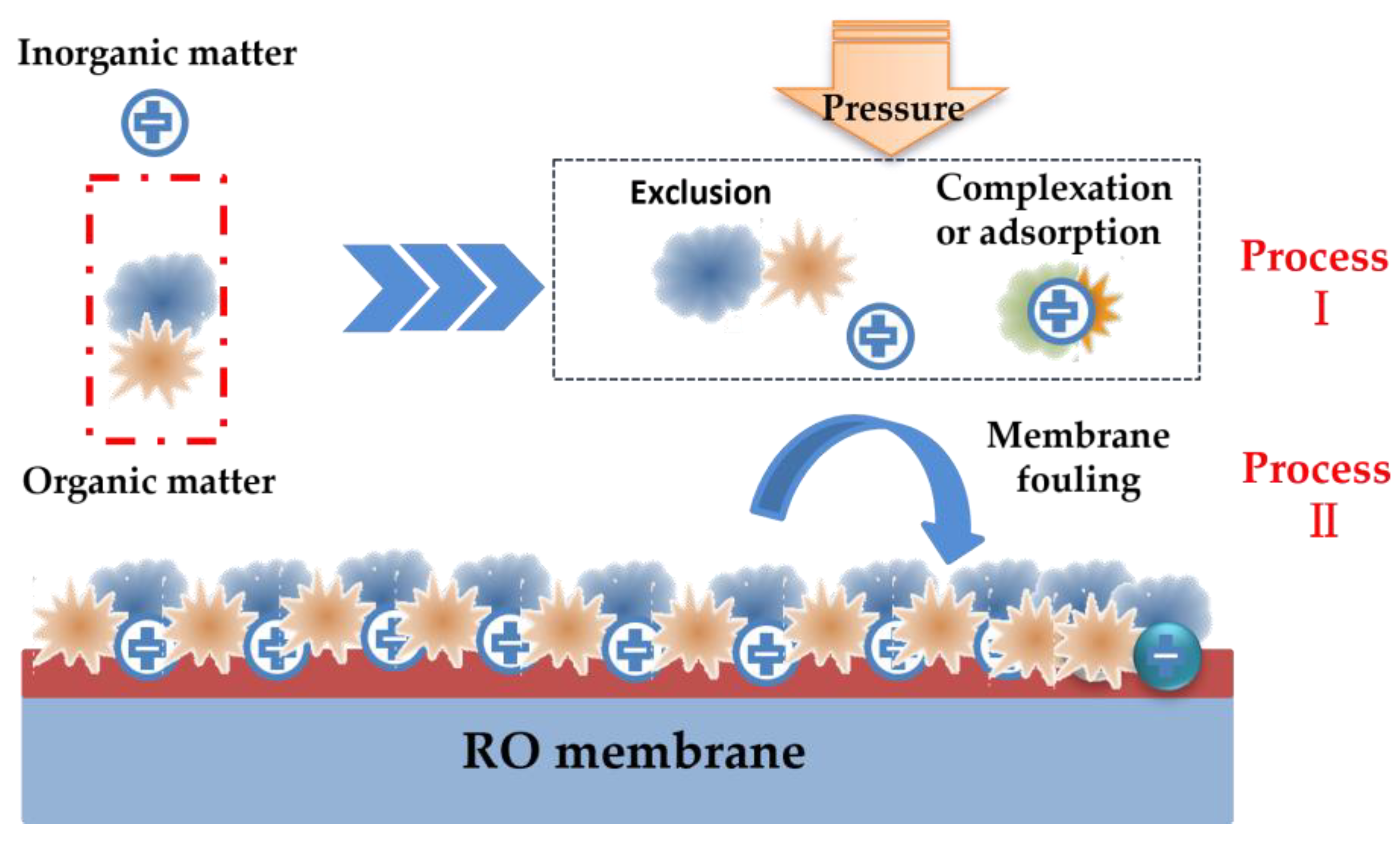
:1. Introduction
2. Interactions between Inorganic and Organic Matter
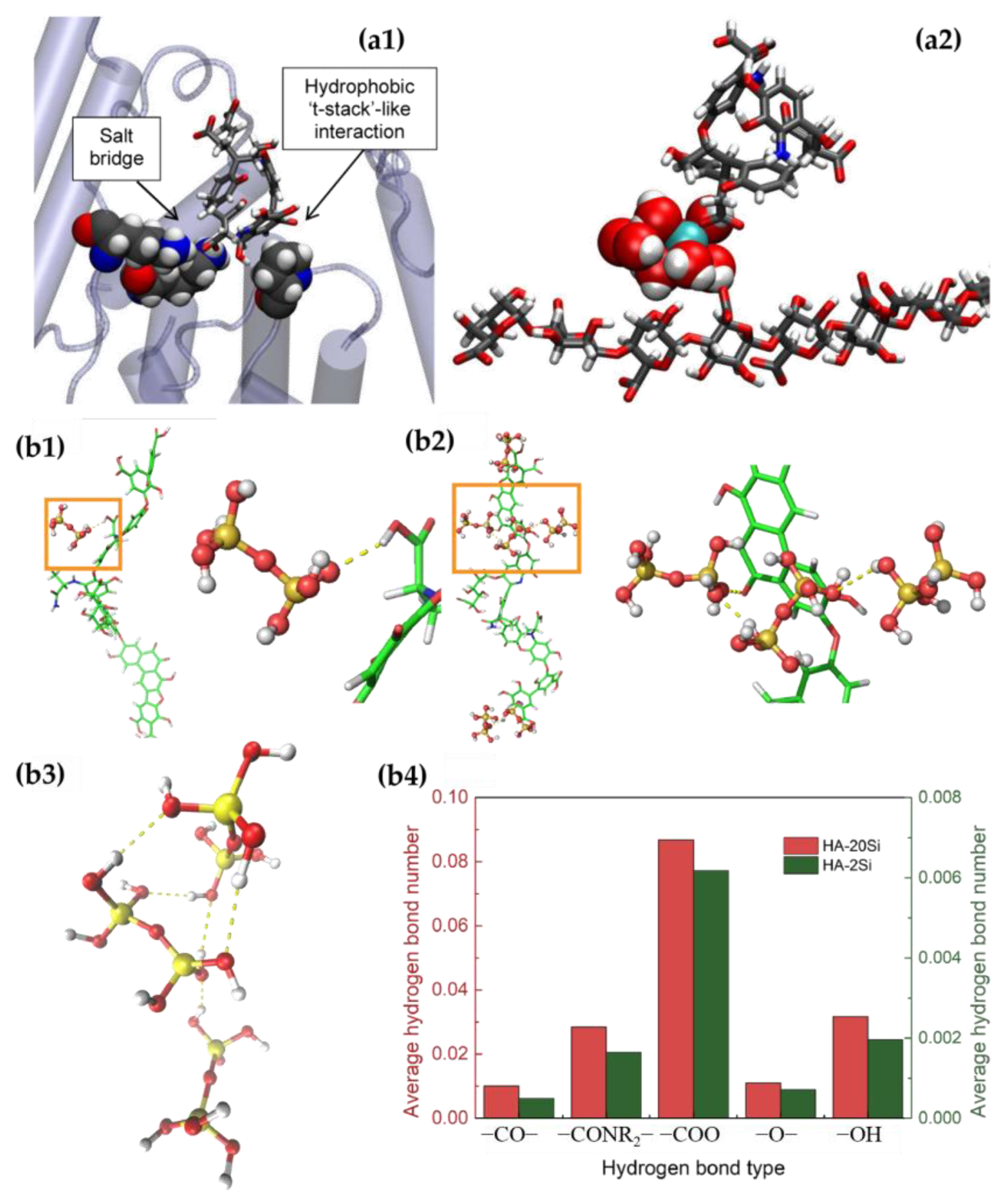
2.1. Humic Acid (HA)
- Ligand interaction: Some inorganic substances can form ligand bonds with functional groups (e.g., carboxylic acids, hydroxy acids, phenols, etc.) in humic acids. This coordination can change the structure of humic acids, affecting their solubility, charge density, and chemical reactivity.
- Adsorption: Inorganic substances can be adsorbed on the surface of humic acid molecules to form an adsorption layer. This adsorption can affect the surface properties, adsorption capacity, and environmental behavior of humic acids.
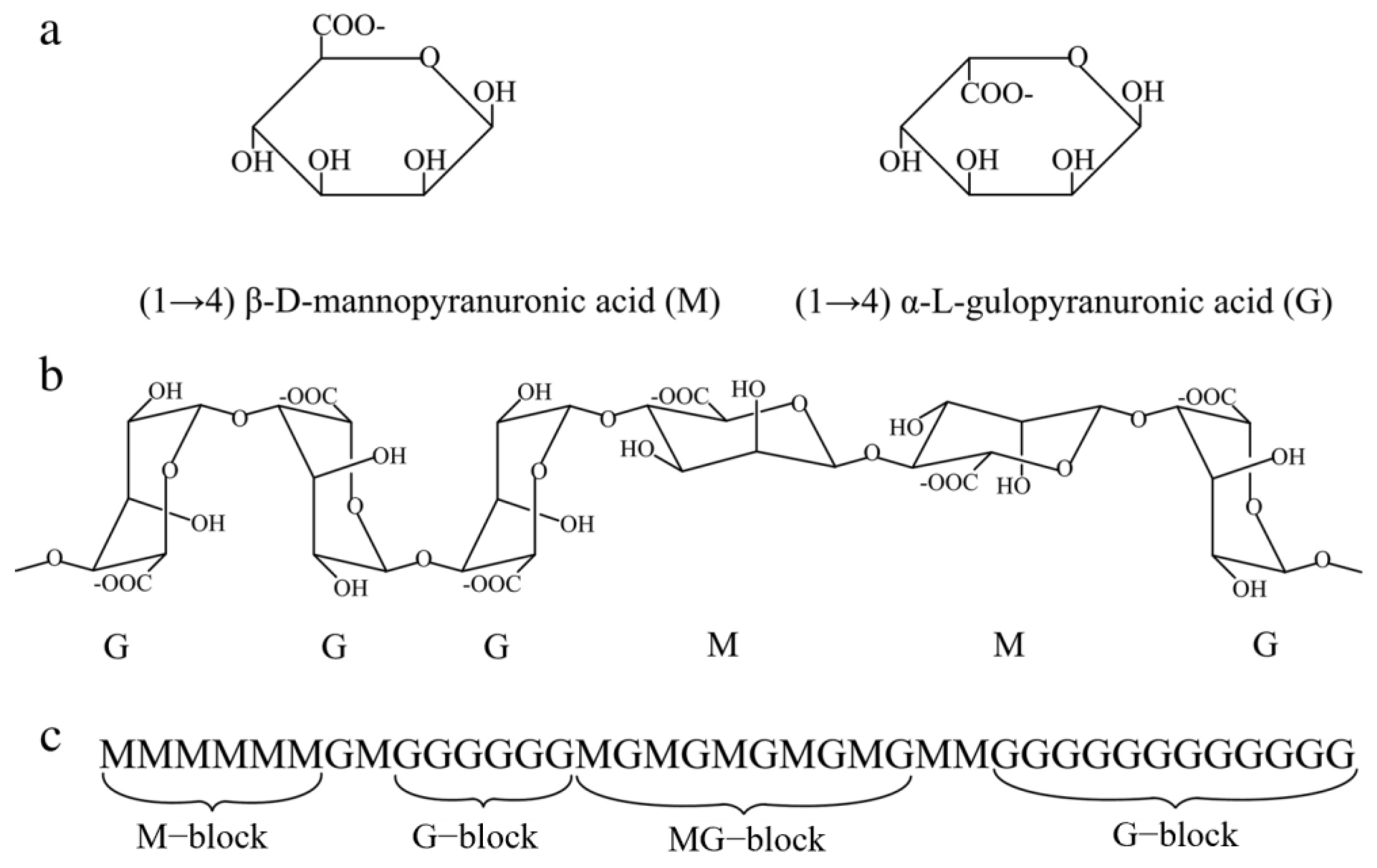
2.2. Polysaccharides
- Ion-exchange interactions: Inorganic ions can undergo an ion exchange with charged groups (e.g., carboxyl groups, hydroxyl groups, etc.) in polysaccharides. This can change the charge state of the polysaccharide, affecting the ionic balance of the solution and the colloidal properties of the polysaccharide.
- Hydrogen bonding: Inorganic substances can form hydrogen bonds with hydrogen bonding acceptors (e.g., hydroxyl, amine groups, etc.) in polysaccharides. This hydrogen bonding can stabilize the structure of polysaccharides and affect their solubility, viscosity, and gel-forming ability.
- Coordination: Some inorganic ions can form coordination bonds with functional groups (e.g., hydroxyl, amine groups, etc.) in polysaccharides. This coordination can affect the structure and stability of polysaccharides, but also change their optical properties and catalytic activity.
- Van der Waals force interactions: There are also van der Waals force interactions between inorganic substances and polysaccharides, such as intermolecular attraction, electrostatic interactions, etc., which can affect their mutual adsorption and cohesion properties.
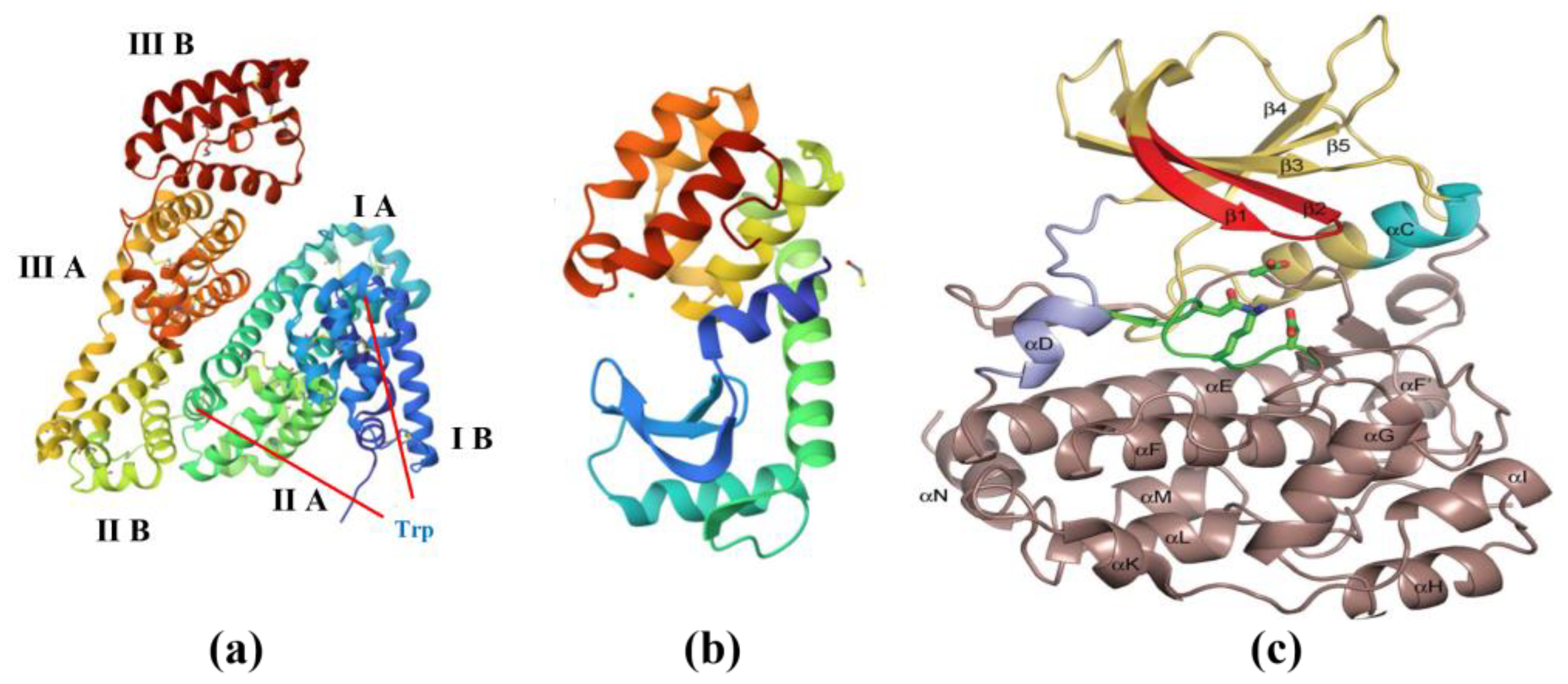
2.3. Protein
- Ionic interactions: Inorganic substances can have ionic interactions, such as salt-bridging, with charged amino acid residues in proteins. This interaction can affect the secondary structure and stability of proteins.
- Hydrogen bonding: Inorganic substances can have hydrogen bonding interactions with amino acid residues in proteins, including the carbonyl, hydroxyl, and amino groups of amino acids. The formation of hydrogen bonds can affect the folding and stability of proteins.
- Non-covalent interactions: Inorganic substances can also interact with proteins through non-covalent interactions such as van der Waals forces, hydrophobic effects, and hydrophobic interactions. These interactions can affect the structure, folding, and interactions of proteins.
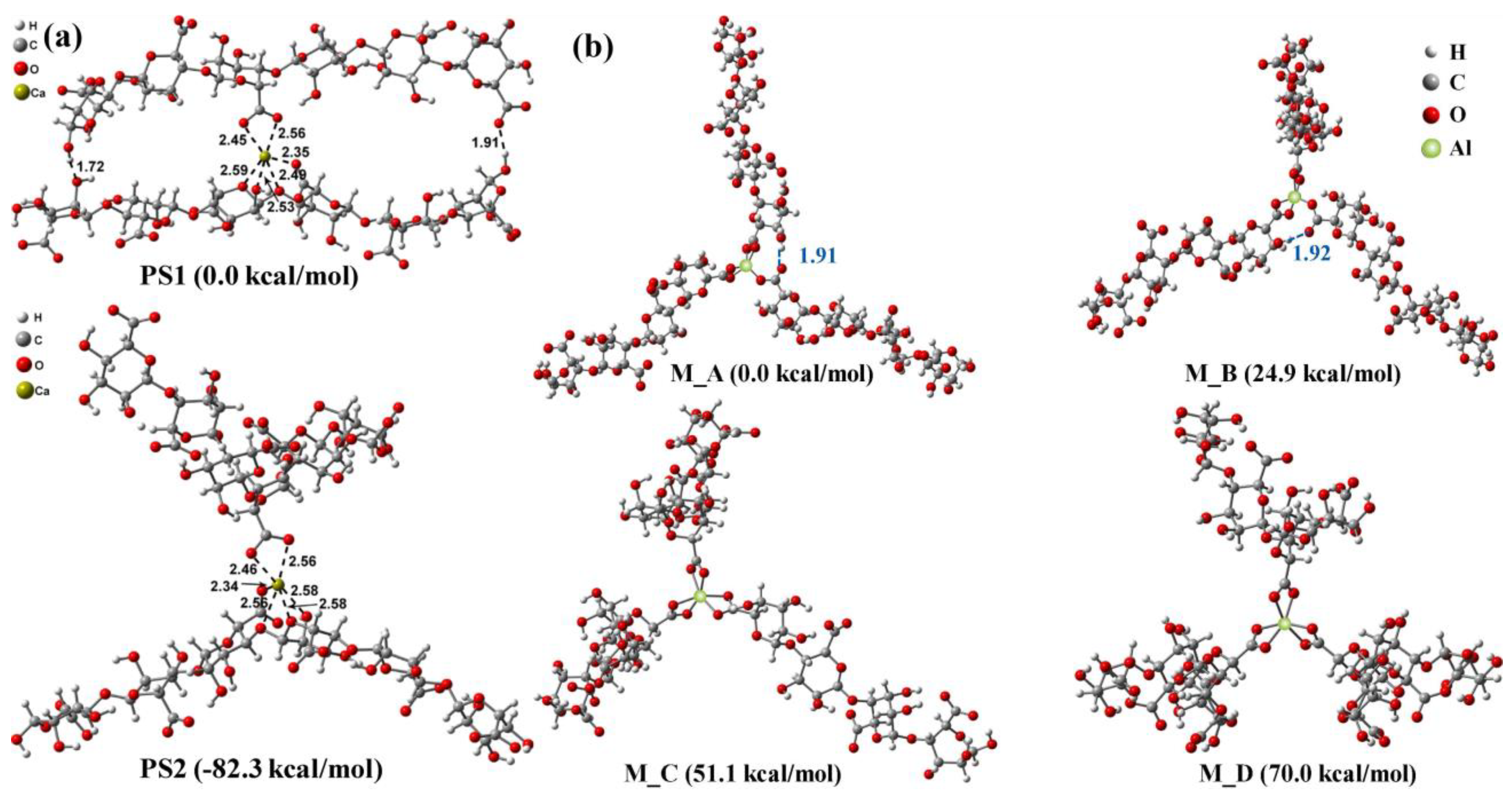
2.4. Molecular Simulation
2.4.1. MD
2.4.2. DFT
3. Membrane Fouling Behavior Based on Inorganic–Organic Interactions
| Organic Matter | Membrane Type | Organic Matter Concentration | Inorganic Matter | Filtration Time | Variation of Normalized Flux Compared with Organic Matter Fouling Only | The Trend of Permeate Flux | |
|---|---|---|---|---|---|---|---|
| Species | Concentration | ||||||
| HA | UF | 10 mg/L [68] | Al3+ | 0.3 mM | 7.5 min | +10.21% | increase |
| 50 mg/L [72] | Mg2+ | 0.025 mM | 60 min | −2.66% | / | ||
| Ca2+ | −9.37% | ||||||
| Fe3+ | −9.03% | ||||||
| 10 mg/L DOC [71] | Ca2+ | 1 mM | 120 min | −43.49% | increase | ||
| 10 mM | −16.27% | ||||||
| 100 mM | +8.30% | ||||||
| 20 mg/L [70] | Ca2+ | 0.02 mM | 5 min | −34.47% | decrease | ||
| 0.2 mM | −49.07% | ||||||
| Mg2+ | 0.02 mM | −27.33% | decrease | ||||
| 0.2 mM | −44.10% | ||||||
| Al3+ | 0.02 mM | −7.55% | increase | ||||
| 0.2 mM | +4.40% | ||||||
| Fe3+ | 0.02 mM | −30.19% | decrease | ||||
| 0.2 mM | −21.38% | ||||||
| RO | 10 mg/L [73] | Ca2+ | 1 mM | 45 h | −24.12% | decrease | |
| 3 mM | −30.35% | ||||||
| 5 mM | −35.77% | ||||||
| 50 mg/L [16] | Si | 6 mM | 10 h | −38.29% | / | ||
| Polysaccharides | UF | 50 mg/L SA MM-block | Ca2+ [12,74] | 1 mM | 48 h | −53.10% | decrease |
| 2 mM | −63.20% | ||||||
| 50 mg/L SA GG-block | 1 mM | 24 h | −80.50% | decrease | |||
| 2 mM | −83.86% | ||||||
| 50 mg/L SA MG-block | 1 mM | 72 h | −10.31% | decrease | |||
| 2 mM | −12.67% | ||||||
| 50 mg/L SA [45] | Ca2+ | 0.13 mM | 200 min | −39.12% | decrease | ||
| 0.25 mM | −42.36% | ||||||
| 0.50 mM | −44.51% | ||||||
| 0.75 mM | −45.17% | ||||||
| 1.00 mM | −46.41% | ||||||
| 1.5 mM | −42.36% | increase | |||||
| 5.00 mM | −41.11% | ||||||
| 10.00 mM | −23.31% | ||||||
| Mg2+ | 0.13 mM | −28.11% | decrease | ||||
| 0.25 mM | −31.86% | ||||||
| 0.50 mM | −33.37% | ||||||
| 0.75 mM | −36.71% | ||||||
| 1.00 mM | −39.22% | ||||||
| 1.5 mM | −46.21% | ||||||
| 5.00 mM | −49.37% | ||||||
| 10.00 mM | −51.73% | ||||||
| RO | 10 mg/L Gellan gum | Ca2+ [38] | 1 mM | 45 h | −34.31% | decrease | |
| 3 mM | −50.59% | ||||||
| 10 mg/L SA | 1 mM | −46.61% | decrease | ||||
| 3 mM | −47.31% | ||||||
| Si [46] | 2 mM | 1000 min | −25.30% | / | |||
| BSA | UF | 20 mg/L [69] | Al3+ | 0.1 mg/L | 10 min | +27.33% | increase |
| 0.3 mg/L | +29.36% | ||||||
| 0.9 mg/L | +9.22% | ||||||
| 1.5 mg/L | +7.69% | ||||||
| 500 ppm [52] | Fe3+ | 0.1 mM | 100 min | −12.31% | / | ||
| Ca2+ | 0 | / | |||||
| / | |||||||
| RO | 50 mg/L [75] | Mg2+ | 1 mM | 25 h | −30.51% | / | |
| Ca2 | −36.86% | / | |||||
| 35 mg/L [53] | Si | 2.8 mM | 500 mins | −46.66% | / | ||
| 300 mg/L [48] | Ca2+ | 0.5 mM | 20 h | −2.17% | decrease | ||
| 1 mM | −18.66% | ||||||
| 2 mM | −21.39% | ||||||
4. Conclusions and Perspectives
4.1. Conclusions
- Inorganic matters like Ca2+, Mg2+, Al3+, and silica readily interact with organic foulants like humic acids, polysaccharides, and proteins via mechanisms including ionic bonding, hydrogen bonding, coordination, and van der Waals forces. These facilitate the formation of larger aggregates that exacerbate membrane fouling, especially in the RO system.
- Molecular modeling techniques, such as MD and DFT, are valuable tools for gaining molecular insights into membrane fouling, facilitating a clearer understanding of organic membrane fouling mechanisms.
- Polysaccharide fouling is primarily governed by the formation of TEP, which is induced by the bridging of inorganic ions between polysaccharide chains.
- Inorganic coagulants, such as aluminum (Al) and iron (Fe) salts, are capable of alleviating UF organic membrane fouling by forming loose and porous fouling layers through complexation with organic matter.
4.2. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Obaidi, M.A.; Kara-Zaïtri, C.; Mujtaba, I.M. Wastewater treatment by spiral wound reverse osmosis: Development and validation of a two dimensional process model. J. Clean. Prod. 2017, 140, 1429–1443. [Google Scholar] [CrossRef]
- Liu, J.; Duan, L.; Gao, Q.; Zhao, Y.; Gao, F. Removal of Typical PPCPs by Reverse Osmosis Membranes: Optimization of Treatment Process by Factorial Design. Membranes 2023, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; Cheng, X.; Chen, H.; Liu, Q.; Yao, P.; Ngo, H.H.; Nghiem, L.D. Mitigation of reverse osmosis membrane fouling by electrochemical-microfiltration- activated carbon pretreatment. J. Membr. Sci. 2022, 656, 120615. [Google Scholar] [CrossRef]
- Gu, H.; Bartman, A.R.; Uchymiak, M.; Christofides, P.D.; Cohen, Y. Self-adaptive feed flow reversal operation of reverse osmosis desalination. Desalination 2013, 308, 63–72. [Google Scholar] [CrossRef]
- Yao, M.; Duan, L.; Song, Y.; Hermanowicz, S.W. Degradation mechanism of Ibuprofen via a forward osmosis membrane bioreactor. Bioresour. Technol. 2021, 321, 124448. [Google Scholar] [CrossRef]
- Chon, K.; Cho, J.; Kim, S.J.; Jang, A. The role of a combined coagulation and disk filtration process as a pre-treatment to microfiltration and reverse osmosis membranes in a municipal wastewater pilot plant. Chemosphere 2014, 117, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Duan, L.; Wei, J.; Qian, F.; Hermanowicz, S.W. Carbamazepine removal from wastewater and the degradation mechanism in a submerged forward osmotic membrane bioreactor. Bioresour. Technol. 2020, 314, 123732. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Hermanowicz, S.W. Influence of water transport characteristics on membrane internal conductive structure in forward osmosis microbial fuel cell. J. Mol. Liq. 2023, 380, 121704. [Google Scholar] [CrossRef]
- Li, S.; Duan, L.; Zhang, H.; Li, M.; Zhao, Y.; Xing, F. Inhibition Strategies of Reverse Solute Flux in Osmotic Microbial Fuel Cells: Take Forward Osmosis as Reference. Acs EsT Water 2023, 3, 2835–2848. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Yang, B.; Xiao, K.; Zhao, H. Separation, anti-fouling, and chlorine resistance of the polyamide reverse osmosis membrane: From mechanisms to mitigation strategies. Water Res. 2021, 195, 116976. [Google Scholar] [CrossRef]
- Gao, Q.; Duan, L.; Liu, J.; Zhang, H.; Zhao, Y. Evaluation and optimization of reverse osmosis pretreatment technology using the modified intermediate blocking model. J. Clean. Prod. 2023, 417, 138029. [Google Scholar] [CrossRef]
- Wang, R.; Liang, D.; Liu, X.; Fan, W.; Meng, S.; Cai. Effect of magnesium ion on polysaccharide fouling. Chem. Eng. J. 2020, 379, 122351. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, H.; Shen, L.; Liao, B.; Wu, X.; Li, R. Effect of calcium ions on fouling properties of alginate solution and its mechanisms. J. Membr. Sci. 2017, 525, 320–329. [Google Scholar] [CrossRef]
- Teng, J.; Chen, Y.; Ma, G.; Hong, H.; Sun, T.; Liao, B.; Lin, H. Membrane fouling by alginate in polyaluminum chloride (PACl) coagulation/microfiltration process: Molecular insights. Sep. Purif. Technol. 2020, 236, 116294. [Google Scholar] [CrossRef]
- Gao, Q.; Duan, L.; Jia, Y.; Zhang, H.; Liu, J.; Yang, W. Differences in the Effect of Mn2+ on the Reverse Osmosis Membrane Fouling Caused by Different Types of Organic Matter: Experimental and Density Functional Theory Evidence. Membranes 2023, 13, 823. [Google Scholar] [CrossRef]
- Li, D.; Lin, W.; Shao, R.; Shen, Y.; Zhu, X.; Huang, X. Interaction between humic acid and silica in reverse osmosis membrane fouling process: A spectroscopic and molecular dynamics insight. Water Res. 2021, 206, 117773. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Alhawari, A.; Alanezi, A.A.; Altaee, A. A Review of Fouling Mechanisms, Control Strategies and Real-Time Fouling Monitoring Techniques in Forward Osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Chen, V. Characterization of protein fouling on membranes: Opportunities and challenges. J. Membr. Sci. 2004, 242, 169–188. [Google Scholar] [CrossRef]
- Liao, Y.; Bokhary, A.; Maleki, E.; Liao, B. A review of membrane fouling and its control in algal-related membrane processes. Bioresour. Technol. 2018, 264, 343–358. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q. Algal fouling of microfiltration and ultrafiltration membranes and control strategies: A review. Sep. Purif. Technol. 2018, 203, 193–208. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Teng, J.; Deng, Y.; Zhou, X.; Yang, W.; Huang, Z.; Zhang, H.; Zhang, M.; Lin, H. A critical review on thermodynamic mechanisms of membrane fouling in membrane—Based water treatment process. Front. Environ. Sci. Eng. 2023, 17, 129. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Schmitt, F.; Do, K.U. Prediction of membrane fouling using artificial neural networks for wastewater treated by membrane bioreactor technologies: Bottlenecks and possibilities. Environ. Sci. Pollut. Res. 2017, 24, 22885–22913. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; Melian-Martel, N.; Nuez, I. Short Review on Predicting Fouling in RO Desalination. Membranes 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Amy, G.; Croue, J.P.; Buisson, H. Identification and understanding of fouling in low-pressure membrane (MF/UF) filtration by natural organic matter (NOM). Water Res. 2004, 38, 4511–4523. [Google Scholar] [CrossRef]
- Guan, Y.; Qian, C.; Chen, W.; Huang, B.; Wang, Y.; Yu, H. Interaction between humic acid and protein in membrane fouling process: A spectroscopic insight. Water Res. 2018, 145, 146–152. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, T.; Hu, G.; Ma, J.; Song, R.; Li, J. Efficient removal of perfluorooctane sulphonate by nanofiltration: Insights into the effect and mechanism of coexisting inorganic ions and humic acid. J. Membr. Sci. 2020, 610, 118176. [Google Scholar] [CrossRef]
- Wang, L.; He, D.; Chen, W.; Yu, H. Probing the roles of Ca2+ and Mg2+ in humic acids-induced ultrafiltration membrane fouling using an integrated approach. Water Res. 2015, 81, 325–332. [Google Scholar] [CrossRef]
- Wall, N.A.; Choppin, G.R. Humic acids coagulation: Influence of divalent cations. Appl. Geochem. 2003, 18, 1573–1582. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Quan, X.; Fan, X.; Zhao, H. Performing a microfiltration integrated with photocatalysis using an Ag-TiO2/HAP/Al2O3 composite membrane for water treatment: Evaluating effectiveness for humic acid removal and anti-fouling properties. Water Res. 2010, 44, 6104–6114. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.; Li, C.; Li, J.; Dong, Z.; Zhang, M.; Qi, P.; Bai, X.; Jiang, K. Exploring and comparing the roles of Ca2+ and Mg2+ in small-sized natural organics-induced charged nanofiltration membrane fouling. Sep. Purif. Technol. 2020, 251, 117415. [Google Scholar] [CrossRef]
- Yu, T.; Sun, H.; Chen, Z.; Wang, Y.; Huo, Z.; Ikuno, N.; Ishii, K.; Jin, Y.; Hu, H.; Wu, Y.; et al. Different bacterial species and their extracellular polymeric substances (EPSs) significantly affected reverse osmosis (RO) membrane fouling potentials in wastewater reclamation. Sci. Total Environ. 2018, 644, 486–493. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Yu, T.; Zhao, X.; Tong, X.; Bai, Y.; Huo, Z.; Hu, H. Effects of chlorine disinfection on the membrane fouling potential of bacterial strains isolated from fouled reverse osmosis membranes. Sci. Total Environ. 2019, 693, 133579. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Nagaraja, N.; Skillman, L.; Li, D.; Ho, G. Comparison of polysaccharide fouling in forward osmosis and reverse osmosis separations. Desalination 2017, 402, 174–184. [Google Scholar] [CrossRef]
- Tong, X.; Zhao, X.; Wu, Y.; Bai, Y.; Ikuno, N.; Ishii, K.; Hu, H. The molecular structures of polysaccharides affect their reverse osmosis membrane fouling behaviors. J. Membr. Sci. 2021, 625, 118984. [Google Scholar] [CrossRef]
- Zheng, X.; Khan, M.T.; Croue, J. Contribution of effluent organic matter (EfOM) to ultrafiltration (UF) membrane fouling: Isolation, characterization, and fouling effect of EfOM fractions. Water Res. 2014, 65, 414–424. [Google Scholar] [CrossRef]
- Winters, H.; Chong, T.H.; Fane, A.G.; Krantz, W.; Rzechowicz, M.; Saeidi, N. The involvement of lectins and lectin-like humic substances in biofilm formation on RO membranes—Is TEP important? Desalination 2016, 399, 61–68. [Google Scholar] [CrossRef]
- Linares, R.V.; Yangali-Quintanilla, V.; Li, Z.; Amy, G. NOM and TEP fouling of a forward osmosis (FO) membrane: Foulant identification and cleaning. J. Membr. Sci. 2012, 421, 217–224. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Passow, U.; Castrillon, S.R.; Elimelech, M. Transparent Exopolymer Particles: From Aquatic Environments and Engineered Systems to Membrane Biofouling. Environ. Sci. Technol. 2015, 49, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Meng, X.; Fan, W.; Liang, D.; Wang, L.; Zhang, W.; Liu, Y. The role of transparent exopolymer particles (TEP) in membrane fouling: A critical review. Water Res. 2020, 181, 115930. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Y. Alginate block fractions and their effects on membrane fouling. Water Res. 2013, 47, 6618–6627. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, R.; Meng, X.; Wang, Y.; Fan, W.; Liang, D.; Zhang, M.; Liao, Y.; Tang, C. Reaction heterogeneity in the bridging effect of divalent cations on polysaccharide fouling. J. Membr. Sci. 2022, 641, 119933. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Elimelech, M. Complexation between dissolved silica and alginate molecules: Implications for reverse osmosis membrane fouling. J. Membr. Sci. 2020, 605, 118109. [Google Scholar] [CrossRef]
- Tian, J.; Ernst, M.; Cui, F.; Jekel, M. Effect of different cations on UF membrane fouling by NOM fractions. Chem. Eng. J. 2013, 223, 547–555. [Google Scholar] [CrossRef]
- Wui, S.A.; Menachem, E. Protein (BSA) fouling of reverse osmosis membranes: Implications for wastewater reclamation. J. Membr. Sci. 2007, 296, 83–92. [Google Scholar]
- Kelly, S.T.; Zydney, A.L. Mechanisms for BSA fouling during microfiltration. J. Membr. Sci. 1995, 107, 115–127. [Google Scholar] [CrossRef]
- Mo, H.; Tay, K.G.; Ng, H.Y. Fouling of reverse osmosis membrane by protein (BSA): Effects of pH, calcium, magnesium, ionic strength and temperature. J. Membr. Sci. 2008, 315, 28–35. [Google Scholar] [CrossRef]
- Tran-Ha, M.H.; Santos, V.; Wiley, D.E. The effect of multivalent cations on membrane-protein interactions during cleaning with CTAB. J. Membr. Sci. 2005, 251, 179–188. [Google Scholar] [CrossRef]
- Hao, Y.; Moriya, A.; Ohmukai, Y.; Matsuyama, H.; Maruyama, T. Effect of metal ions on the protein fouling of hollow-fiber ultrafiltration membranes. Sep. Purif. Technol. 2013, 111, 137–144. [Google Scholar] [CrossRef]
- Quay, A.N.; Tong, T.; Hashmi, S.M.; Zhou, Y.; Zhao, S.; Elimelech, M. Combined Organic Fouling and Inorganic Scaling in Reverse Osmosis: Role of Protein-Silica Interactions. Environ. Sci. Technol. 2018, 52, 9145–9153. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Myat, D.T.; Stewart, M.B.; Mergen, M.; Zhao, O.; Orbell, J.D.; Gray, S. Experimental and computational investigations of the interactions between model organic compounds and subsequent membrane fouling. Water Res. 2014, 48, 108–118. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, H.; Lin, H.; Shen, L.; Yu, H.; Ma, G.; Chen, J.; Liao, B. Mechanistic insights into alginate fouling caused by calcium ions based on terahertz time-domain spectra analyses and DFT calculations. Water Res. 2018, 129, 337–346. [Google Scholar] [CrossRef]
- Tu, Q.; Ibrahimi, W.; Ren, S.; Wu, J.; Li, S. A Molecular Dynamics Study on Rotational Nanofluid and Its Application to Desalination. Membranes 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Ebro, H.; Kim, Y.M.; Kim, J.H. Molecular dynamics simulations in membrane-based water treatment processes: A systematic overview. J. Membr. Sci. 2013, 438, 112–125. [Google Scholar] [CrossRef]
- Lach, J.; Goclon, J.; Rodziewicz, P. Structural flexibility of the sulfur mustard molecule at finite temperature from Car-Parrinello molecular dynamics simulations. J. Hazard. Mater. 2016, 306, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Marry, V.; Dubois, E.; Malikova, N.; Durand-Vidal, S.; Longeville, S.; Breu, J. Water Dynamics in Hectorite Clays: Infuence of Temperature Studied by Coupling Neutron Spin Echo and Molecular Dynamics. Environ. Sci. Technol. 2011, 45, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, Y.; Liu, H. Classical density functional analysis of ion selectivity in nanopores: The coupling between hard-sphere and electrostatic interactions. Chem. Eng. J. 2022, 444, 136673. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, E.; Qin, Y.; Liu, X.; Zou, Y.; Wu, H.; Zhu, T. Density functional theory (DFT) studies of vanadium-titanium based selective catalytic reduction (SCR) catalysts. J. Environ. Sci. 2020, 90, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, D.; Li, C.; Wang, Y.; Yang, Q. Density functional theory study of mercury adsorption and oxidation on CuO (111) surface. Chem. Eng. J. 2014, 258, 128–135. [Google Scholar] [CrossRef]
- Kang, X.; Peng, J.; Ragauskas, A.J.; Ren, X.; Si, C.; Wang, S.; Song, X. Competitive effects of glucan’s main hydrolysates on biochar formation: A combined experiment and density functional theory analysis. Bioresour. Technol. 2022, 359, 127427. [Google Scholar] [CrossRef]
- Bresnahan, C.G.; McAlexander, H.R.; Woodley, C.M.; Shukla, M.K. Density functional theory explorations of parathion and paraoxon hydrolysis as a function of the underlying alkaline environment. Environ. Sci. Process. Impacts 2022, 24, 2249–2262. [Google Scholar] [CrossRef]
- Chen, Z.; Ito, K.; Yanagishita, H.; Oshima, N.; Suzuki, R.; Kobayashi, Y. Correlation Study between Free-Volume Holes and Molecular Separations of Composite Membranes for Reverse Osmosis Processes by Means of Variable-Energy Positron Annihilation Techniques. J. Phys. Chem. C 2011, 115, 18055–18060. [Google Scholar] [CrossRef]
- Ma, B.; Yu, W.; Liu, H.; Yao, J.; Qu, J. Effect of iron/aluminum hydrolyzed precipitate layer on ultrafiltration membrane. Desalination 2013, 330, 16–21. [Google Scholar] [CrossRef]
- Baiwen, M.; Xing, W.; Chengzhi, H.; William, A.J.; Huijuan, L.; Jiuhui, Q. Antifouling by pre-deposited Al hydrolytic flocs on ultrafiltration membrane in the presence of humic acid and bovine serum albumin. J. Membr. Sci. 2017, 538, 34–40. [Google Scholar]
- Ma, B.; Ding, Y.; Li, W.; Hu, C.; Yang, M.; Liu, H.; Qu, J. Ultrafiltration membrane fouling induced by humic acid with typical inorganic salts. Chemosphere 2018, 197, 793–802. [Google Scholar] [CrossRef]
- Miao, R.; Wu, Y.; Wang, P.; Wu, G.; Wang, L.; Li, X.; Wang, J.; Lv, Y.; Liu, T. New insights into the humic acid fouling mechanism of ultrafiltration membranes for different Ca2+ thorn dosage ranges: Results from micro- and macro-level analyses. Water Sci. Technol. 2018, 77, 2265–2273. [Google Scholar] [CrossRef]
- Hao, Y.; Moriya, A.; Maruyama, T.; Ohmukai, Y.; Matsuyama, H. Effect of metal ions on humic acid fouling of hollow fiber ultrafiltration membrane. J. Membr. Sci. 2011, 376, 247–253. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, J.; He, G.; Wang, T.; Hou, D.; Luan, Z. Perfluorooctane sulfonate removal by nanofiltration membrane the role of calcium ions. Chem. Eng. J. 2013, 233, 224–232. [Google Scholar] [CrossRef]
- Meng, S.; Winters, H.; Liu, Y. Ultrafiltration behaviors of alginate blocks at various calcium concentrations. Water Res. 2015, 83, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wen, T.; Li, Y.; Li, A.; Long, C. Alleviating reverse osmosis membrane fouling caused by biopolymers using pre-ozonation. J. Membr. Sci. 2020, 595, 117546. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Duan, L.; Jia, Y.; Zhang, H.; Liu, J.; Yang, W. A Comprehensive Analysis of the Impact of Inorganic Matter on Membrane Organic Fouling: A Mini Review. Membranes 2023, 13, 837. https://doi.org/10.3390/membranes13100837
Gao Q, Duan L, Jia Y, Zhang H, Liu J, Yang W. A Comprehensive Analysis of the Impact of Inorganic Matter on Membrane Organic Fouling: A Mini Review. Membranes. 2023; 13(10):837. https://doi.org/10.3390/membranes13100837
Chicago/Turabian StyleGao, Qiusheng, Liang Duan, Yanyan Jia, Hengliang Zhang, Jianing Liu, and Wei Yang. 2023. "A Comprehensive Analysis of the Impact of Inorganic Matter on Membrane Organic Fouling: A Mini Review" Membranes 13, no. 10: 837. https://doi.org/10.3390/membranes13100837
APA StyleGao, Q., Duan, L., Jia, Y., Zhang, H., Liu, J., & Yang, W. (2023). A Comprehensive Analysis of the Impact of Inorganic Matter on Membrane Organic Fouling: A Mini Review. Membranes, 13(10), 837. https://doi.org/10.3390/membranes13100837