Characterisation and Colour Response of Smart Sago Starch-Based Packaging Films Incorporated with Brassica oleracea Anthocyanin
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Film Preparation
2.3. Colour Change with pH Buffers
2.4. Characterisation of Films
2.4.1. Colour Measurement of Films
2.4.2. Moisture Content and Solubility and Swelling Degree
2.4.3. Thickness and Mechanical Properties
2.4.4. Fourier Transform Infrared (FTIR)
2.4.5. Scanning Electron Microscopy (SEM)
2.4.6. Thermogravimetric Analysis (TGA)
3. Results and Discussion
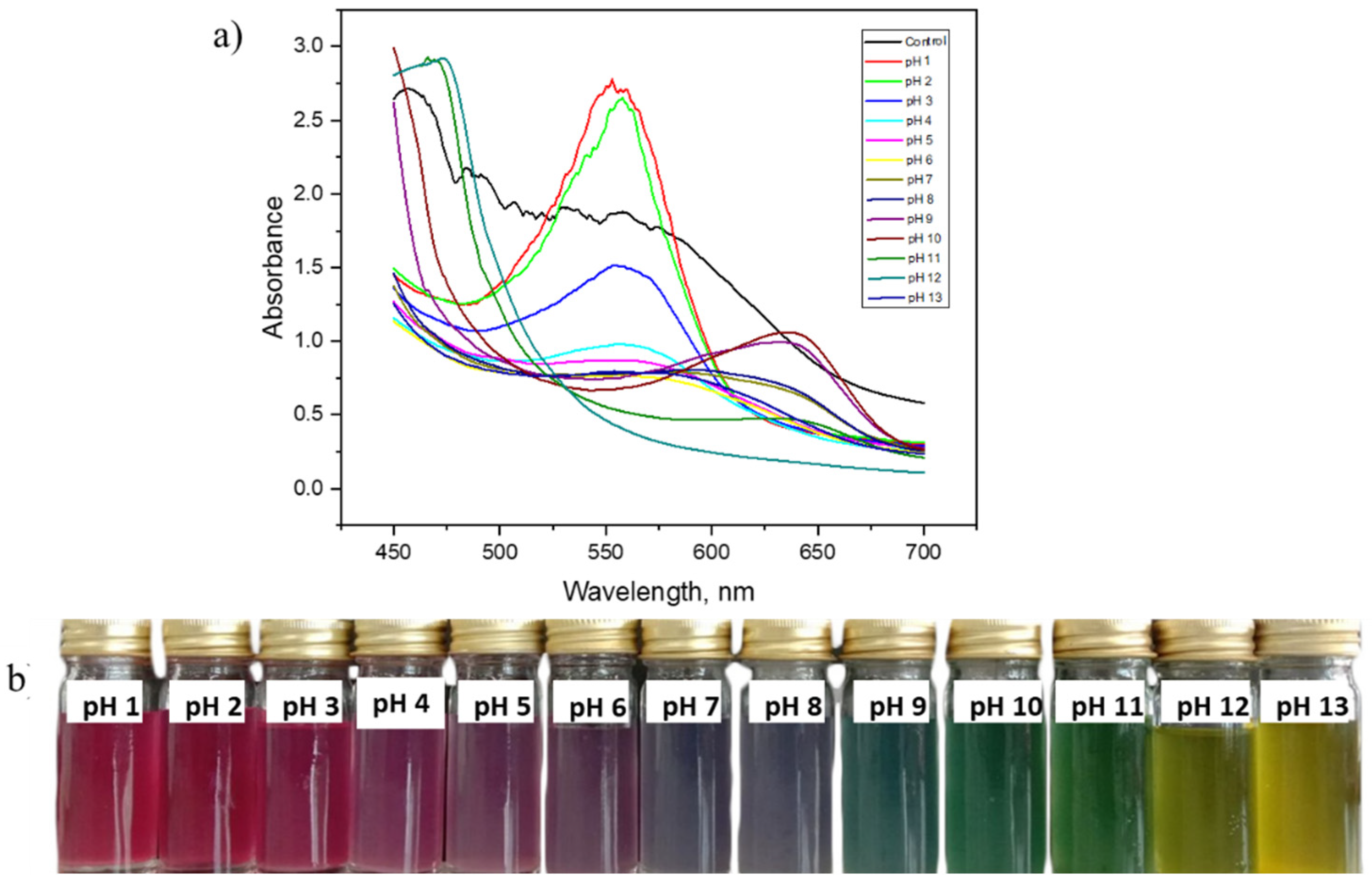
3.1. Colour Variation of RCA in Different pH Buffer Solutions
3.2. Characterisation of Films
3.2.1. Colourimetric Analysis
3.2.2. The Physical Appearance of Films
3.2.3. Thickness
3.2.4. Water Content, Solubility, and Swelling Degree
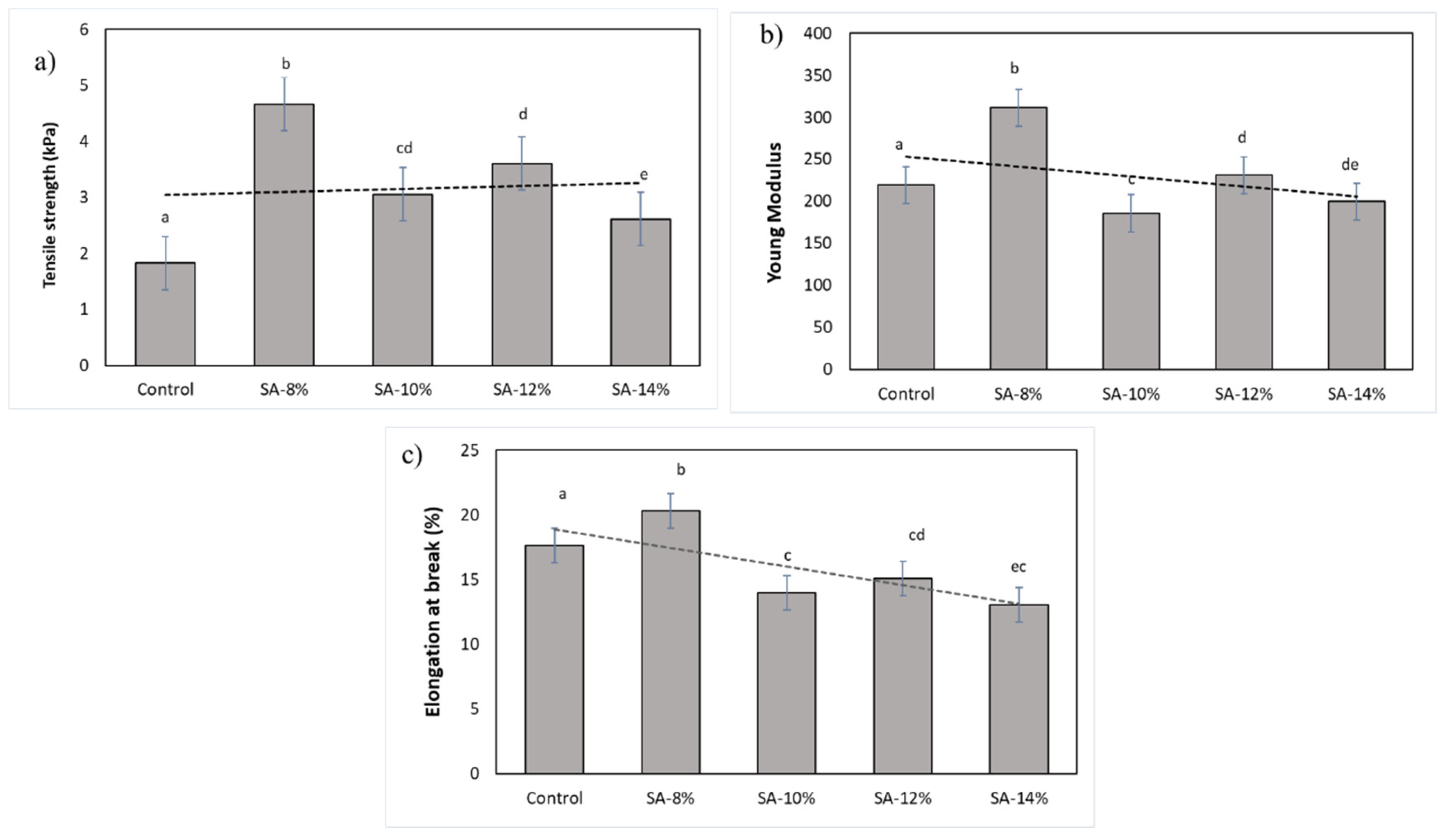
3.2.5. Mechanical Properties
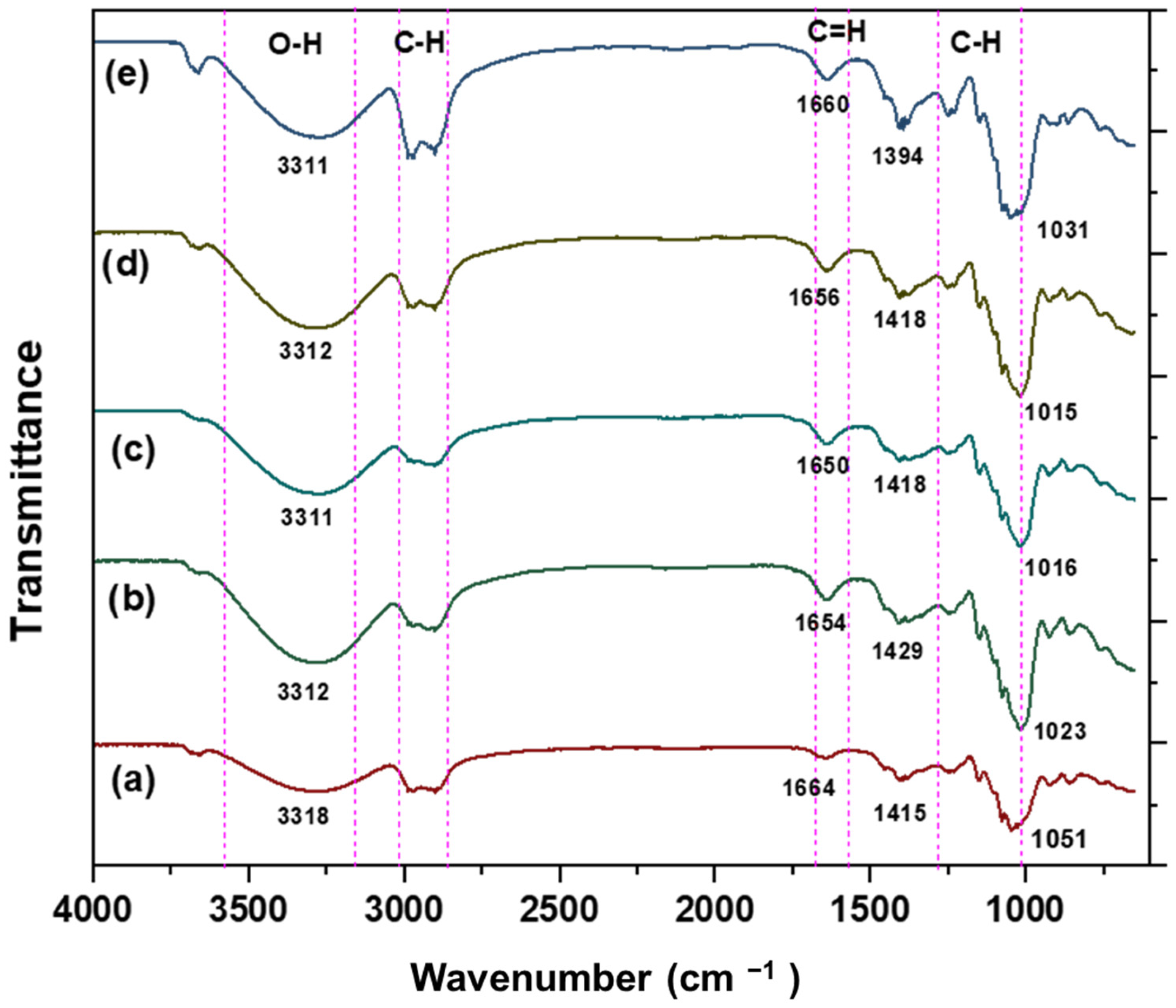
3.2.6. Chemical Characterisation by Using Fourier Transform Infrared (FTIR)
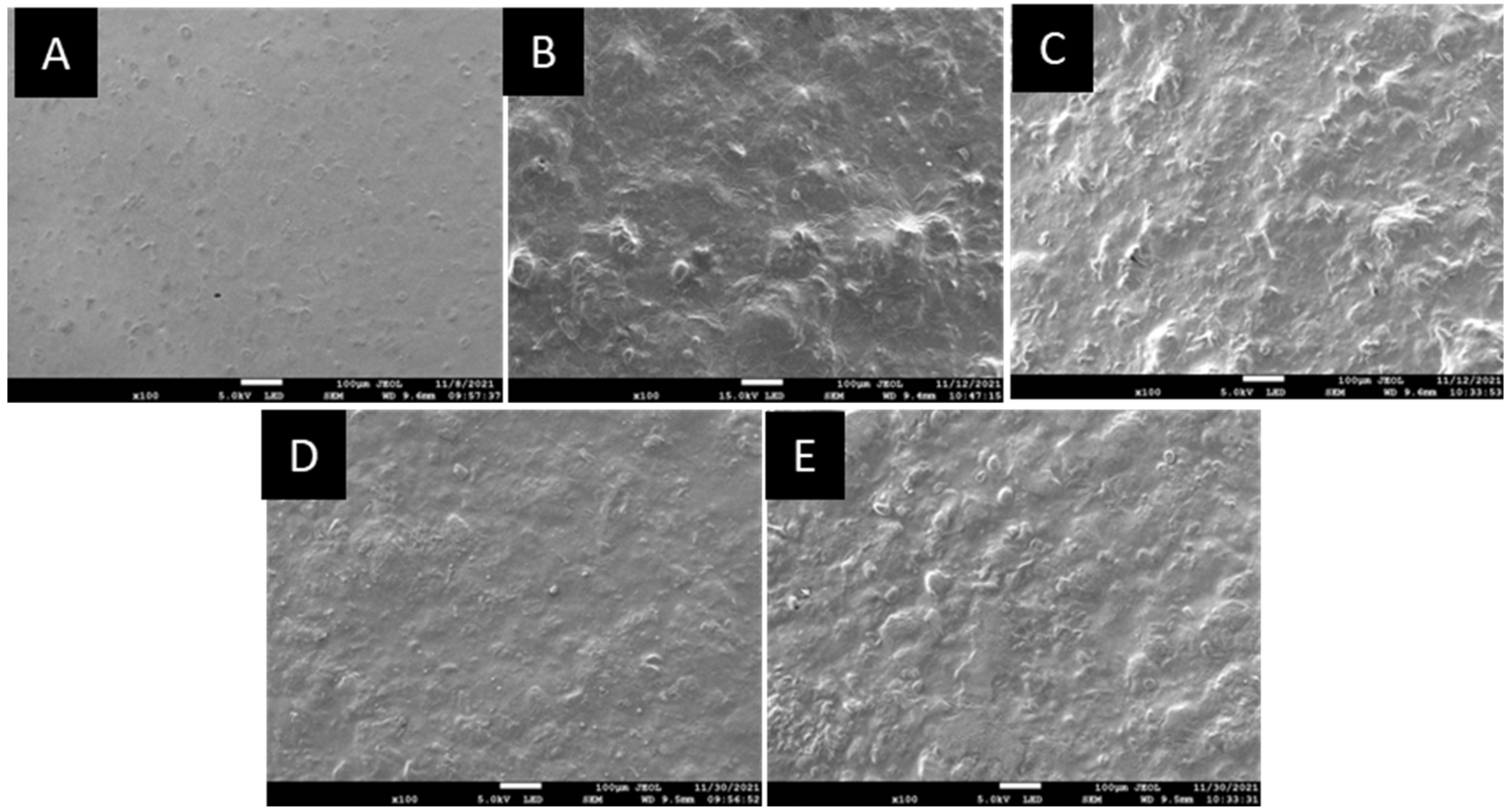
3.2.7. Micrograph Study by Using Scanning Electron Microscopy (SEM)
3.2.8. Thermal Characterisation by Using Thermogravimetric Analysis (TGA)
3.3. Colour Responses of pH-Sensitive Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilarinho, F.; Sendón, R.; van der Kellen, V.A.; Vaz, M.F.; Silva, A.S. Bisphenol A in food as a result of its migration from food packaging. Trends Food Sci. Technol. 2019, 91, 33–65. [Google Scholar] [CrossRef]
- Heidbreder, L.M.; Bablok, I.; Drews, S.; Menzel, C. Tackling the plastic problem: A review on perceptions, behaviors, and interventions. Sci. Total Environ. 2019, 668, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Musa, M.N.I.; Marimuthu, T.; Mohd Rashid, H.N.; Sambasevam, K.P. Development of pH indicator film composed of corn starch-glycerol and anthocyanin from hibiscus sabdariffa. Malays. J. Chem. 2020, 22, 19–24. [Google Scholar]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef]
- Khairuddin, N.; Siddique, B.M.; Muhamad, I.I. Physicochemical Properties and Antibacterial Effect of Lysozyme Incorporated in a Wheat-Based Active Packaging Film. Arab. J. Sci. Eng. 2017, 42, 2229–2239. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Ehara, H.; Toyoda, Y.; Johnson, D.V. Sago Palm: Multiple Contributions to Food Security and Sustainable Livelihoods; Springer: Berlin, Germany, 2018. [Google Scholar]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart gelatin films prepared using red cabbage (Brassica oleracea L.) extracts as solvent. Food Hydrocoll. 2018, 89, 674–681. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łetowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Junka, N.; Rattanamechaiskul, C.; Wongs-Aree, C.; Kanlayanarat, S. Comparative study of organic solvents and extraction conditions on colour and antioxidant capacity in red cabbage. Int. Food Res. J. 2017, 24, 518–524. [Google Scholar]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Ticha, M.B.; Haddar, W.; Meksi, N.; Guesmi, A.; Mhenni, M.F. Improving dyeability of modified cotton fabrics by the natural aqueous extract from red cabbage using ultrasonic energy. Carbohydr. Polym. 2016, 154, 287–295. [Google Scholar] [CrossRef]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of anthocyanin associated purple sweet potato starch and peel-based pH indicator films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The potential of anthocyanins in smart, active; bioactive eco-friendly polymer-based films: A review. Food Res. Int. 2020, 142, 110202. [Google Scholar] [CrossRef]
- Chigurupati, N.; Saiki, L.; Gayser, C.; Dash, A.K. Evaluation of red cabbage dye as a potential natural color for pharmaceutical use. Int. J. Pharm. 2002, 241, 293–299. [Google Scholar] [CrossRef]
- Mei, L.X.; Nafchi, A.M.; Ghasemipour, F.; Easa, A.M.; Jafarzadeh, S.; Al-Hassan, A.A. Characterization of pH sensitive sago starch films enriched with anthocyanin-rich torch ginger extract. Int. J. Biol. Macromol. 2020, 164, 4603–4612. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yun, D.; Xu, F.; Li, C.; Chen, D.; Liu, J. Impact of storage conditions on the structure and functionality of starch/polyvinyl alcohol films containing Lycium ruthenicum anthocyanins. Food Packag. Shelf Life 2021, 29, 100693. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Stoll, L. Effect of anthocyanins addition on the mechanical properties of starch-maltodextrin biodegradable films. In Proceedings of the 18th World Congress of Food Science and Technology—IUFoST 2016, Dublin, Ireland, 21–25 August 2016. [Google Scholar]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2020, 61, 2297–2325. [Google Scholar] [CrossRef]
- Vo, T.; Dang, T.; Chen, B.H. Synthesis of Intelligent pH Indicative Fims from Chitosan/Poly(vinyl alcohol)/Anthocyanin Extracted from Red Cabbage. Polymers 2019, 11, 1088. [Google Scholar] [CrossRef]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of active and intelligent films based on cassava starch and Chinese bayberry (Myrica rubra Sieb. et Zucc.) anthocyanins. RSC Adv. 2019, 9, 30905–30916. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, T.S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef]
- Valdir Aniceto, P.; Queiroz de Arruda, I.Z.; Stefani, R. Active Chitosan/PVA Films with Anthocyanins from Brassica Oleraceae (Red Cabbage) as Time-Temperature Indicators for Application in Intelligent Food Packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar]
- Eskandarabadi, S.M.; Mahmoudian, M.; Farah, K.R.; Abdali, A.; Nozad, E.; Enayati, M. Active intelligent packaging film based on ethylene vinyl acetate nanocomposite containing extracted anthocyanin, rosemary extract and ZnO/Fe-MMT nanoparticles. Food Packag. Shelf Life 2018, 22, 100389. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; Dias, A.R.G.; Zavareze, E.R. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Carvalho, V.V.L.; Gonçalves, J.O.; Silva, A.; Cadaval, T.R.; Pinto, A.A.L.; Lopes, T.J. Separation of anthocyanins extracted from red cabbage by adsorption onto chitosan films. Int. J. Biol. Macromol. 2019, 131, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef]
- Amin, M.R.; Chowdhury, M.A.; Kowser, M.A. Characterization and performance analysis of composite bioplastics synthesized using titanium dioxide nanoparticles with corn starch. Heliyon 2019, 5, e02009. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, optical and mechanical properties of Chitosan–Starch films with natural extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Thermal, mechanical, and physical properties of seaweed/sugar palm fibre reinforced thermoplastic sugar palm Starch/Agar hybrid composites. Int. J. Biol. Macromol. 2017, 97, 606–615. [Google Scholar] [CrossRef]
- Mohebi, E.; Marquez, L. Intelligent packaging in meat industry: An overview of existing solutions. J. Food Sci. Technol. 2015, 52, 3947–3964. [Google Scholar] [CrossRef]
- Hock Eng, K.; Azlan, A.; Teng Tang, S.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar]
- Abedi-Firoozjah, R.; Yousefi, S.; Heydari, M.; Seyedfatehi, F.; Jafarzadeh, S.; Mohammadi, R.; Rouhi, M.; Garavand, F. Application of Red Cabbage Anthocyanins as pH-Sensitive Pigments in Smart Food Packaging and Sensors. Polymers 2022, 14, 1629. [Google Scholar] [CrossRef]
- Khajavi, M.Z.; Mohammadi, R.; Ahmadi, S.; Farhoodi, M.; Yousefi, M. Strategies for controlling release of plastic compounds into foodstuffs based on application of nanoparticles and its potential health issues. Trends Food Sci. Technol. 2019, 90, 1–12. [Google Scholar] [CrossRef]



| Films | g/L |
|---|---|
| Control | 0 |
| SA-8% | 80 |
| SA-10% | 100 |
| SA-12% | 120 |
| SA-14% | 140 |
| Films | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Control | 45.27 | 0.57 | 0.6 | 0.0 |
| SA-8% | 42.58 | 3.83 | 2.86 | 4.80 |
| SA-10% | 39.56 | 3.59 | 1.82 | 6.57 |
| SA-12% | 39.31 | 3.52 | 1.13 | 6.67 |
| SA-14% | 38.52 | 2.69 | 0.75 | 7.08 |
| Sample | Thickness (mm) | Water Content (%) | Water Solubility (%) | Swelling Degree (%) |
|---|---|---|---|---|
| Control | 0.12 ± 0.04 a | 40.38 ± 8.39 a | 32.37 ± 6.96 a | 130.31 ± 23.69 a |
| SA-8% | 0.17 ± 0.01 b | 31.39 ± 1.68 ab | 56.62 ± 11.73 b | 74.80 ± 53.68 b |
| SA-10% | 0.19 ± 0.01 bc | 30.17 ± 0.79 bc | 55.60 ± 5.26 bc | 79.46 ± 46.06 bc |
| SA-12% | 0.22 ± 0.02 d | 29.40 ± 0.42 cd | 57.95 ± 8.81 cd | 79.52 ± 53.35 cd |
| SA-14% | 0.23 ± 0.02 de | 23.95 ± 0.32 e | 59.45 ± 0.01 de | 99.48 ± 3.09 e |
| Samples | Peak Temperature (°C) | Weight Loss (%) |
|---|---|---|
| Control film | 190.83 | 91.38 |
| SA-8% | 301.89 | 47.62 |
| SA-10% | 299.91 | 63.31 |
| SA-12% | 301.85 | 53.32 |
| SA-14% | 302.61 | 58.34 |
| Sample | pH | Colour | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|
| SA-8% | 3 |  | 46.9 | 4.3 | 0.8 | 10.7 |
| 5 |  | 45.1 | 1.6 | 1.1 | 12.8 | |
| 9 |  | 43.6 | 0 | 0.9 | 14.6 | |
| 11 |  | 45.2 | 0.2 | 2.0 | 12.9 | |
| 13 |  | 51.9 | 0.1 | 5.2 | 7.31 | |
| SA-10% | 3 |  | 45.1 | 4.9 | 0.7 | 4.4 |
| 5 |  | 43.6 | 2.2 | 0.7 | 6.1 | |
| 9 |  | 44.2 | 0.1 | 0.8 | 6.7 | |
| 11 |  | 43.7 | −0.2 | 1.8 | 7.0 | |
| 13 |  | 51.2 | 0.1 | 4.8 | 5.0 | |
| SA-12% | 3 |  | 42.6 | 5.6 | 0.1 | 5.8 |
| 5 |  | 44.6 | 2.0 | 0.8 | 1.6 | |
| 9 |  | 44.8 | −0.2 | 0.5 | 0.3 | |
| 11 |  | 44.2 | −0.2 | 2.0 | 1.9 | |
| 13 |  | 47.3 | 0.5 | 7.3 | 6.8 | |
| SA-14% | 3 |  | 43.0 | 3.9 | 0.6 | 2.3 |
| 5 |  | 43.2 | 2.1 | 0.8 | 2.8 | |
| 9 |  | 42.0 | 0.6 | 0.5 | 3.2 | |
| 11 |  | 40.7 | −0.7 | 0.3 | 4.1 | |
| 13 |  | 42.6 | 1.8 | 4.4 | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Hamzah, N.H.; Khairuddin, N.; Muhamad, I.I.; Hassan, M.A.; Ngaini, Z.; Sarbini, S.R. Characterisation and Colour Response of Smart Sago Starch-Based Packaging Films Incorporated with Brassica oleracea Anthocyanin. Membranes 2022, 12, 913. https://doi.org/10.3390/membranes12100913
Che Hamzah NH, Khairuddin N, Muhamad II, Hassan MA, Ngaini Z, Sarbini SR. Characterisation and Colour Response of Smart Sago Starch-Based Packaging Films Incorporated with Brassica oleracea Anthocyanin. Membranes. 2022; 12(10):913. https://doi.org/10.3390/membranes12100913
Chicago/Turabian StyleChe Hamzah, Nurul Husna, Nozieana Khairuddin, Ida Idayu Muhamad, Mohd Ali Hassan, Zainab Ngaini, and Shahrul Razid Sarbini. 2022. "Characterisation and Colour Response of Smart Sago Starch-Based Packaging Films Incorporated with Brassica oleracea Anthocyanin" Membranes 12, no. 10: 913. https://doi.org/10.3390/membranes12100913
APA StyleChe Hamzah, N. H., Khairuddin, N., Muhamad, I. I., Hassan, M. A., Ngaini, Z., & Sarbini, S. R. (2022). Characterisation and Colour Response of Smart Sago Starch-Based Packaging Films Incorporated with Brassica oleracea Anthocyanin. Membranes, 12(10), 913. https://doi.org/10.3390/membranes12100913






