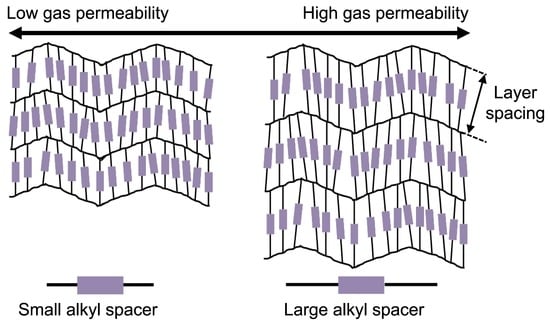
Tuning the Gas Separation Performances of Smectic Liquid Crystalline Polymer Membranes by Molecular Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membrane Preparation
2.3. Characterization
2.4. Gas Sorption
2.5. Single Gas Membrane Performances
3. Results and Discussion
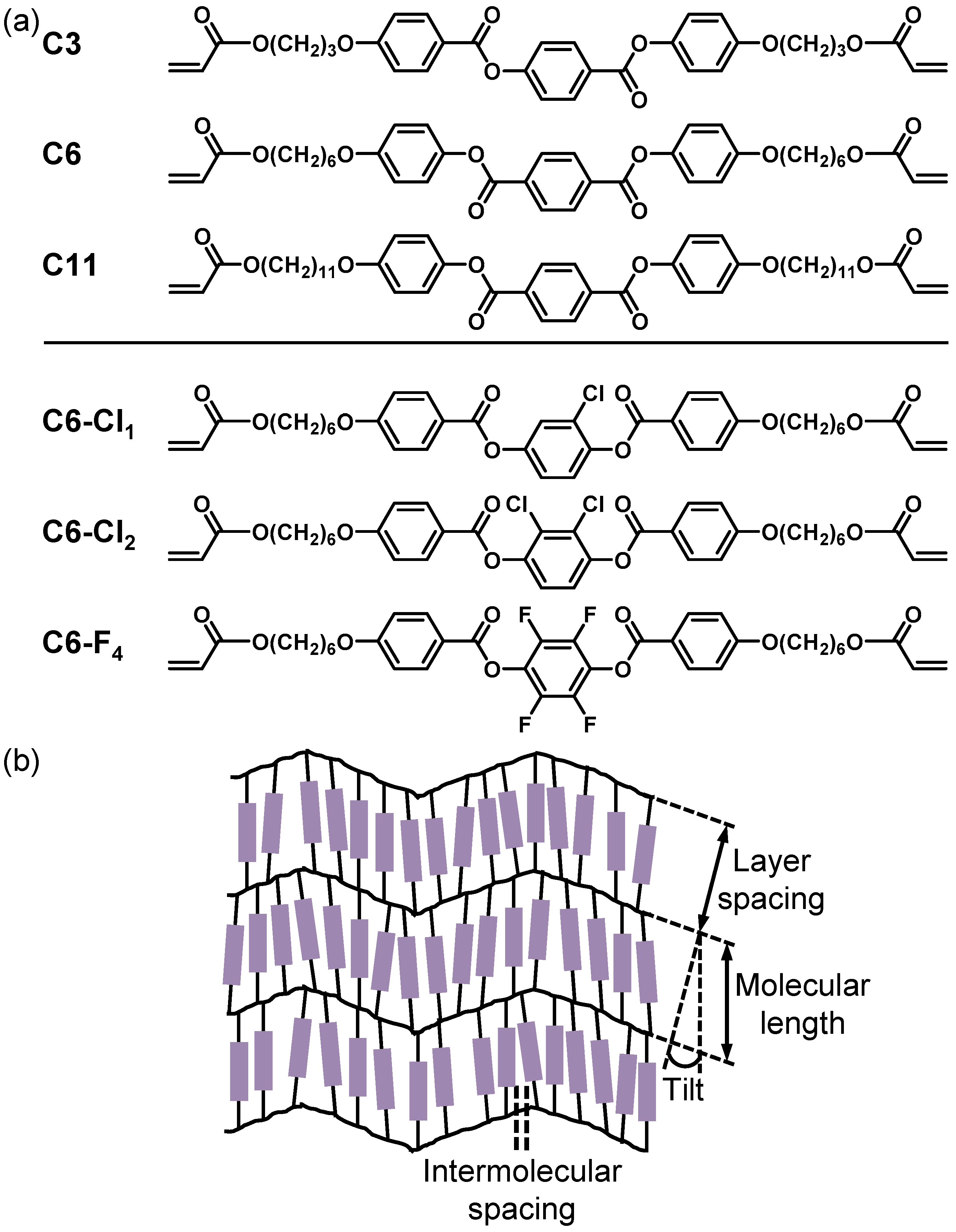
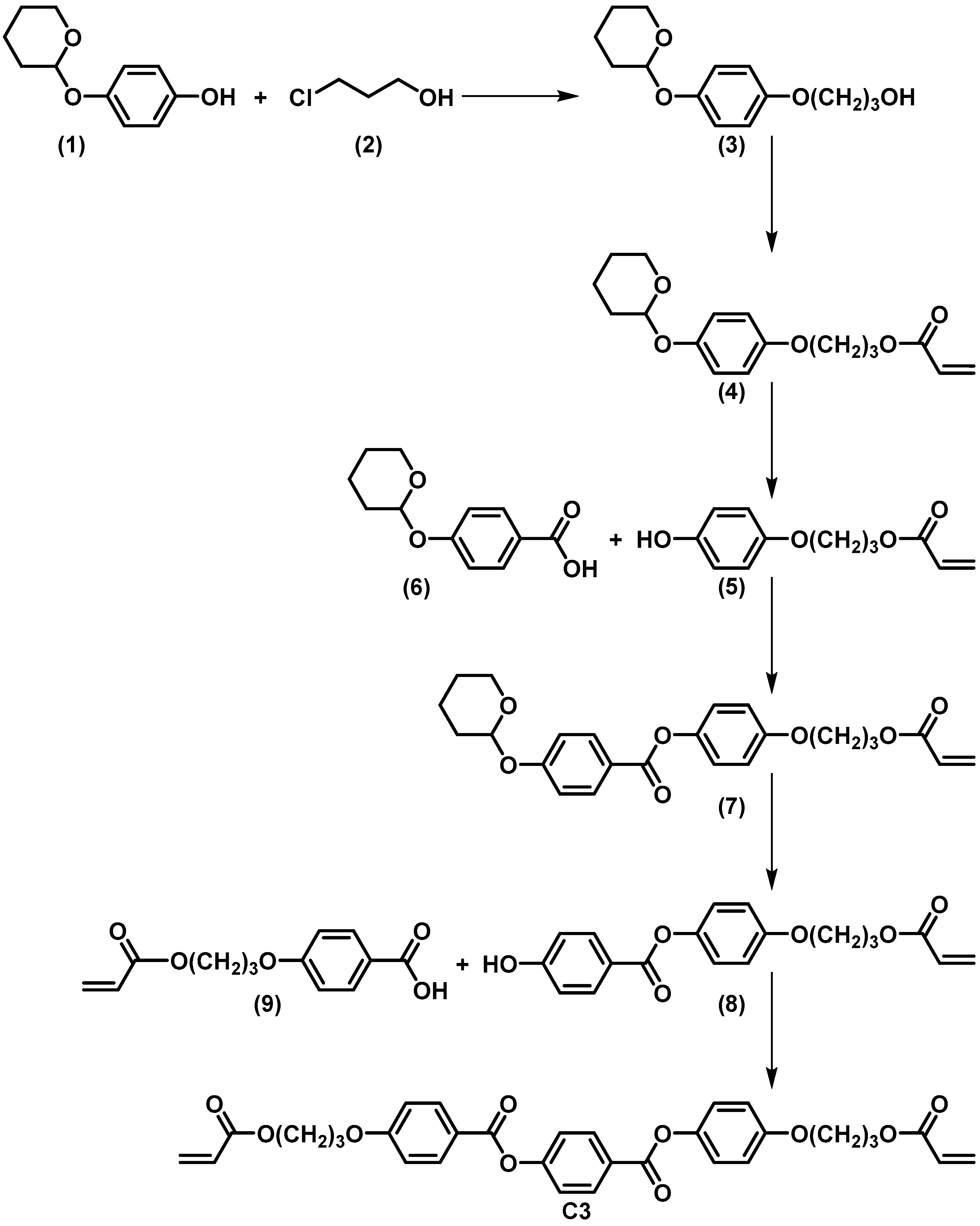
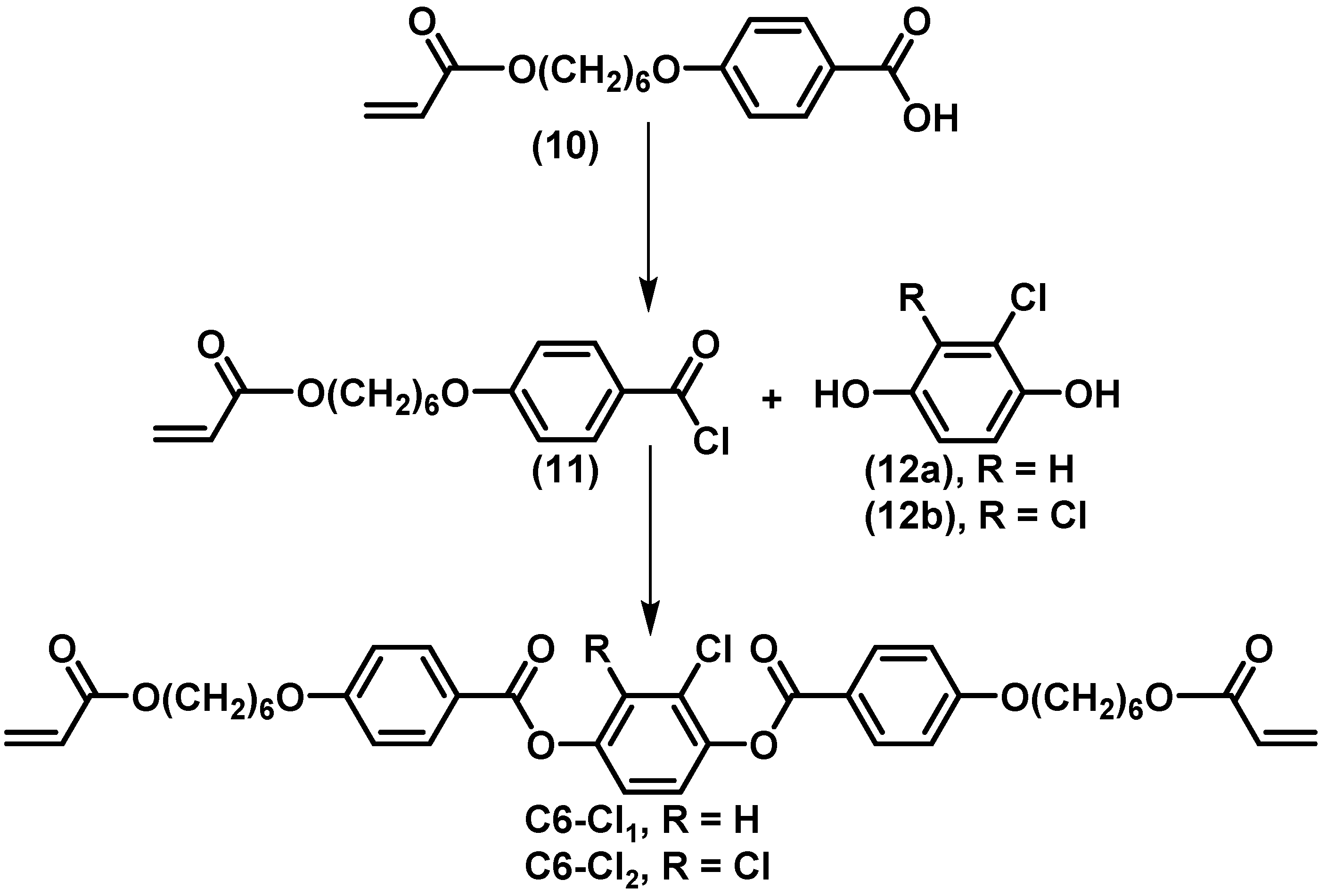
3.1. Preparation and Characterization of the Liquid Crystalline Molecules and Mixtures
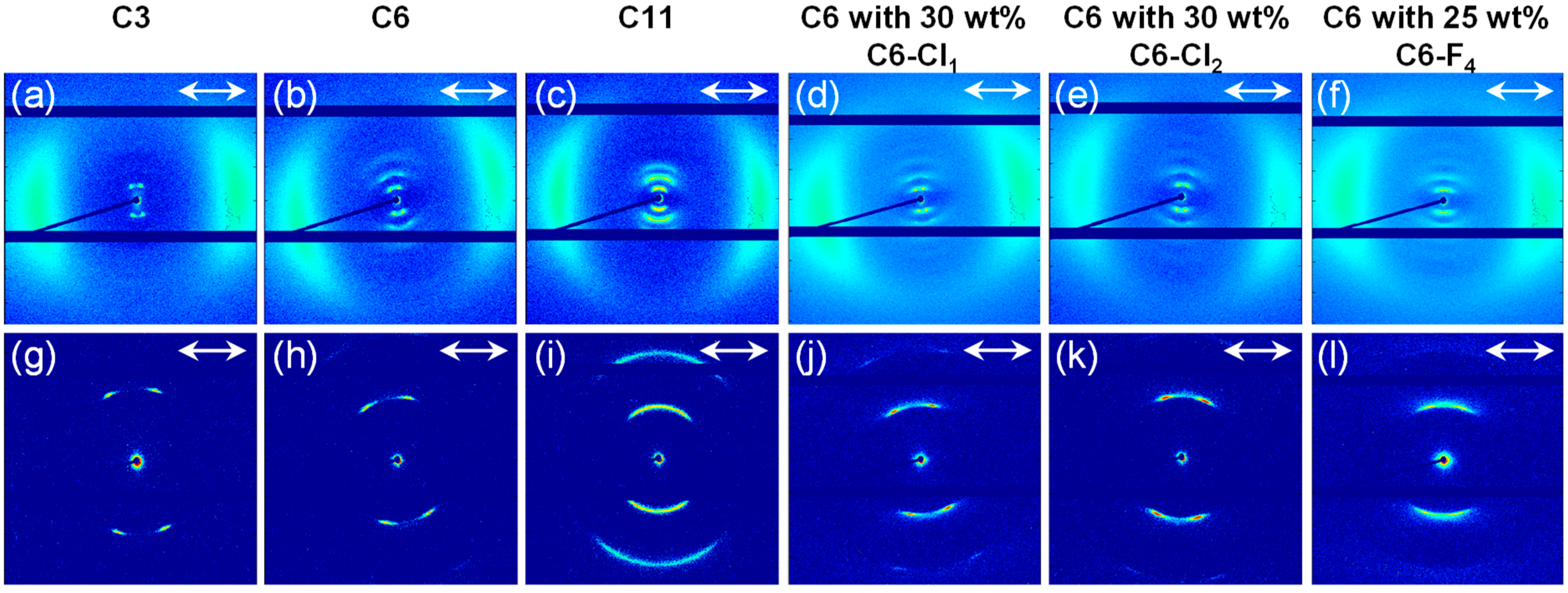
3.2. Preparation and Characterization of Liquid Crystalline Membranes
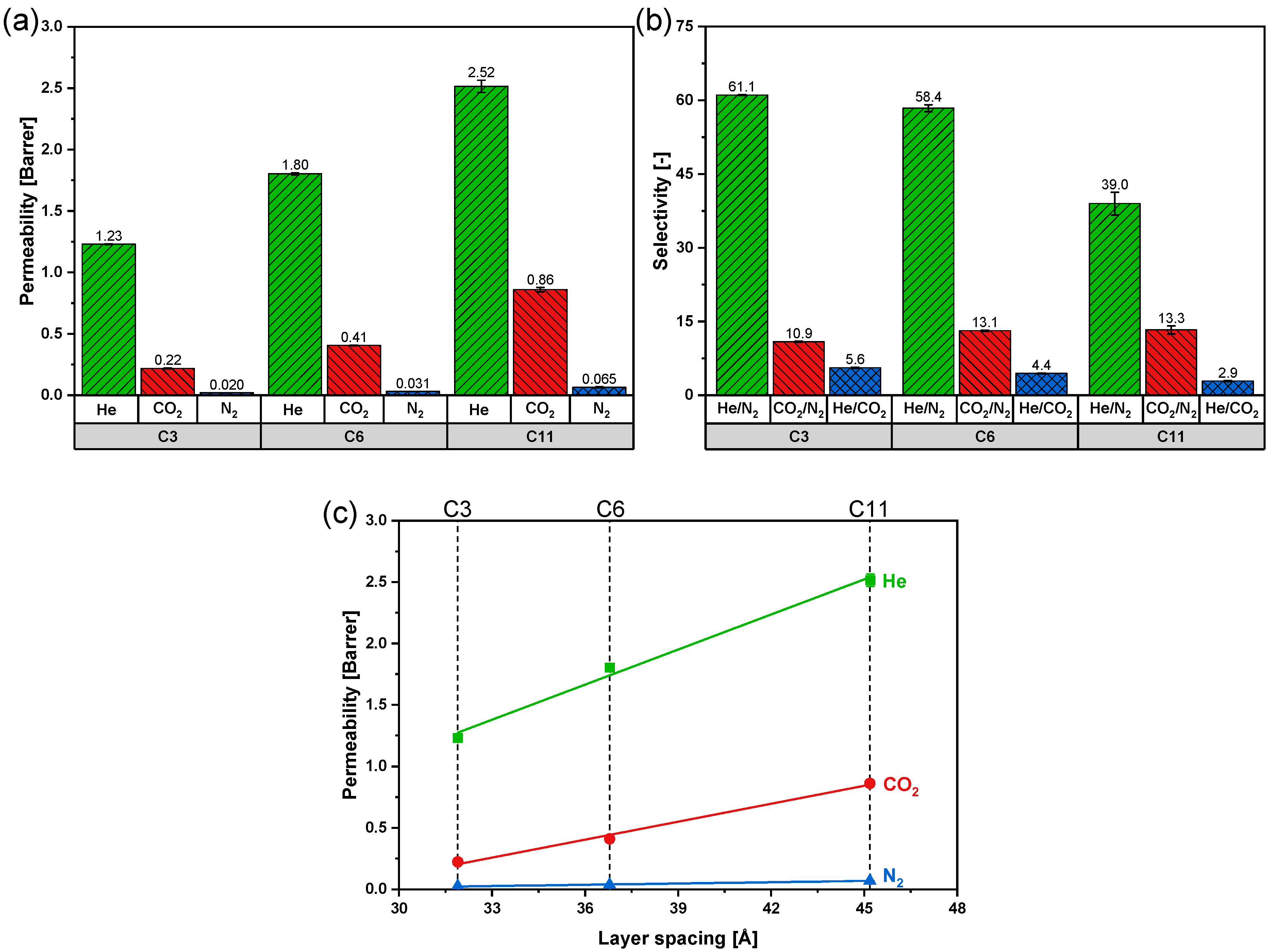
3.3. Effect of Layer Spacing on Single Gas Performances
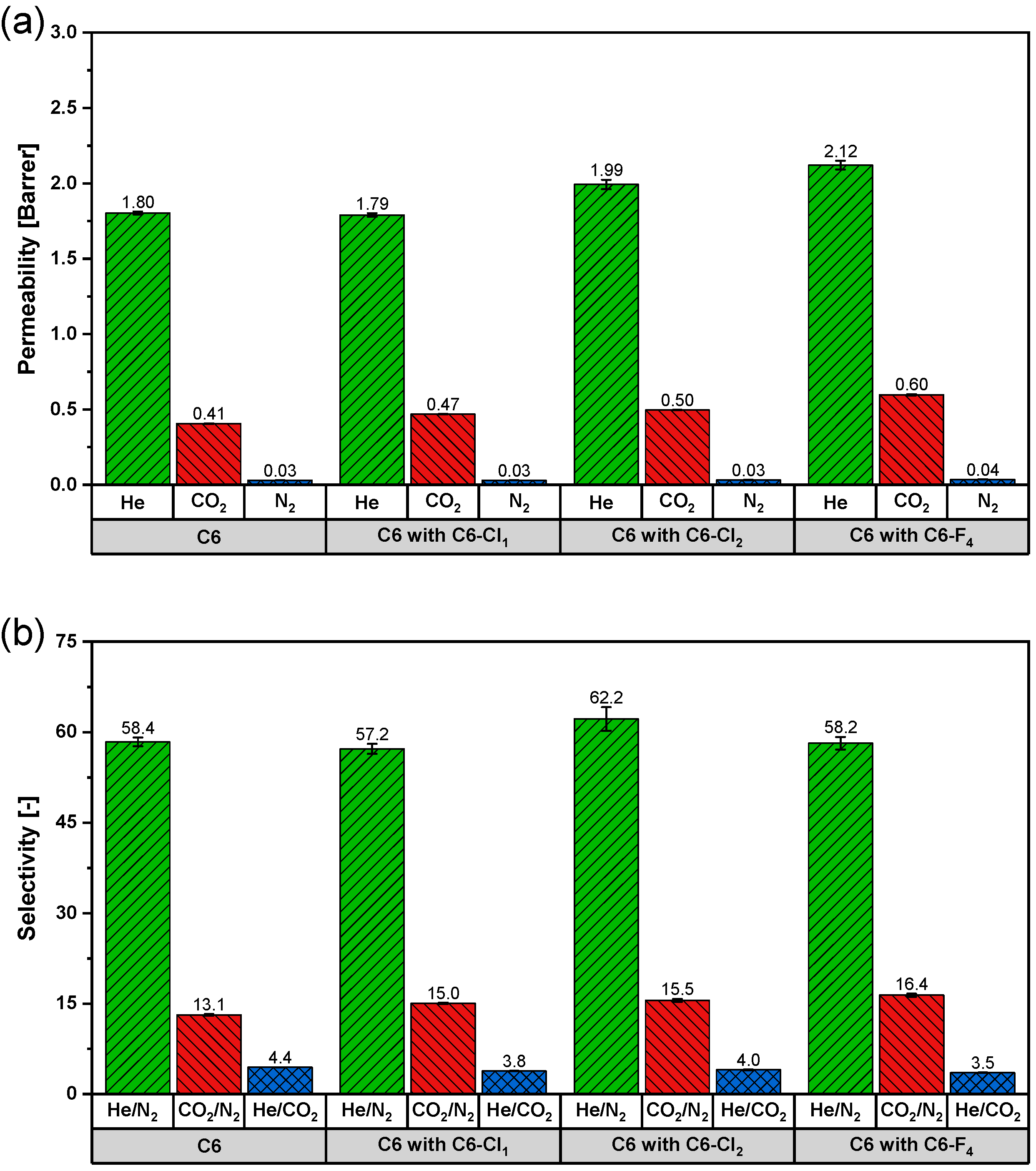
3.4. Effect of Halogenation on Single Gas Performances
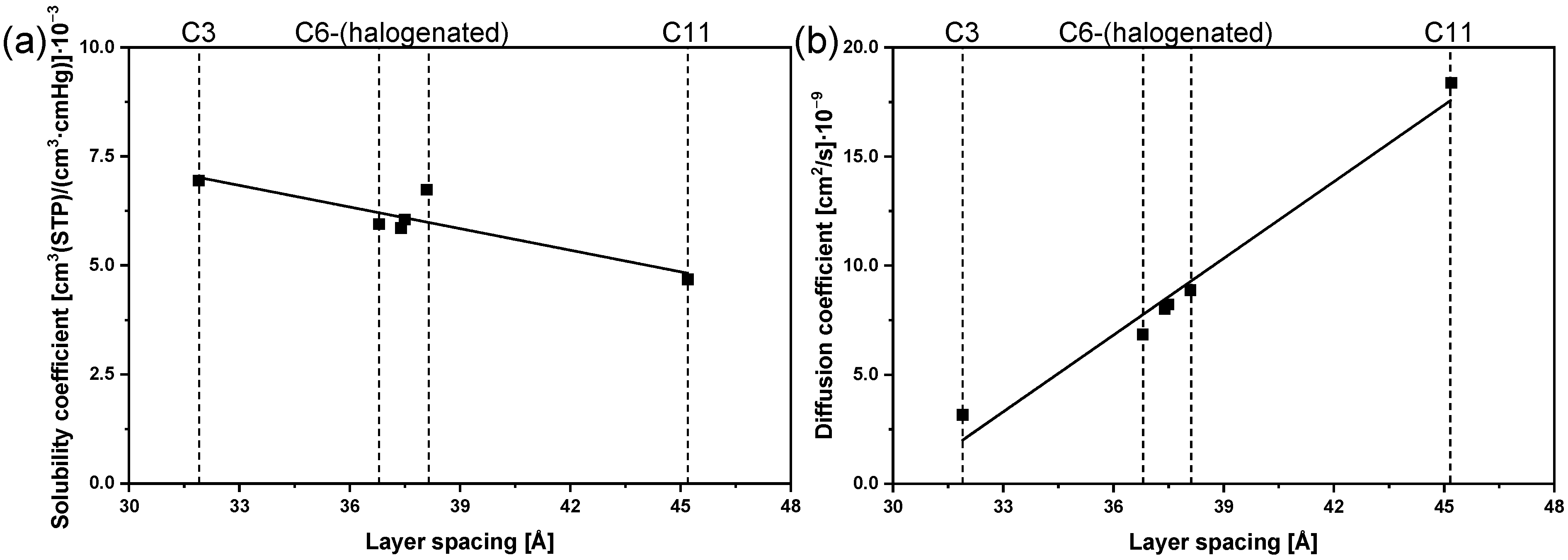
3.5. CO2 Sorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Liu, J.; Lin, H. Thermally Stable, Homogeneous Blends of Cross-Linked Poly(Ethylene Oxide) and Crown Ethers with Enhanced CO2 Permeability. J. Memb. Sci. 2020, 610, 118253. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in High Permeability Polymer-Based Membrane Materials for CO2 Separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-Based Technologies for Biogas Separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-Efficient Polymeric Gas Separation Membranes for a Sustainable Future: A Review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The Removal of CO2 and N2 from Natural Gas: A Review of Conventional and Emerging Process Technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. High Performance Polymer Membranes for CO2 Separation. Curr. Opin. Chem. Eng. 2013, 2, 238–244. [Google Scholar] [CrossRef]
- Hamid, M.R.A.; Jeong, H.K. Recent Advances on Mixed-Matrix Membranes for Gas Separation: Opportunities and Engineering Challenges. Korean J. Chem. Eng. 2018, 35, 1577–1600. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Aminabhavi, T.M. Separation of Carbon Dioxide from Natural Gas Mixtures through Polymeric Membranes—A Review. Sep. Purif. Rev. 2007, 36, 113–174. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Burns, R.L.; Steel, K.M.; Burns, S.D.; Koros, W.J. Explanation of a Selectivity Maximum, as a Function of the Material Structure for Organic Gas Separation Membranes. Ind. Eng. Chem. Res. 2004, 43, 5942–5949. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Zhou, M.; Kidd, T.J.; Noble, R.D.; Gin, D.L. Supported Lyotropic Liquid-Crystal Polymer Membranes: Promising Materials for Molecular-Size-Selective Aqueous Nanofiltration. Adv. Mater. 2005, 17, 1850–1853. [Google Scholar] [CrossRef]
- Bögels, G.M.; Lugger, J.A.M.; Goor, O.J.G.M.; Sijbesma, R.P. Size-Selective Binding of Sodium and Potassium Ions in Nanoporous Thin Films of Polymerized Liquid Crystals. Adv. Funct. Mater. 2016, 26, 8023–8030. [Google Scholar] [CrossRef]
- Dischinger, S.M.; Rosenblum, J.; Noble, R.D.; Gin, D.L.; Linden, K.G. Application of a Lyotropic Liquid Crystal Nano Filtration Membrane for Hydraulic Fracturing Flowback Water: Selectivity and Implications for Treatment. J. Memb. Sci. 2017, 543, 319–327. [Google Scholar] [CrossRef]
- Marets, N.; Kuo, D.; Torrey, J.R.; Sakamoto, T.; Henmi, M.; Katayama, H.; Kato, T. Highly Efficient Virus Rejection with Self-Organized Membranes Based on a Crosslinked Bicontinuous Cubic Liquid Crystal. Adv. Healthc. Mater. 2017, 6, 1700252. [Google Scholar] [CrossRef] [PubMed]
- Henmi, M.; Nakatsuji, K.; Ichikawa, T.; Tomioka, H.; Sakamoto, T.; Yoshio, M.; Kato, T. Self-Organized Liquid-Crystalline Nanostructured Membranes for Water Treatment: Selective Permeation of Ions. Adv. Mater. 2012, 24, 2238–2241. [Google Scholar] [CrossRef]
- Sasaki, T.; Hazato, H.; Katsuragi, A.; Nakazawa, Y. Photorefractive Effect of Photoconductive-Polymer-Stabilized Ferroelectric Liquid Crystals. Mol. Cryst. Liq. Cryst. 2009, 503, 81–98. [Google Scholar] [CrossRef]
- Li, C.; Cho, J.; Yamada, K.; Hashizume, D.; Araoka, F.; Takezoe, H.; Aida, T.; Ishida, Y. Macroscopic Ordering of Helical Pores for Arraying Guest Molecules Noncentrosymmetrically. Nat. Commun. 2015, 6, 8418. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Suzuki, Y.; Sakamoto, T.; Yoshio, M.; Torii, S.; Katayama, H.; Kato, T. Polymerizable Photocleavable Columnar Liquid Crystals for Nanoporous Water Treatment Membranes. ACS Macro Lett. 2019, 8, 1303–1308. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ogawa, T.; Nada, H.; Nakatsuji, K.; Mitani, M.; Soberats, B.; Kawata, K.; Yoshio, M.; Tomioka, H.; Sasaki, T.; et al. Development of Nanostructured Water Treatment Membranes Based on Thermotropic Liquid Crystals: Molecular Design of Sub-Nanoporous Materials. Adv. Sci. 2018, 5, 1700405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kuringen, H.P.C.; Eikelboom, G.M.; Shishmanova, I.K.; Broer, D.J.; Schenning, A.P.H.J. Responsive Nanoporous Smectic Liquid Crystal Polymer Networks as Efficient and Selective Adsorbents. Adv. Funct. Mater. 2014, 24, 5045–5051. [Google Scholar] [CrossRef]
- Liang, T.; Van Kuringen, H.P.C.; Mulder, D.J.; Tan, S.; Wu, Y.; Borneman, Z.; Nijmeijer, K.; Schenning, A.P.H.J. Anisotropic Dye Adsorption and Anhydrous Proton Conductivity in Smectic Liquid Crystal Networks: The Role of Cross-Link Density, Order, and Orientation. ACS Appl. Mater. Interfaces 2017, 9, 35218–35225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Nemade, P.R.; Lu, X.; Zeng, X.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. New Type of Membrane Material for Water Desalination Based on a Cross-Linked Bicontinuous Cubic Lyotropic Liquid Crystal Assembly. J. Am. Chem. Soc. 2007, 129, 9574–9575. [Google Scholar] [CrossRef]
- Bara, J.E.; Kaminski, A.K.; Noble, R.D.; Gin, D.L. Influence of Nanostructure on Light Gas Separations in Cross-Linked Lyotropic Liquid Crystal Membranes. J. Memb. Sci. 2007, 288, 13–19. [Google Scholar] [CrossRef]
- Kloos, J.; Jansen, N.; Houben, M.; Casimiro, A.; Lub, J.; Borneman, Z.; Schenning, A.P.H.J.; Nijmeijer, K. On the Order and Orientation in Liquid Crystalline Polymer Membranes for Gas Separation. Chem. Mater. 2021, 33, 8323–8333. [Google Scholar] [CrossRef]
- Houben, S.J.A.; Kloos, J.; Borneman, Z.; Schenning, A.P.H.J. Switchable Gas Permeability of a Polypropylene-Liquid Crystalline Composite Film. J. Polym. Sci. 2022, 60, 803–811. [Google Scholar] [CrossRef]
- Kloos, J.; Jansen, N.; Houben, M.; Nijmeijer, K.; Schenning, A.P.H.J.; Borneman, Z. Molecular Order Determines Gas Transport through Smectic Liquid Crystalline Polymer Membranes with Different Chemical Compositions. ACS Appl. Polym. Mater. submitt.
- Ghosal, K.; Freeman, B.D. Gas Separation Using Polymer Membranes: An Overview. Polym. Adv. Technol. 1994, 5, 673–697. [Google Scholar] [CrossRef]
- Hellums, M.W.; Koros, W.J.; Husk, G.R.; Paul, D.R. Fluorinated Polycarbonates for Gas Separation Applications. J. Memb. Sci. 1989, 46, 93–112. [Google Scholar] [CrossRef]
- Wang, X.; Wilson, T.J.; Maroon, C.R.; Laub, J.A.; Rheingold, S.E.; Vogiatzis, K.D.; Long, B.K. Vinyl-Addition Fluoroalkoxysilyl-Substituted Polynorbornene Membranes for CO2/CH4 Separation. ACS Appl. Polym. Mater. 2022. [Google Scholar] [CrossRef]
- Kadirkhan, F.; Goh, P.S.; Ismail, A.F.; Wan Mustapa, W.N.F.; Halim, M.H.M.; Soh, W.K.; Yeo, S.Y. Recent Advances of Polymeric Membranes in Tackling Plasticization and Aging for Practical Industrial CO2/CH4 Applications—A Review. Membranes 2022, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, R.A.M.; Lub, J.; Tol, A.J.W. Effect of the Orientation of the Ester Bonds on the Properties of Three Isomeric Liquid Crystal Diacrylates before and after Polymerization. Macromolecules 1995, 28, 3313–3327. [Google Scholar] [CrossRef]
- Ikenaga, M.; Kubota, D.; Yamamoto, T. Liquid Crystal Composition, Liquid Crystal Element, and Liquid Crystals Display Device. U.S. Patent 2013/0256595 A1, 3 October 2013. [Google Scholar]
- Dallacker, F.; van Wersch, J. Synthese von Apiolanaloga, II. Chem. Ber. 1972, 105, 3301–3305. [Google Scholar] [CrossRef]
- Sikirić, M.; Primožič, I.; Talmon, Y.; Filipović-Vinceković, N. Effect of the Spacer Length on the Association and Adsorption Behavior of Dissymmetric Gemini Surfactants. J. Colloid Interface Sci. 2005, 281, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Rallapalli, P.; Prasanth, K.P.; Patil, D.; Somani, R.S.; Jasra, R.V.; Bajaj, H.C. Sorption Studies of CO2, CH4, N2, CO, O2 and Ar on Nanoporous Aluminum Terephthalate [MIL-53(Al)]. J. Porous Mater. 2011, 18, 205–210. [Google Scholar] [CrossRef]
- Houben, M.; Borneman, Z.; Nijmeijer, K. Plasticization Behavior of Crown-Ether Containing Polyimide Membranes for the Separation of CO2. Sep. Purif. Technol. 2021, 255, 117307. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Arun, A.; Gaymans, R.J.; Nijmeijer, K.; Wessling, M. Tuning of Mass Transport Properties of Multi-Block Copolymers for CO2 Capture Applications. J. Memb. Sci. 2010, 359, 54–63. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.V. Pebax®/Polyethylene Glycol Blend Thin Film Composite Membranes for CO2 Separation: Performance with Mixed Gases. Sep. Purif. Technol. 2008, 62, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Z.; Chung, T.S. CO2-Selective Membranes for Hydrogen Purification and the Effect of Carbon Monoxide (CO) on Its Gas Separation Performance. Int. J. Hydrogen Energy 2012, 37, 6001–6011. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G.; Clark, K.; Lin, H. Maximizing Ether Oxygen Content in Polymers for Membrane CO2 Removal from Natural Gas. ACS Appl. Mater. Interfaces 2019, 11, 10933–10940. [Google Scholar] [CrossRef]
- Hu, C.C.; Chang, C.S.; Ruaan, R.C.; Lai, J.Y. Effect of Free Volume and Sorption on Membrane Gas Transport. J. Memb. Sci. 2003, 226, 51–61. [Google Scholar] [CrossRef]
- Haraya, K.; Hwang, S.T. Permeation of Oxygen, Argon and Nitrogen through Polymer Membranes. J. Memb. Sci. 1992, 71, 13–27. [Google Scholar] [CrossRef]
- Thornton, A.W.; Nairn, K.M.; Hill, A.J.; Hill, J.M. New Relation between Diffusion and Free Volume: I. Predicting Gas Diffusion. J. Memb. Sci. 2009, 338, 29–37. [Google Scholar] [CrossRef]
| Compound/ Mixtures | Isotropic Temperature [°C] | Polymerization Temperature [°C] | Cooling Rate [°C/min] |
|---|---|---|---|
| C3 | 180 | 100 | 3 |
| C6 | 155 | 130 | 3 |
| C11 | 140 | 122 | 3 |
| C6 with 30 wt% C6-Cl1 | 140 | 110 | 3 |
| C6 with 30 wt% C6-Cl2 | 145 | 115 | 3 |
| C6 with 25 wt% C6-F4 | 140 | 108 | 3 |
| Compound | Isotropic [°C] | Nematic [°C] | Smectic [°C] |
|---|---|---|---|
| C3 | >174 | 174–113 | 113–48 |
| C6 | >146 | 146–142 | 142–113 |
| C11 | >132 | 132–131 | 131–116 |
| C6-Cl1 | >110 | 110–99 | - |
| C6-Cl2 | >139 | 139–104 | - |
| C6-F4 | >109 | 109–104 | - |
| Membranes | Morphology | Tilt Angle [°] | Layer Spacing [Å] | Intermolecular Spacing [Å] |
|---|---|---|---|---|
| C3 | SmC | 21 | 31.9 | 4.6 |
| C6 | SmC | 20 | 36.8 | 4.6 |
| C11 | SmA/C | 18 | 45.2 | 4.6 |
| C6 with 30 wt% C6-Cl1 | SmC | 20 | 37.4 | 4.5 |
| C6 with 30 wt% C6-Cl2 | SmC | 22 | 37.5 | 4.6 |
| C6 with 25 wt% C6-F4 | SmA/C | 20 | 38.1 | 4.5 |
| Membrane | Layer Spacing [Å] | P | S | D |
|---|---|---|---|---|
| C3 | 31.9 | 0.22 | 6.94 | 3.16 |
| C6 | 36.8 | 0.41 | 5.95 | 6.85 |
| C11 | 45.2 | 0.86 | 4.68 | 18.37 |
| C6 with 30 wt% C6-Cl1 | 37.4 | 0.47 | 5.86 | 8.01 |
| C6 with 30 wt% C6-Cl2 | 37.5 | 0.50 | 6.05 | 8.22 |
| C6 with 25 wt% C6-F4 | 38.1 | 0.60 | 6.74 | 8.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloos, J.; Houben, M.; Lub, J.; Nijmeijer, K.; Schenning, A.P.H.J.; Borneman, Z. Tuning the Gas Separation Performances of Smectic Liquid Crystalline Polymer Membranes by Molecular Engineering. Membranes 2022, 12, 805. https://doi.org/10.3390/membranes12080805
Kloos J, Houben M, Lub J, Nijmeijer K, Schenning APHJ, Borneman Z. Tuning the Gas Separation Performances of Smectic Liquid Crystalline Polymer Membranes by Molecular Engineering. Membranes. 2022; 12(8):805. https://doi.org/10.3390/membranes12080805
Chicago/Turabian StyleKloos, Joey, Menno Houben, Johan Lub, Kitty Nijmeijer, Albert P. H. J. Schenning, and Zandrie Borneman. 2022. "Tuning the Gas Separation Performances of Smectic Liquid Crystalline Polymer Membranes by Molecular Engineering" Membranes 12, no. 8: 805. https://doi.org/10.3390/membranes12080805
APA StyleKloos, J., Houben, M., Lub, J., Nijmeijer, K., Schenning, A. P. H. J., & Borneman, Z. (2022). Tuning the Gas Separation Performances of Smectic Liquid Crystalline Polymer Membranes by Molecular Engineering. Membranes, 12(8), 805. https://doi.org/10.3390/membranes12080805