Differences in the Effect of Mn2+ on the Reverse Osmosis Membrane Fouling Caused by Different Types of Organic Matter: Experimental and Density Functional Theory Evidence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Reserve Reagent
2.3. Design of Experiments
2.4. RO Membrane Filtration Experiment
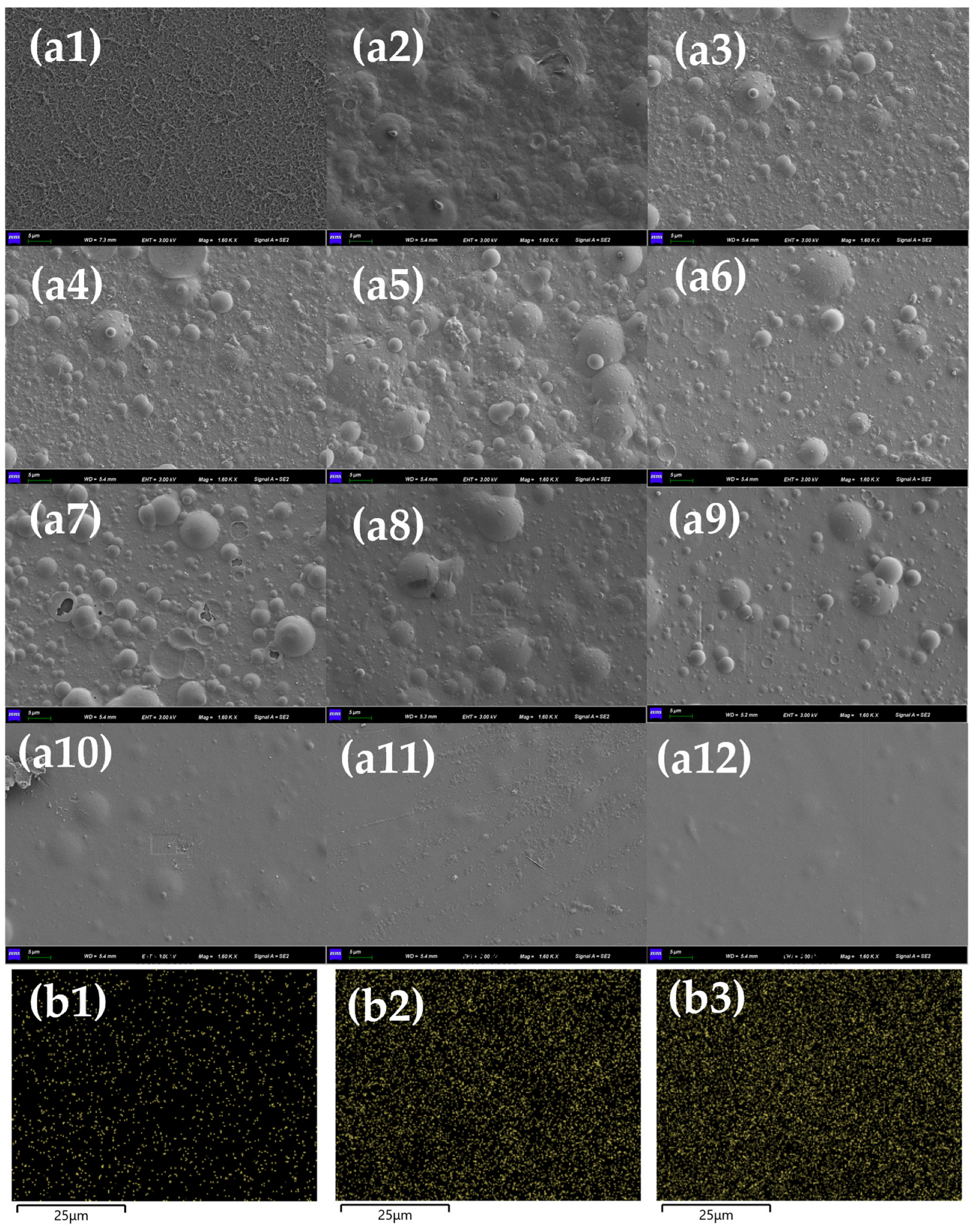
2.5. RO Membrane Characterization
2.6. The Density Functional Theory (DFT) Calculations
3. Results and Discussion
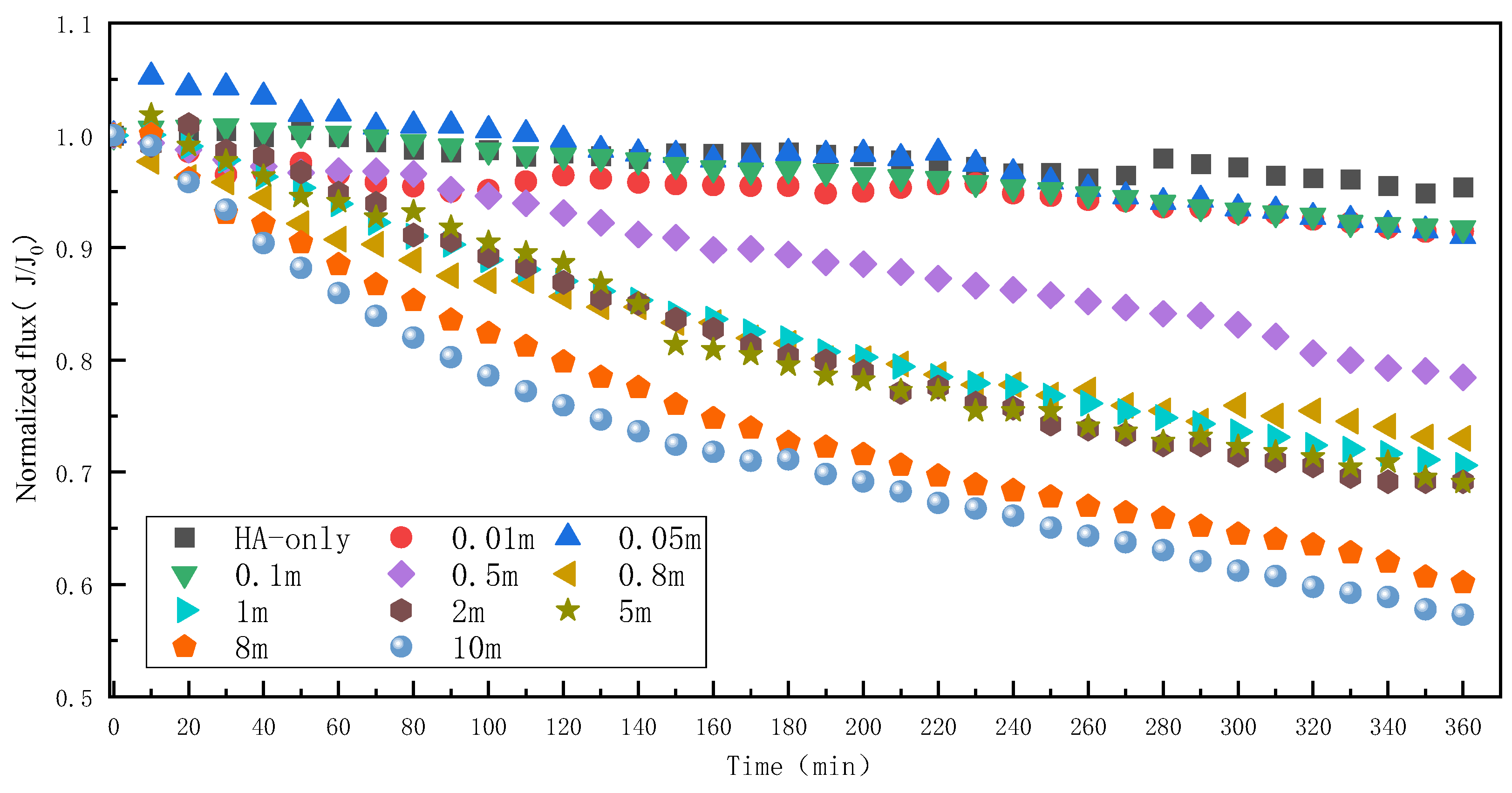
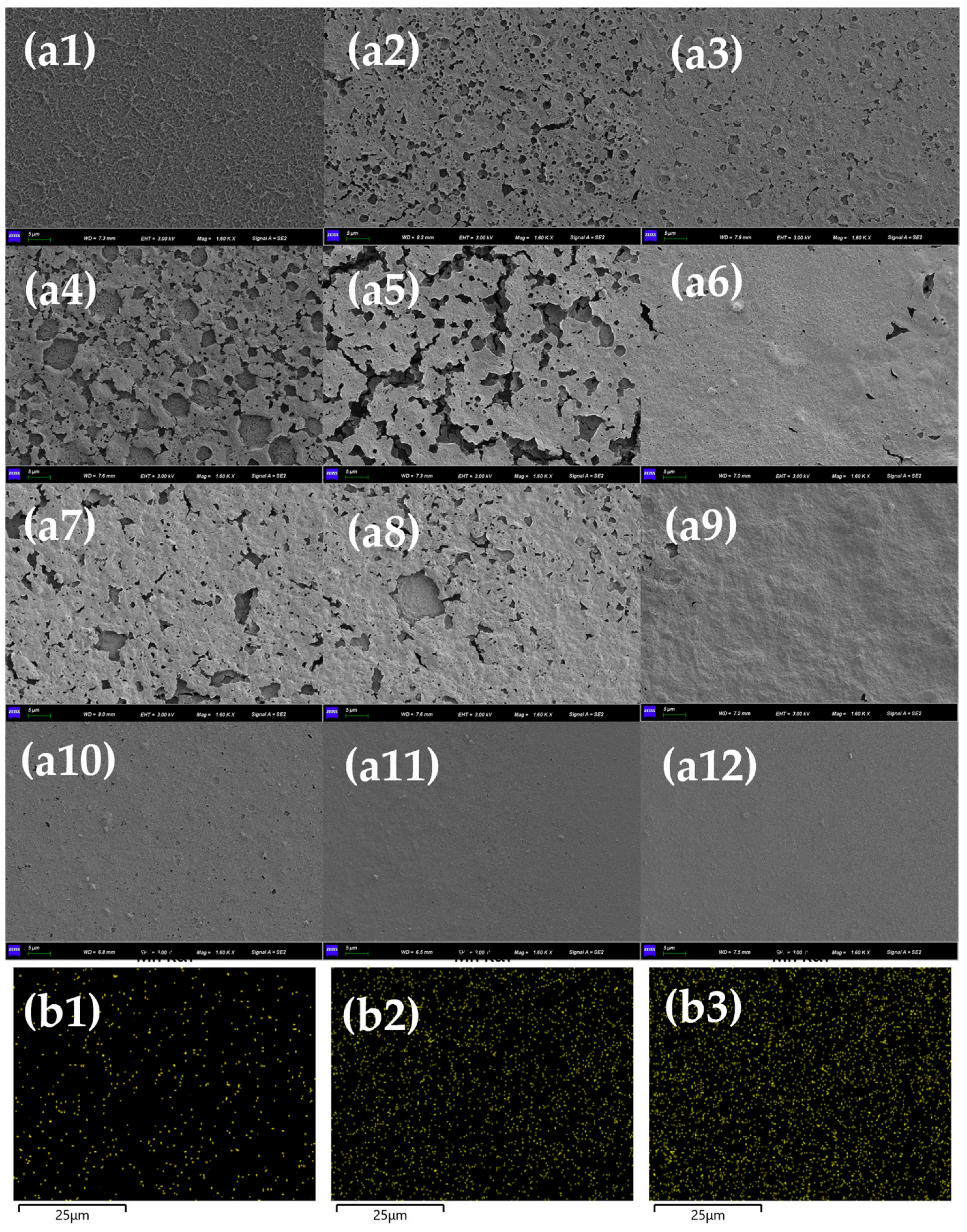
3.1. Effect of Mn2+ on RO Membrane Fouling Caused by HA
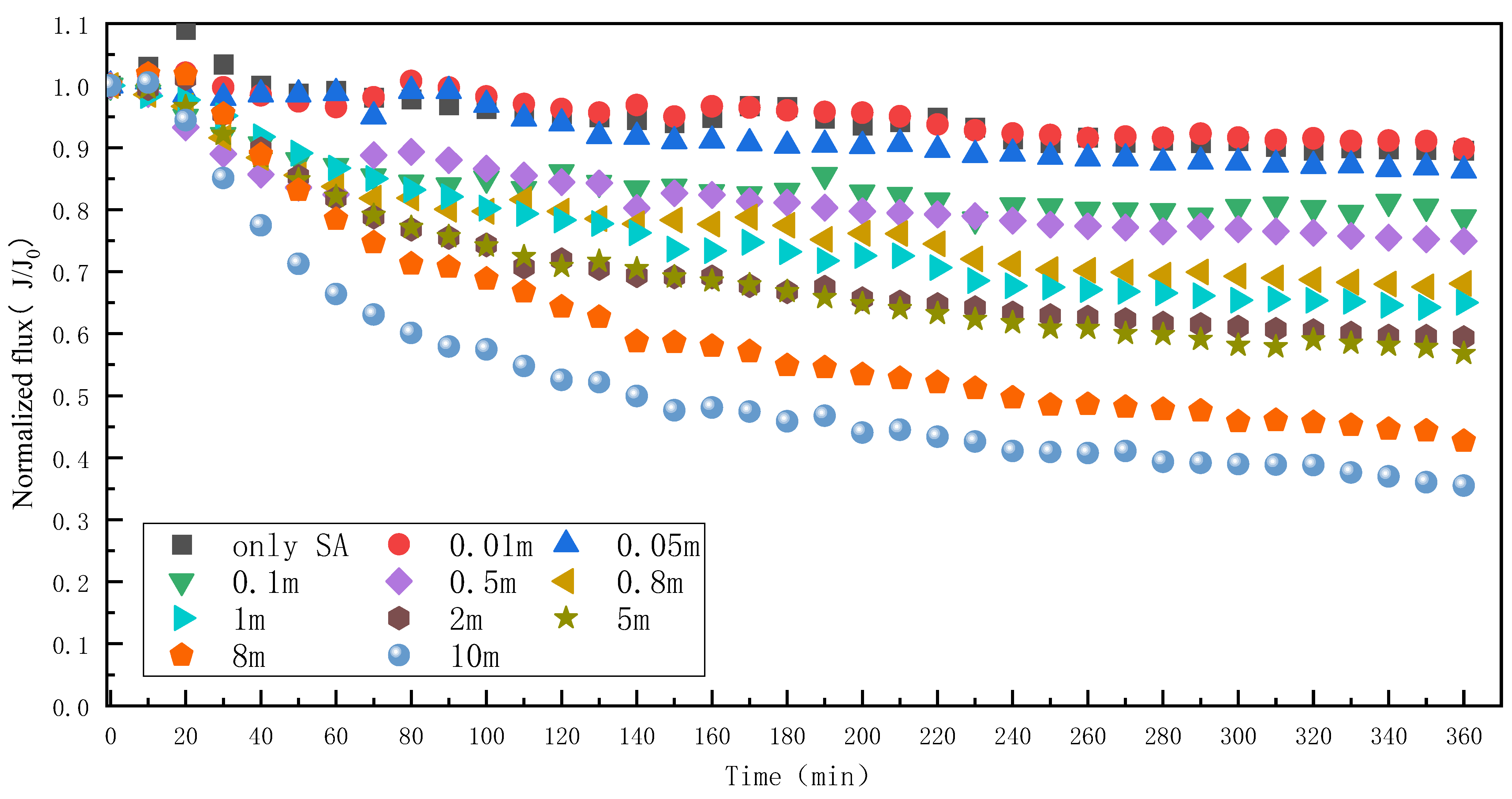
3.2. Effect of Mn2+ on RO Membrane Fouling Caused by SA
3.3. Effect of Mn2+ on RO Membrane Fouling by Caused BSA
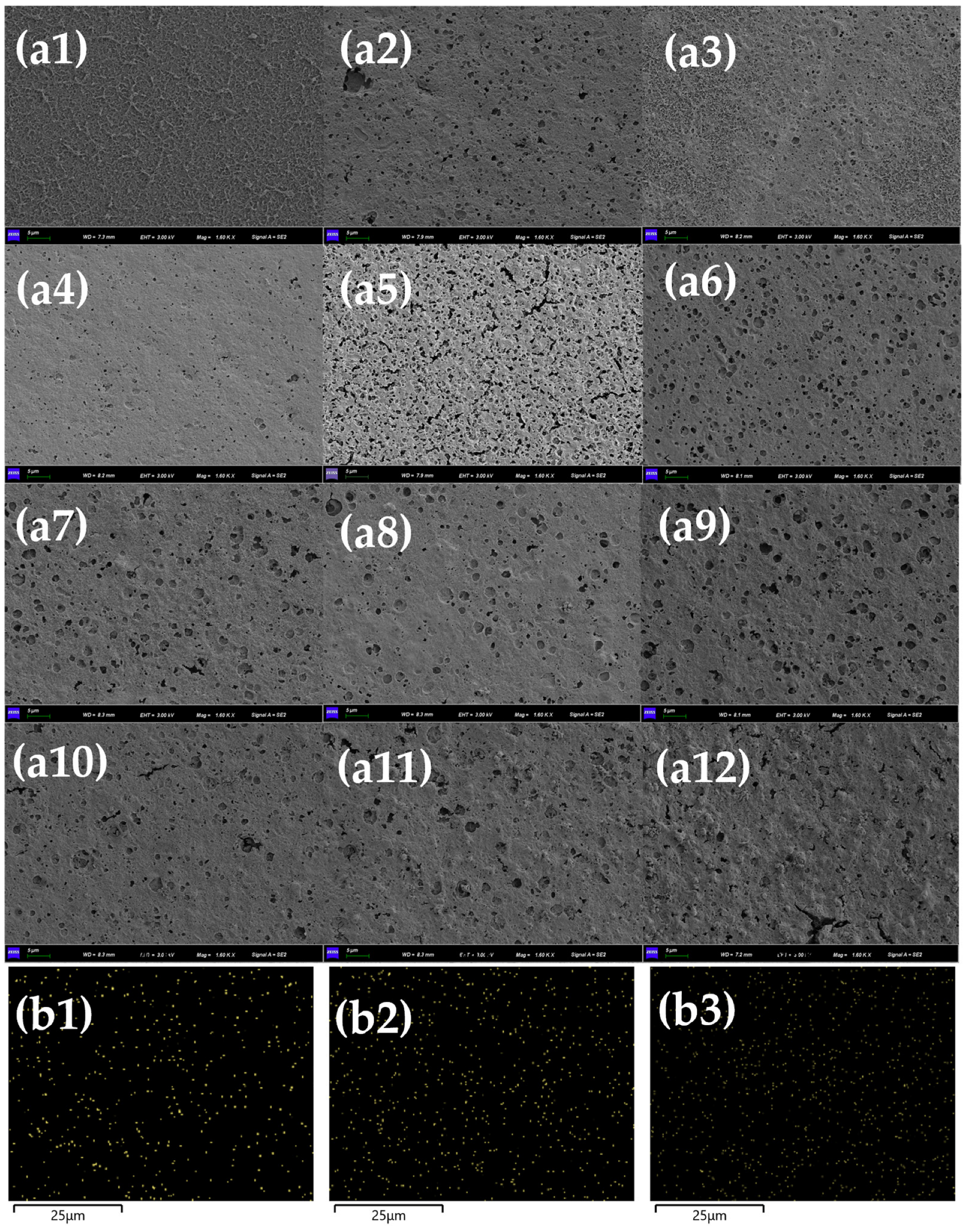
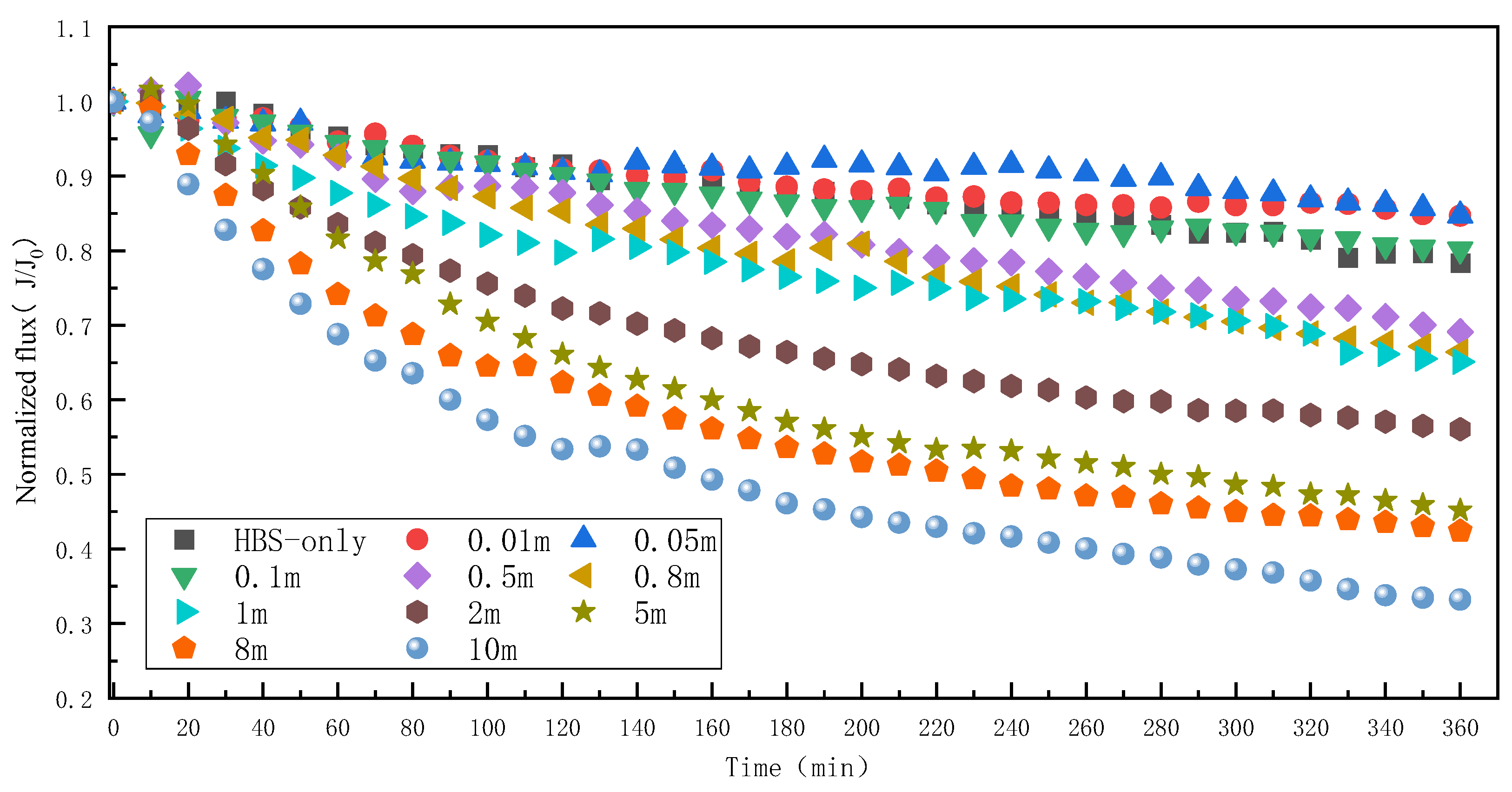
3.4. Effect of Mn2+ on RO Membrane Fouling Caused by HBS
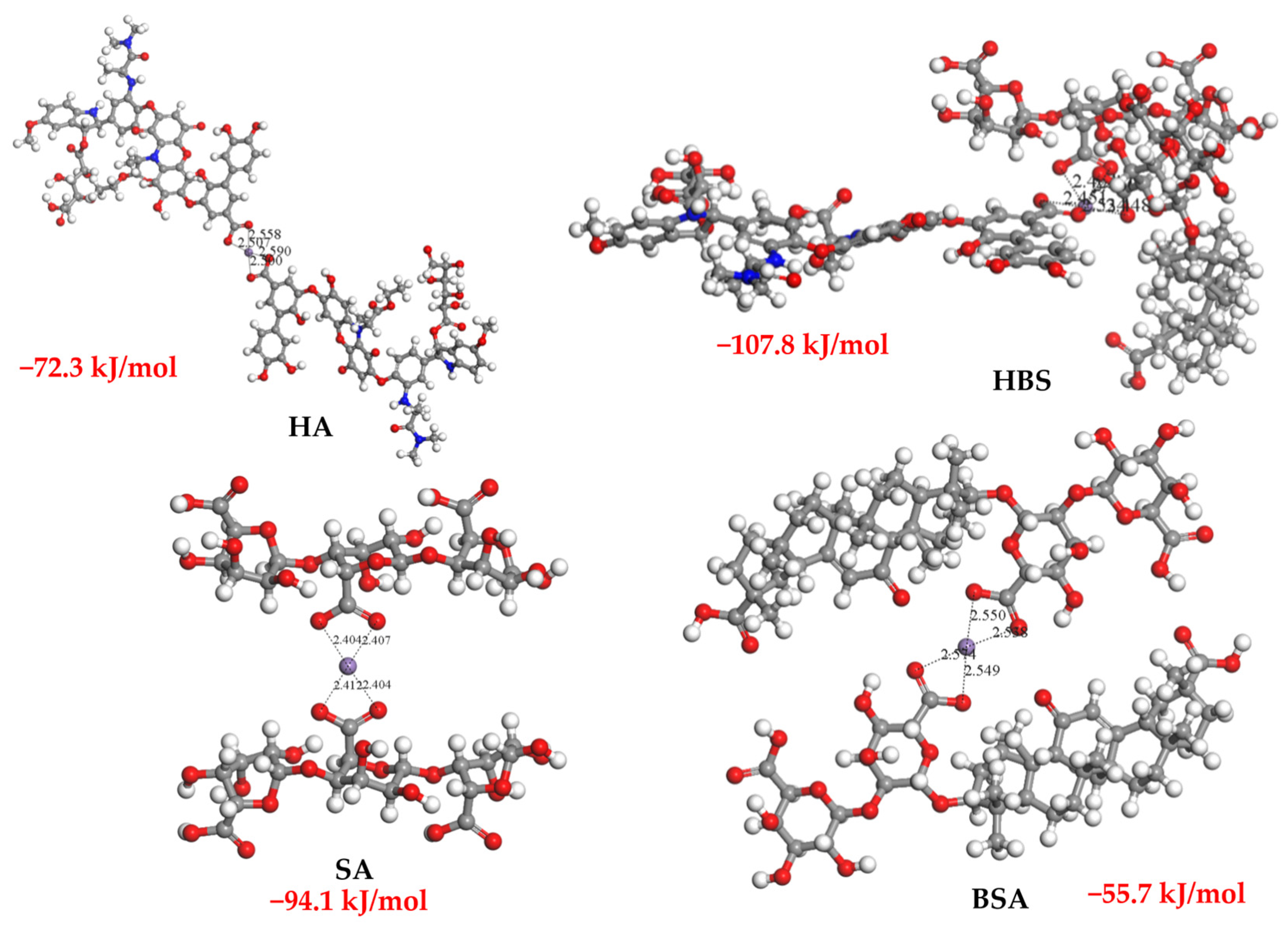
3.5. Validation Using DFT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, J.; Shi, F.; Yi, J.; Fu, H. Analysis of the factors that affect the production of municipal solid waste in China. J. Clean. Prod. 2020, 259, 120808. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; He, P.-J.; Shao, L.-M. Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis. Water Res. 2011, 45, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, S.; Behrendt, J.; Otterpohl, R. Comparison of NF-RO and RO-NF for the Treatment of Mature Landfill Leachates: A Guide for Landfill Operators. Membranes 2018, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, L.; Gao, Q.; Zhao, Y.; Gao, F. Removal of Typical PPCPs by Reverse Osmosis Membranes: Optimization of Treatment Process by Factorial Design. Membranes 2023, 13, 355. [Google Scholar] [CrossRef]
- Sir, M.; Podhola, M.; Patocka, T.; Honzajková, Z.; Kocurek, P.; Kubal, M.; Kuraš, M. The effect of humic acids on the reverse osmosis treatment of hazardous landfill leachate. J. Hazard. Mater. 2012, 207, 86–90. [Google Scholar] [CrossRef]
- Peters, T.A. Purification of landfill leachate with reverse osmosis and nanofiltration. Desalination 1998, 119, 289–293. [Google Scholar] [CrossRef]
- Li, D.; Lin, W.; Shao, R.; Shen, Y.-X.; Zhu, X.; Huang, X. Interaction between humic acid and silica in reverse osmosis membrane fouling process: A spectroscopic and molecular dynamics insight. Water Res. 2021, 206, 117773. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, H.; Lin, H.; Shen, L.; Yu, H.; Ma, G.; Chen, J.; Liao, B.-Q. Mechanistic insights into alginate fouling caused by calcium ions based on terahertz time-domain spectra analyses and DFT calculations. Water Res. 2018, 129, 337–346. [Google Scholar] [CrossRef]
- Tong, X.; Zhao, X.-H.; Wu, Y.-H.; Bai, Y.; Ikuno, N.; Ishii, K.; Hu, H.-Y. The molecular structures of polysaccharides affect their reverse osmosis membrane fouling behaviors. J. Membr. Sci. 2021, 625, 118984. [Google Scholar] [CrossRef]
- Xin, Y.; Bligh, M.W.; Kinsela, A.S.; Waite, T.D. Effect of iron on membrane fouling by alginate in the absence and presence of calcium. J. Membr. Sci. 2016, 497, 289–299. [Google Scholar] [CrossRef]
- Teng, J.; Chen, Y.; Ma, G.; Hong, H.; Sun, T.; Liao, B.-Q.; Lin, H. Membrane fouling by alginate in polyaluminum chloride (PACl) coagulation/microfiltration process: Molecular insights. Sep. Purif. Technol. 2020, 236, 116294. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B.-Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Moriya, A.; Ohmukai, Y.; Matsuyama, H.; Maruyama, T. Effect of metal ions on the protein fouling of hollow-fiber ultrafiltration membranes. Sep. Purif. Technol. 2013, 111, 137–144. [Google Scholar] [CrossRef]
- Yin, Z.; Shao, Q.; Wen, T.; Li, A.; Long, C. Insights into the coupling pre-ozonation with coagulation pre-treatment for mitigating biopolymer fouling of reverse osmosis membrane: Role of Ca2+. J. Membr. Sci. 2021, 639, 119768. [Google Scholar] [CrossRef]
- Beinabaj, S.M.H.; Heydariyan, H.; Aleii, H.M.; Hosseinzadeh, A. Concentration of heavy metals in leachate, soil, and plants in Tehran’s landfill: Investigation of the effect of landfill age on the intensity of pollution. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef]
- Kaiya, Y.; Itoh, Y.; Fujita, K.; Takizawa, S. Study on fouling materials in the membrane treatment process for potable water. Desalination 1996, 106, 71–77. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, X.; Huang, K.; Wang, J.; Guo, Y.; Xie, B.; Li, G.; Liang, H. Can ultrafiltration singly treat the iron—And manganese-containing groundwater? J. Hazard. Mater. 2021, 409, 124983. [Google Scholar] [CrossRef]
- Tang, X.; Wang, J.; Zhang, H.; Yu, M.; Guo, Y.; Li, G.; Liang, H. Respective role of iron and manganese in direct ultrafiltration: From membrane fouling to flux improvements. Sep. Purif. Technol. 2021, 259, 118174. [Google Scholar] [CrossRef]
- Choo, K.H.; Lee, H.; Choi, S.J. Iron and manganese removal and membrane fouling during UF in conjunction with prechlorination for drinking water treatment. J. Membr. Sci. 2005, 267, 18–26. [Google Scholar] [CrossRef]
- Gray, S.; Ritchie, C.; Tran, T.; Bolto, B. Effect of NOM characteristics and membrane type on microfiltration performance. Water Res. 2007, 41, 3833–3841. [Google Scholar] [CrossRef]
- Tu, Q.; Ibrahimi, W.; Ren, S.; Wu, J.; Li, S. A Molecular Dynamics Study on Rotational Nanofluid and Its Application to Desalination. Membranes 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Myat, D.T.; Stewart, M.B.; Mergen, M.; Zhao, O.; Orbell, J.D.; Gray, S. Experimental and computational investigations of the interactions between model organic compounds and subsequent membrane fouling. Water Res. 2014, 48, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector Augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Zhang, X.; Tong, X.; Yu, T.; Wang, Y.; Ikuno, N.; Ishii, K.; Hu, H. Ozonation as an efficient pretreatment method to alleviate reverse osmosis membrane fouling caused by complexes of humic acid and calcium ion. Front. Environ. Sci. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Ye, W.; Bernstein, N.J.; Lin, J.; Jordens, J.; Zhao, S.; Tang, C.Y.; Van der Bruggen, B. Theoretical and experimental study of organic fouling of loose nanofiltration membrane. J. Taiwan Inst. Chem. E 2018, 93, 509–518. [Google Scholar] [CrossRef]
- Wang, L.-F.; He, D.-Q.; Chen, W.; Yu, H.-Q. Probing the roles of Ca2+ and Mg2+ in humic acids-induced ultrafitltration membrane fouling using an integrated approach. Water Res. 2015, 81, 325–332. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, K.; Huang, X. Characterizing the roles of organic and inorganic foulants in RO membrane fouling development: The case of coal chemical wastewater treatment. Sep. Purif. Technol. 2019, 210, 1008–1016. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Passow, U.; Castrillón, S.R.-V.; Elimelech, M. Transparent Exopolymer Particles: From Aquatic Environments and Engineered Systems to Membrane Biofouling. Environ. Sci. Technol. 2015, 49, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.T.; Zydney, A.L. Mechanisms for BSA fouling during microfiltration. J. Membr. Sci. 1995, 107, 115–127. [Google Scholar] [CrossRef]
- Mo, H.; Tay, K.G.; Ng, H.Y. Fouling of reverse osmosis membrane by protein (BSA): Effects of pH, calcium, magnesium, ionic strength and temperature. J. Membr. Sci. 2008, 315, 28–35. [Google Scholar] [CrossRef]
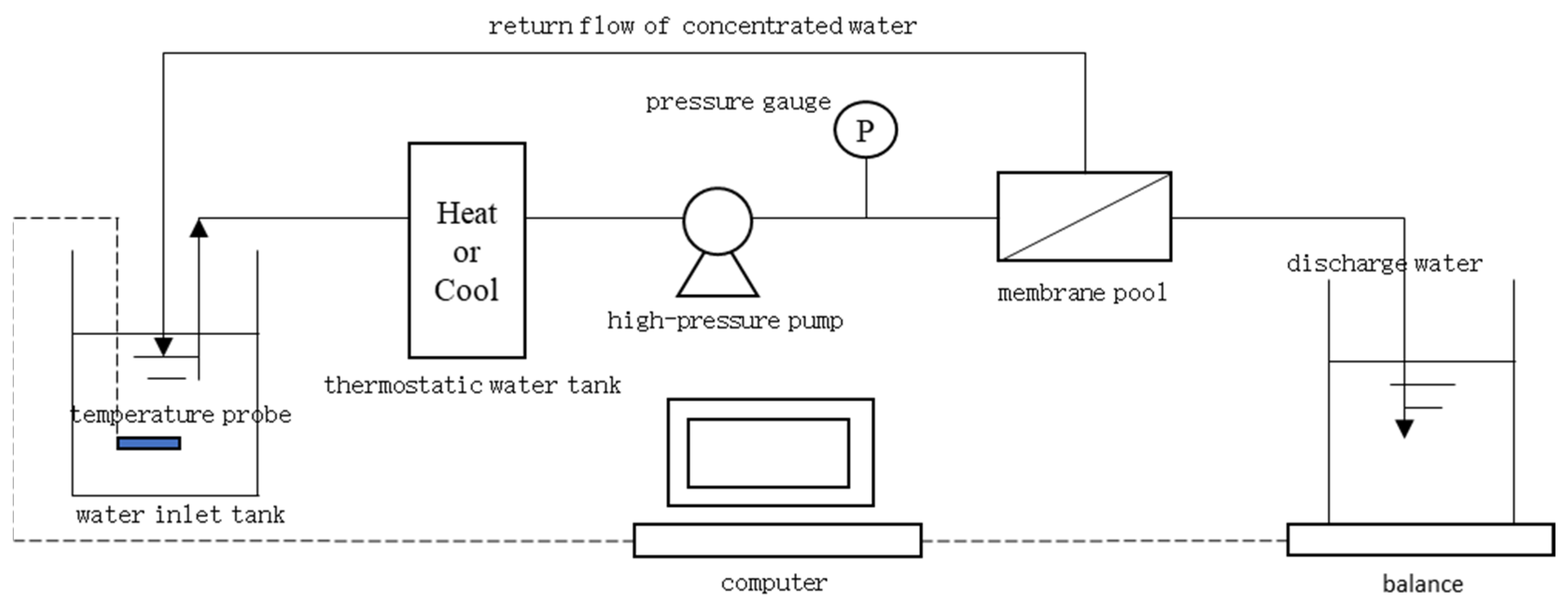


| Item | Parameter |
|---|---|
| Membrane Size | 24 cm2 |
| Operation method | Cross-flow filtration + concentrate recirculation |
| RO Membrane material | Polyamide composite membrane |
| Operating pressure | 12 bar |
| Filtration time | 6–12 h |
| Crossflow rate | about 9.6 cm/s |
| Working voltage | 220 V |
| Working temperature | 25 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Duan, L.; Jia, Y.; Zhang, H.; Liu, J.; Yang, W. Differences in the Effect of Mn2+ on the Reverse Osmosis Membrane Fouling Caused by Different Types of Organic Matter: Experimental and Density Functional Theory Evidence. Membranes 2023, 13, 823. https://doi.org/10.3390/membranes13100823
Gao Q, Duan L, Jia Y, Zhang H, Liu J, Yang W. Differences in the Effect of Mn2+ on the Reverse Osmosis Membrane Fouling Caused by Different Types of Organic Matter: Experimental and Density Functional Theory Evidence. Membranes. 2023; 13(10):823. https://doi.org/10.3390/membranes13100823
Chicago/Turabian StyleGao, Qiusheng, Liang Duan, Yanyan Jia, Hengliang Zhang, Jianing Liu, and Wei Yang. 2023. "Differences in the Effect of Mn2+ on the Reverse Osmosis Membrane Fouling Caused by Different Types of Organic Matter: Experimental and Density Functional Theory Evidence" Membranes 13, no. 10: 823. https://doi.org/10.3390/membranes13100823
APA StyleGao, Q., Duan, L., Jia, Y., Zhang, H., Liu, J., & Yang, W. (2023). Differences in the Effect of Mn2+ on the Reverse Osmosis Membrane Fouling Caused by Different Types of Organic Matter: Experimental and Density Functional Theory Evidence. Membranes, 13(10), 823. https://doi.org/10.3390/membranes13100823








