Bisphenol A Removal Using Visible Light Driven Cu2O/PVDF Photocatalytic Dual Layer Hollow Fiber Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Cu2O/PVDF DLHF Membrane
2.3. Membrane Characterization
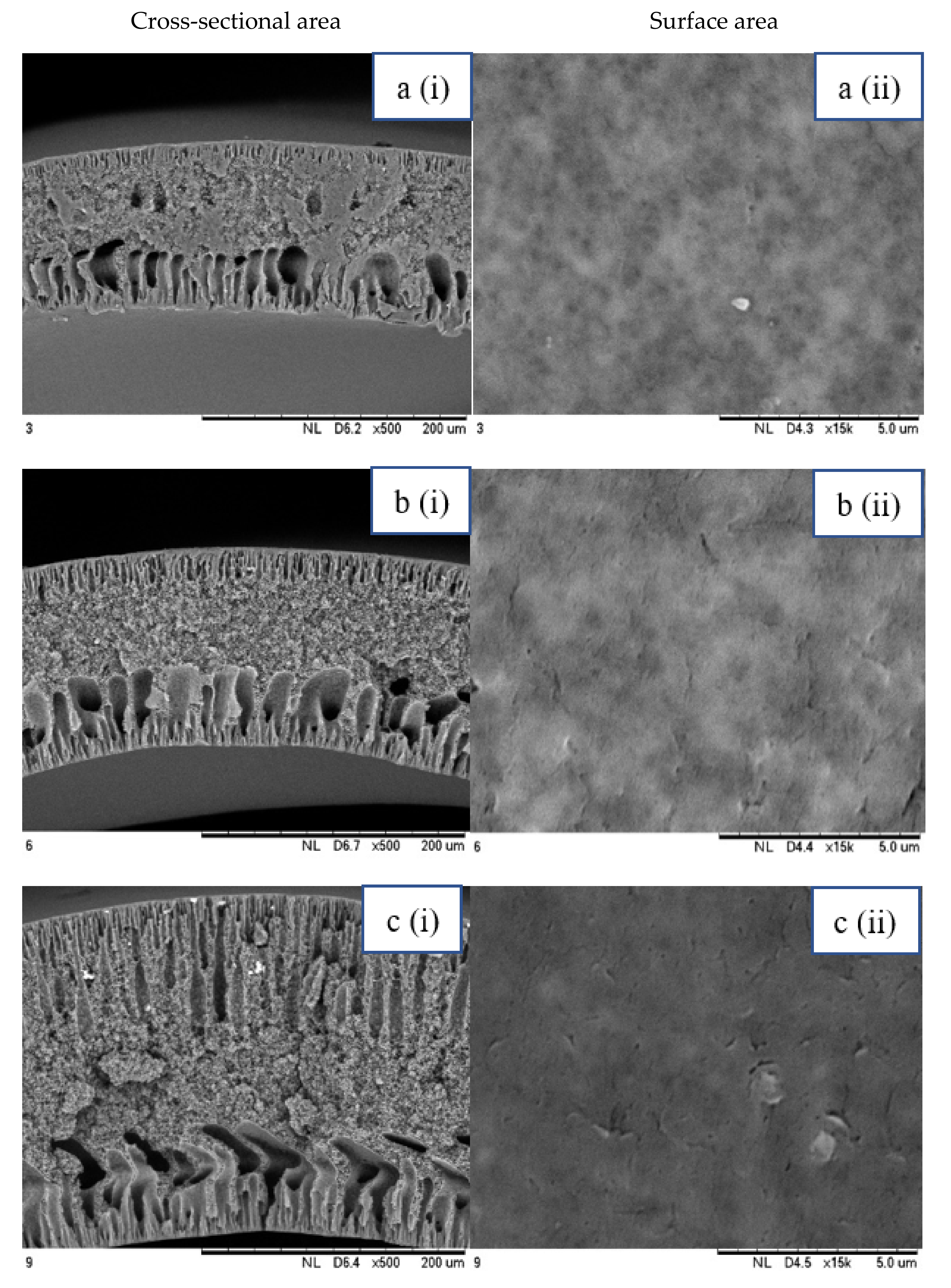
2.3.1. Morphological Structure
2.3.2. Crystalline Property
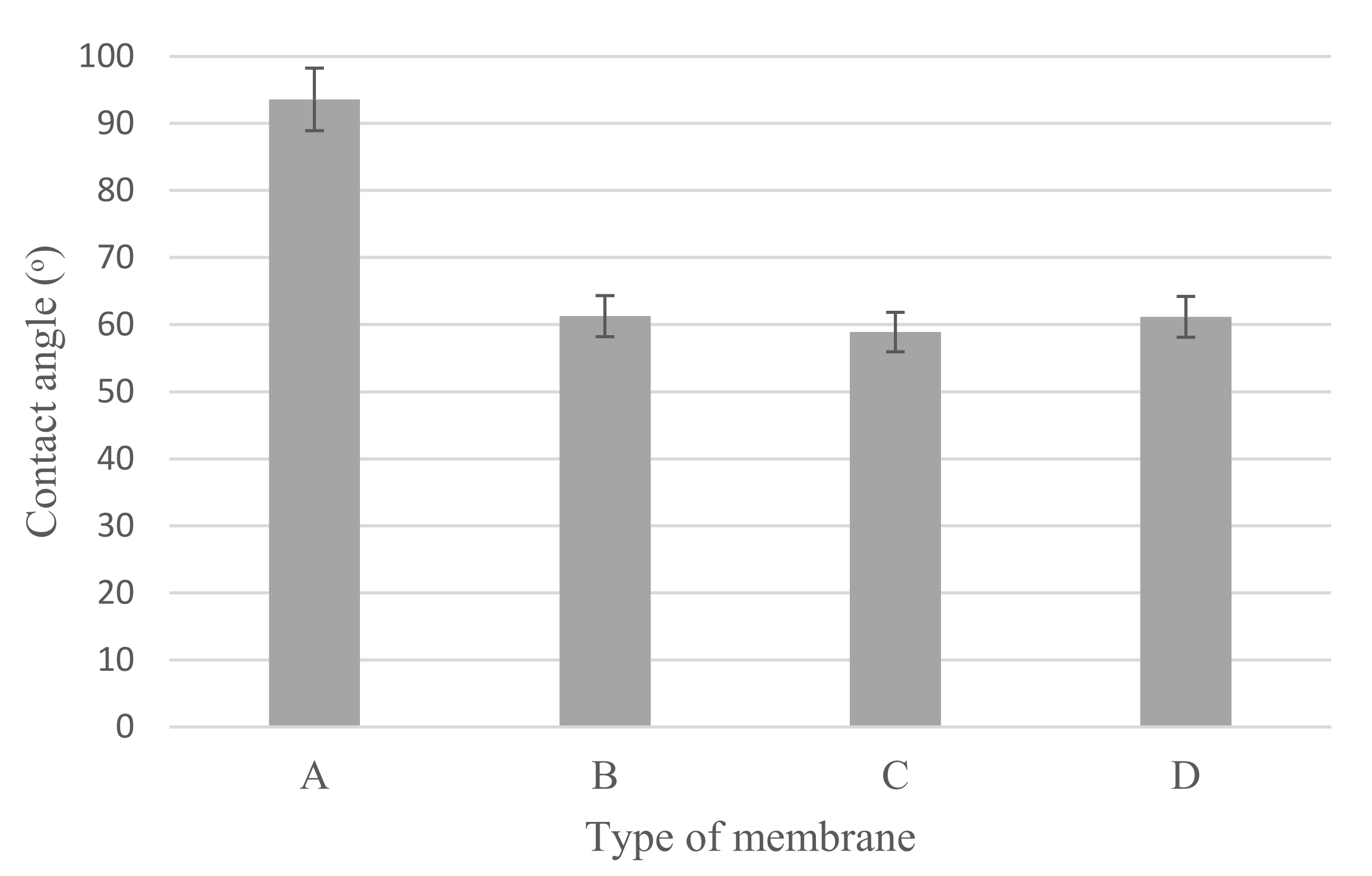
2.3.3. Hydrophilicity
2.3.4. Water Flux
2.3.5. Porosity
2.3.6. Surface Roughness
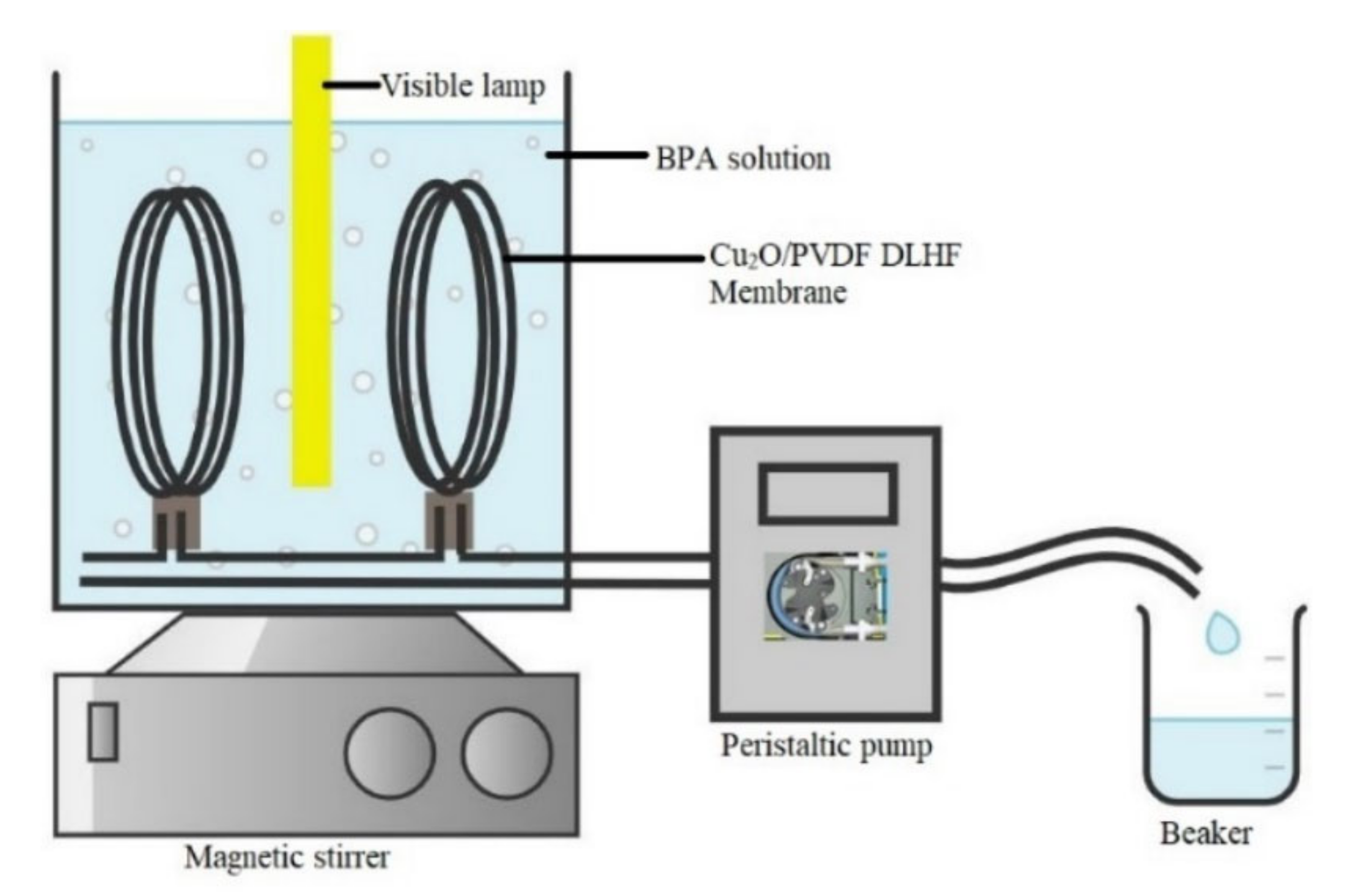
2.4. Performance Evaluation
2.5. Leaching Compound Detection
3. Results and Discussion
3.1. The Effect of Different Outer Dope Flowrate
3.1.1. Morphological Structure
3.1.2. Crystalline Property
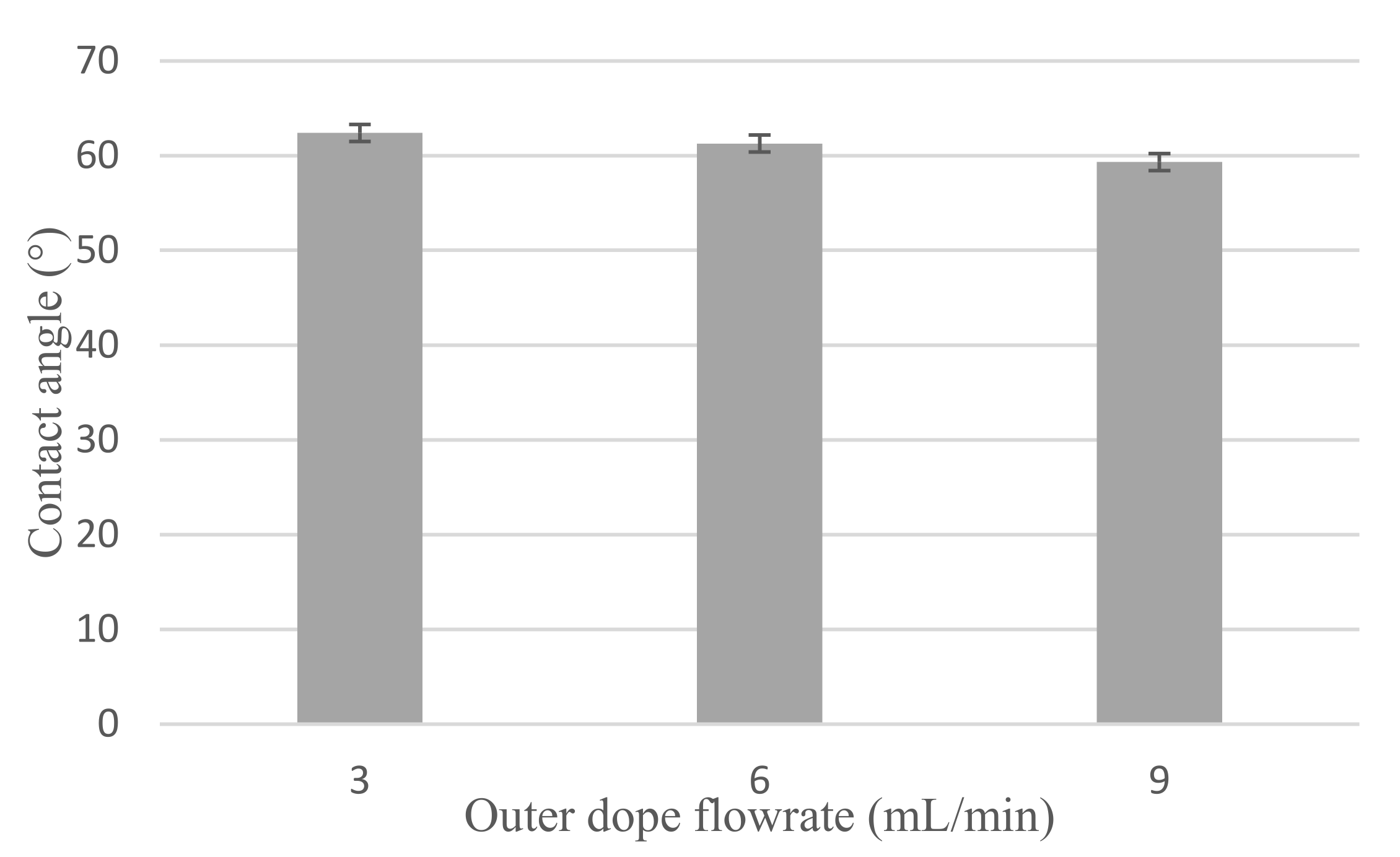
3.1.3. Hydrophilicity
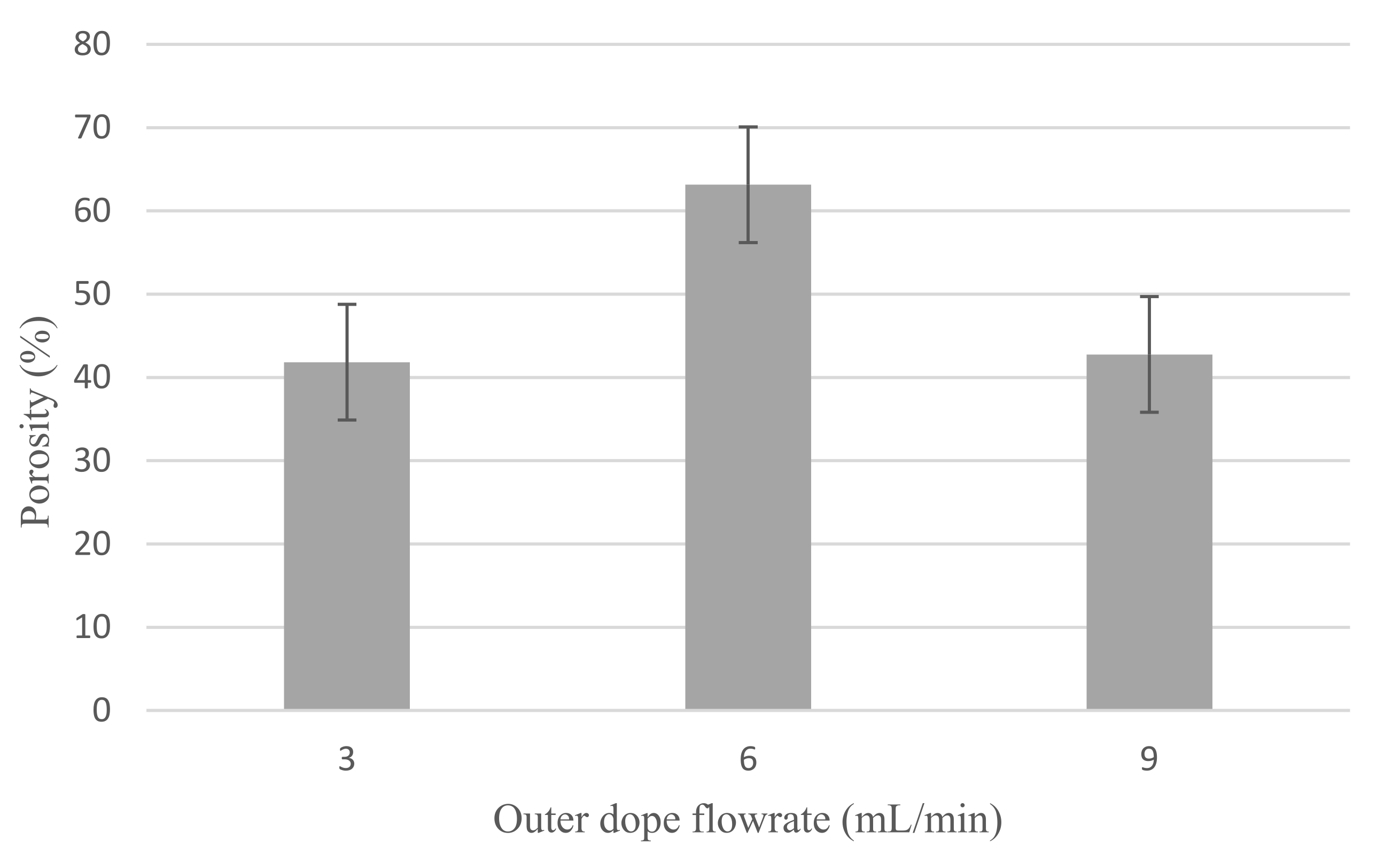
3.1.4. Porosity
3.1.5. Water Flux
3.2. The Effect of Different Cu2O/PVDF Ratio Loading
3.2.1. Morphological Structure
3.2.2. Hydrophilicity
3.2.3. Porosity
3.2.4. Water Flux
3.2.5. Surface Roughness
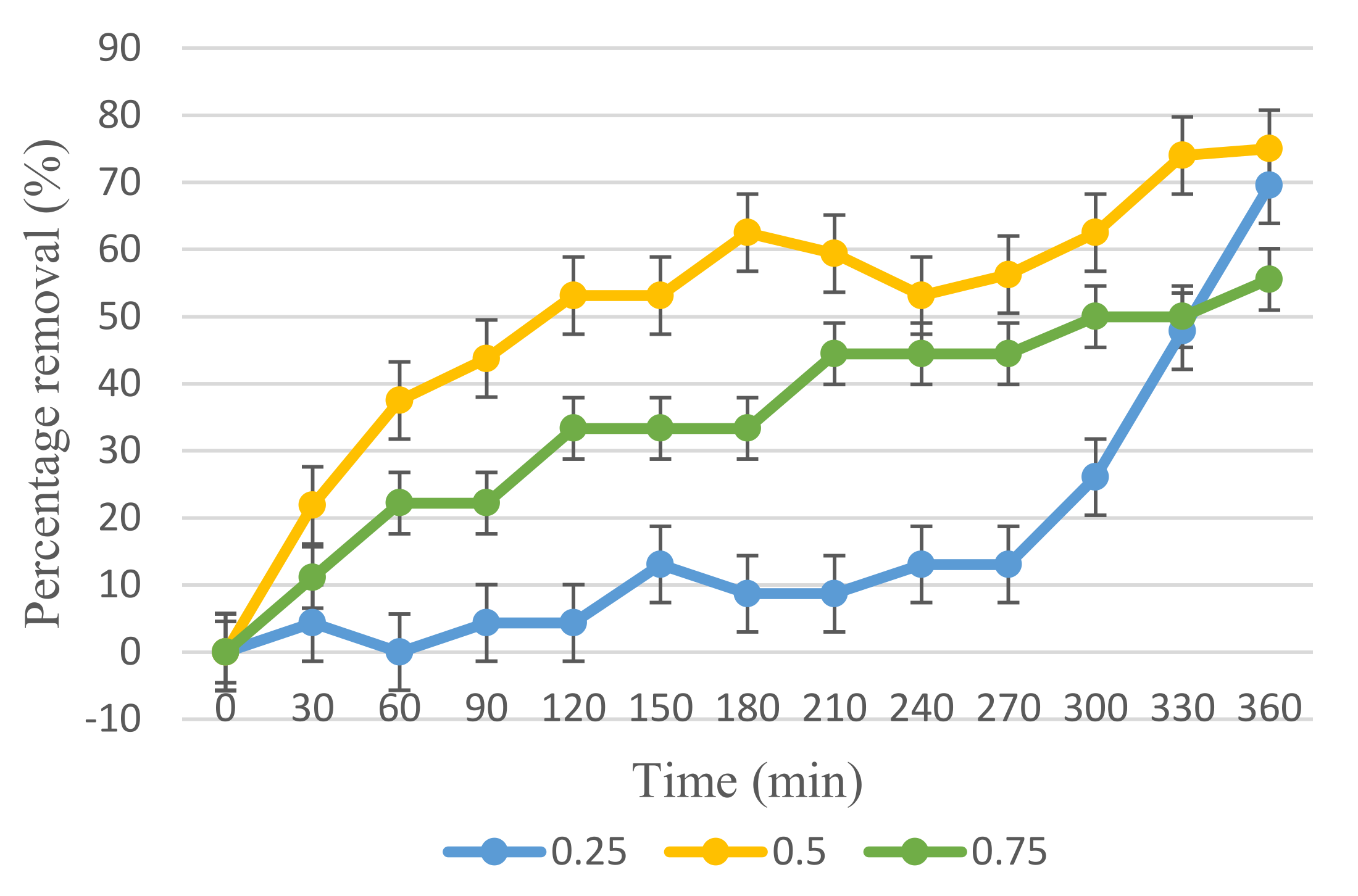
3.3. Photocatalytic Activity
3.4. Leaching Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global assessment of bisphenol a in the environment: Review and analysis of its occurrence and bioaccumulation. Dose-Response 2015, 13, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E.; Bisphenol, A. Nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-friendly removal methods for endocrine disrupting chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Santhi, V.A.; Sakai, N.; Ahmad, E.D.; Mustafa, A.M. Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci. Total Environ. 2012, 427–428, 332–338. [Google Scholar] [CrossRef] [PubMed]
- International, Food Safety, Authorities Network (INFOSAN). BISPHENOL A (BPA)—Current State of Knowledge and Future Actions by WHO and FAO; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009; 6p, Available online: http://www.who.int/foodsafety/publications/fs_management/No_05_Bisphenol_A_Nov09_en.pdf?ua=1 (accessed on 16 December 2021).
- Losada-Garcia, N.; Rodriguez-Otero, A.; Palomo, J.M. Fast degradation of bisphenol a in water by nanostructured cunps@calb biohybrid catalysts. Nanomaterials 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Scopel, C.F.V.; Sousa, C.; Machado, M.R.F.; Dos Santos, W.G. Bpa toxicity during development of zebrafish embryo. Braz. J. Biol. 2021, 81, 437–447. [Google Scholar] [CrossRef]
- Sasso, A.F.; Pirow, R.; Andra, S.S.; Church, R.; Nachman, R.M.; Linke, S.; Kapraun, D.F.; Schurman, S.H.; Arora, M.; Thayer, K.A.; et al. Pharmacokinetics of bisphenol A in humans following dermal administration. Environ. Int. 2020, 144, 106031. [Google Scholar] [CrossRef] [PubMed]
- Rovani, S.; Santos, J.J.; Guilhen, S.N.; Corio, P.; Fungaro, D.A. Fast, efficient and clean adsorption of bisphenol-A using renewable mesoporous silica nanoparticles from sugarcane waste ash. RSC Adv. 2020, 10, 27706–27712. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K.; Cydzik-Kwiatkowska, A.; Bernat, K.; Wojnowska-Baryła, I. Removal of bisphenol A (BPA) from biologically treated wastewater by microfiltration and nanofiltration. Int. J. Environ. Sci. Technol. 2016, 13, 2239–2248. [Google Scholar] [CrossRef]
- Oh, S.; Choi, D. Microbial, community enhances, biodegradation of bisphenol, a through, selection of sphingomonadaceae. Microb. Ecol. 2019, 77, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Peng, W.; Zhao, J.; Xu, C. Extraction performance of bisphenol A from aqueous solutions by emulsion liquid membrane using response surface methodology. Desalination 2013, 313, 36–43. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Hilal, N.; Al-Zoubib, H.; Darwish, N.A.; Ali, N. Modelling the effects of nanofiltration membrane properties on system cost assessment for desalination applications. Desalination 2007, 206, 215–225. [Google Scholar] [CrossRef]
- Alin, I.; Prisecaru, M.; Stoica, I.; Călin, M.; Cristea, T. Biological, remediation of soil, polluted with, oil products: An overview of available, technologies. Stud. şi Cercet. Univ. Vasile Alecsandri din Bacău 2016, 25, 89–101. Available online: https://www.researchgate.net/publication/309901995_BIOLOGICAL_REMEDIATION_OF_SOIL_POLLUTED_WITH_OIL_PRODUCTS_AN_OVERVIEW_OF_AVAILABLE_TECHNOLOGIES (accessed on 5 January 2020).
- Kumar, A.; Thakur, A.; Panesar, P.S. A review on emulsion liquid membrane (ELM) for the treatment of various industrial effluent streams. Rev. Environ. Sci. Bio/Technol. 2019, 18, 153–182. [Google Scholar] [CrossRef]
- Saravanan, R.; Gracia, F.; Stephen, A. Basic, principles, mechanism, and challenges of photocatalysis. In Nanocomposites for Visible, Light-Induced Photocatalysis; Springer: Cham, Germany, 2017; pp. 19–40. Available online: http://link.springer.com/10.1007/978-3-319-62446-4_2%0Ahttp://files/20/9783319624457-c2.pdf (accessed on 6 January 2020).
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.M.; Dilbaghi, N. Nanotechnology-based water treatment strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of produced water with photocatalysis: Recent advances, affecting factors and future research prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Koiki, B.A.; Arotiba, O.A. Cu2O as an emerging semiconductor in photocatalytic and photoelectrocatalytic treatment of water contaminated with organic substances: A review. RSC Adv. 2020, 10, 36514–36525. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, P.; Wang, W.; Cai, Y.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Enhanced dark adsorption and visible-light-driven photocatalytic properties of narrower-band-gap Cu2S decorated Cu2O nanocomposites for efficient removal of organic pollutants. J. Hazard. Mater. 2020, 384, 121302. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Cheng, W.; Hu, J. Photocatalytic membrane in water purification: Is it stepping closer to be driven by visible light? J. Membr. Sci. 2019, 584, 364–392. [Google Scholar] [CrossRef]
- Tolu, H.; Iren, E.; Altinbas, M. Full scale sanitary landfill leachate treatment by MBR: Flat sheet vs. hollow fiber membrane. J. Membr. Sci. Res. 2021, 7, 118–124. [Google Scholar]
- Zhang, L.-Z. Heat and mass, transfer in hollow, fiber membrane, bundles with randomly, distributed fibers. In Conjugate Heat and Mass Transfer in Heat Mass Exchanger Ducts; Elsevier: Amsterdam, The Netherlands, 2013; pp. 233–254. [Google Scholar]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Morphological study of co-extruded dual-layer hollow fiber membranes incorporated with different TiO2 loadings. J. Memb. Sci. 2015, 479, 123–131. [Google Scholar] [CrossRef]
- Setiawan, L.; Wang, R.; Shi, L.; Li, K.; Fane, A.G. Novel dual-layer hollow fiber membranes applied for forward osmosis process. J. Memb. Sci. 2012, 421–422, 238–246. [Google Scholar] [CrossRef]
- Kamaludin, R.; Othman, M.H.D.; Kadir, S.H.S.A.; Rahman, M.A.; Jaafar, J. The morphological properties study of photocatalytic TiO2/pvdf dual layer hollow fiber membrane for endocrine disrupting compounds degradation. Malays. J. Anal. Sci. 2017, 21, 426–434. [Google Scholar]
- Houis, S.; Engelhardt, E.M.; Wurm, F.; Gries, T. Application of polyvinylidene, fluoride (PVDF) as a biomaterial in medical, textiles. In Medical and Healthcare Textiles; Woodhead Publishing: Cambridge, UK, 2010; pp. 342–352. [Google Scholar]
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Memb. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Sai Guru Srinivasan, S.; Govardhanan, B.; Aabel, P.; Ashok, M.; Santhosh Kumar, M.C. Effect of oxygen partial pressure on the tuning of copper oxide thin films by reactive sputtering for solar light driven photocatalysis. Sol. Energy 2019, 187, 368–378. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Eftekhari, E.; Huo, Z.; Li, X.; Tade, M.O.; Yan, C.; Yan, Z.; Li, C.; Li, Q.; et al. High performance heterojunction photocatalytic membranes formed by embedding Cu2O and TiO2 nanowires in reduced graphene oxide. Catal. Sci. Technol. 2018, 8, 1704–1711. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Park, M.J.; Tijing, L.; Han, D.S.; Phuntsho, S.; Shon, H.K. Modification of nanofiber support layer for thin film composite forward osmosis membranes via layer-by-layer polyelectrolyte deposition. Membranes 2018, 8, 70. [Google Scholar] [CrossRef]
- Ismail, N.J.; Othman, M.H.D.; Kamaludin, R.; Mohamad, I. Characterization of bauxite as a potential, natural photocatalyst for photodegradation of textile dye. Arab. J. Sci. Eng. 2019, 44, 10031–10040. [Google Scholar] [CrossRef]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Sheikh Abdul Kadir, S.H.; Kurniawan, T.A.; Jilani, A. Immobilization techniques of a photocatalyst into and onto a polymer membrane for photocatalytic activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Shaliutina-Kolešová, A.; Bouša, D.; Sofer, Z.; Friess, K. Co0·5Ni0·5FeCrO4 spinel nanoparticles decorated with UiO-66-based metal-organic frameworks grafted onto GO and O-SWCNT for gas adsorption and water purification. Chemosphere 2020, 255, 126966. [Google Scholar] [CrossRef] [PubMed]
- Kamaludin, R.; Othman, M.H.D.; Kadir, S.H.S.A.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Visible-light-driven, photocatalytic N-Doped, TiO2 for degradation of bisphenol, A (BPA) and reactive, Black 5 (RB5) dye. Water Air Soil Pollut. 2018, 229, 363. [Google Scholar] [CrossRef]
- Dzinun, H.; Hafiz, M.; Othman, D.; Fauzi, A.; Hafiz, M.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Fabrication of dual, layer hollow, fibre membranes for photocatalytic, degradation of organic, pollutants. Int. J. Chem. Eng. Appl. 2015, 6, 289–292. [Google Scholar] [CrossRef][Green Version]
- Akbari, R.; Mohammadizadeh, M.R.; Khajeh Aminian, M.; Abbasnejad, M. Hydrophobic, Cu2O surfaces prepared by chemical bath deposition method. Appl. Phys. A Mater. Sci. Process. 2019, 125, 190. [Google Scholar] [CrossRef]
- He, T. Spongelike, structure. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1814–1815. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, L.; Luo, Q.; Li, X.; An, J.; Duan, Y. Highly efficient visible light TiO2 photocatalyst prepared by sol-gel method at temperatures lower than 300 °C. J. Hazard. Mater. 2011, 192, 150–159. [Google Scholar] [CrossRef]
- Kamaludin, R.; Syarifuddin, A.; Puad, M.; Ha, M.; Othman, D. Incorporation of N-doped TiO2 into dual layer hollow fi ber (DLHF) membrane for visible light-driven photocatalytic removal of reactive black 5. Polym. Test. 2019, 78, 105939. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, B.; Zhu, T.; Shi, J.; Xu, Z.; Hu, C.; Wang, J. Facile and low-cost approach towards a PVDF ultrafiltration membrane with enhanced hydrophilicity and antifouling performance via graphene oxide/water-bath coagulation. RSC Adv. 2015, 5, 7880–7889. [Google Scholar] [CrossRef]
- Hssi, A.A.; Atourki, L.; Abouabassi, K.; Elfanaoui, A.; Bouabid, K.; Ihlal, A.; Benmokhtar, S.; Ouafi, M. Growth and characterization of Cu2O for solar cells applications. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 2056, pp. 2–8. [Google Scholar]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Electrochemical sensor for rapid and sensitive detection of tryptophan by a Cu2O nanoparticles- coated reduced graphene oxide nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ramli, M.R.M.; Esham, M.I.M. Effect of additives on hydrophobicity of PVDF membrane in two-stage coagulation baths for desalination. J. Phys. Sci. 2019, 30, 207–221. [Google Scholar] [CrossRef]
- He, M.; Zhang, S.; Su, Y.; Zhang, R.; Liu, Y.; Jiang, Z. Manipulating membrane surface porosity and pore size by in-situ assembly of Pluronic, F127 and tannin. J. Memb. Sci. 2018, 556, 285–292. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Figoli, A. The formation of polyvinylidene fluoride membranes with tailored properties via vapour/non-solvent induced phase separation. Membranes 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Ng, S. Effect of membrane properties on contact angle. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 2016. [Google Scholar]
- Niu, F.; Huang, M.; Cai, T.; Meng, L. Effect of membrane, thickness on properties of FO membranes with nanofibrous, substrate. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, England, 2018; Volume 170. [Google Scholar]
- Dahman, Y.; Javaheri, H.; Chen, J.; Sulaiman, B.A.-C. Nanoparticles. In Nanotechnology and Functional, Materials for Engineers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 93–119. [Google Scholar]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R. Enhancing, morphology and separation, performance of polyamide 6, 6 membranes, by minimal, incorporation of silver, decorated graphene, oxide nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef]
- Van Den Berg, T.; Ulbricht, M. Polymer, nanocomposite ultrafiltration, membranes: The influence of polymeric, additive, dispersion, quality and particle, modification on the integration of zinc, oxide nanoparticles into polyvinylidene, difluoride membranes. Membranes 2020, 10, 197. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Iratni, A.; Toubane, M.; Tala-Ighil, R. Effect of thickness on photocatalytic activity of TiO2 thin films. In Progress in Clean Energy; Springer: Cham, Switzerland, 2015; Volume 1, pp. 763–776. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sugumaran, J.; Shoparwe, N.F. Antifouling, properties of PES membranes by blending with ZnO nanoparticles and NMP—Acetone, mixture as solvent. Membranes 2018, 8, 131. [Google Scholar] [CrossRef]
- Chung, H.H.; Mireles, M.; Kwarta, B.J.; Gaborski, T.R. Use of porous membranes in tissue barrier and co-culture models. Lab Chip 2018, 18, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Hajian, M. Preparation and characterization of polyvinyl butyral/zeolitic imidazolate framework-8 nanocomposite ultrafiltration membranes to improve water flux. Adv. Polym. Technol. 2018, 37, 3607–3618. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nam, S.N.; Son, J.; Oh, J. Tungsten, trioxide (WO3)-assisted photocatalytic, degradation of amoxicillin by simulated, solar irradiation. Sci. Rep. 2019, 9, 3949. [Google Scholar] [CrossRef]
- Munusamy, Y.; Fun, T.Y. Effect of graphene oxide (GO) on water flux of polyvinylidene difluoride (PVDF) membrane in oily wastewater. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2124. [Google Scholar]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of surface roughness on contact angle hysteresis and spreading work. Colloid. Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Junkar, I. Interaction of cells and platelets with biomaterial surfaces treated with gaseous plasma. In Advances in Biomembranes and Lipid, Self-Assembly, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 25–59. [Google Scholar] [CrossRef]
- Busscher, H.J.; van Pelt, A.W.J.; de Boer, P.; de Jong, H.P.; Arends, J. The effect of surface roughening of polymers on measured contact angles of liquids. Colloids Surf. 1984, 9, 319–331. [Google Scholar] [CrossRef]
- David, R.; Fahrni, J.; Marcolli, C.; Mahrt, F.; Brühwiler, D.; Kanji, Z. Review of “the role of contact, angle and pore, width on pore, condensation and freezing”. Atmos. Chem. Phys. 2020, 20, 9419–9440. [Google Scholar] [CrossRef]
- Salim, N.E.; Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Matsuura, T.; Qtaishat, M.R.; Othman, M.H.D.; Rahman, M.C.; Aziz, F.; Yusof, N. Performance of PES/LSMM-OGCN photocatalytic membrane for phenol removal: Effect of OGCN loading. Membranes 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, A.; Sivakumar, V.M.; Thirumarimurugan, M. Influencing, parameters in the photocatalytic, degradation of organic, effluent via nanometal, oxide catalyst: A review. Indian J. Mater. Sci. 2015, 2015, e601827. [Google Scholar] [CrossRef]
- Kamaludin, R.; Rasdi, Z.; Othman, M.H.D.; Kadir, S.H.S.A.; Nor, N.S.M.; Khan, J.; Mohamad Zain, W.N.I.W.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Visible-light active photocatalytic dual layer hollow fiber (DLHF) membrane and its potential in mitigating the detrimental effects of bisphenol A in water. Membranes 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Shareef, U.; Waqas, M. Bisphenol A removal through low-cost, kaolin-based, Ag@TiO2 photocatalytic, hollow fiber, membrane from the liquid, media under visible, light irradiation. J. Nanomater. 2020, 2020, 3541797. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, S.; Ma, G.; Qiu, L.; Sun, H. Application of ceramic, membrane in water and wastewater, treatment. E3S Web Conf. 2018, 53, 4–7. [Google Scholar] [CrossRef]
- Abo, R.; Kummer, N.A.; Merkel, B.J. Optimized photodegradation of Bisphenol A in water using ZnO.; TiO2 and SnO2 photocatalysts under UV radiation as a decontamination procedure. Drink. Water Eng. Sci. 2016, 9, 27–35. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, N.; Xu, X.; Yu, Y.; Wang, Q.; Wang, N.; Han, X.; Yu, H. Degradation of bisphenol a using peroxymonosulfate activated by WO3@MoS2/Ag hollow nanotubes photocatalyst. Chemosphere 2019, 227, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Wu, C.H.; Wu, J.T.; Chen, Y.C. Preparation of immobilized Cu2O using microwave irradiation and its catalytic activity for bisphenol A: Comparisons of Cu2O/H2O2 and visible-light/Cu2O/H2O2 systems. Water Sci. Technol. 2014, 70, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Sathe, P.; Laxman, K.; Myint, M.T.Z.; Dobretsov, S.; Richter, J.; Dutta, J. Bioinspired nanocoatings for biofouling prevention by photocatalytic redox reactions. Sci. Rep. 2017, 7, 3624. [Google Scholar] [CrossRef] [PubMed]




| Ratio | Outer Layer Composition (wt%) | Inner Layer Composition (wt%) | ||||
|---|---|---|---|---|---|---|
| Photocatalyst/Polymer | PVDF | Cu2O | DMAc | PVDF | PEG 6000 | DMAc |
| 0.25 | 15.0 | 3.75 | 81.25 | 15.0 | 3.0 | 82.0 |
| 0.50 | 15.0 | 7.5 | 77.5 | 15.0 | 3.0 | 82.0 |
| 0.75 | 15.0 | 11.25 | 73.75 | 15.0 | 3.0 | 82.0 |
| Outer Layer Dope Flowrate (mL/min) | Outer Layer Thickness (nm) |
|---|---|
| 3 | 13.05 |
| 6 | 21.35 |
| 9 | 89.35 |
| Membrane | Cross Section | Copper |
|---|---|---|
| Neat PVDF membrane | 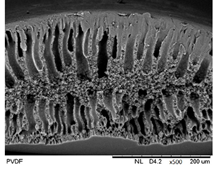 |  |
| 0.25 Cu2O/PVDF DLHF membrane |  |  |
| 0.50 Cu2O/PVDF DLHF membrane | 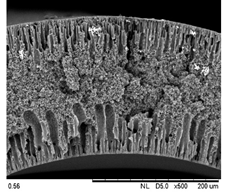 | 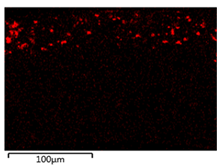 |
| 0.75 Cu2O/PVDF DLHF membrane | 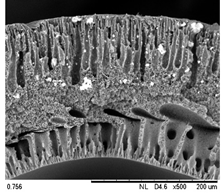 | 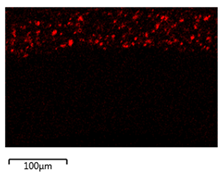 |
| Membrane | Membrane Surface | Surface Roughness (nm) |
|---|---|---|
| Neat PVDF membrane | 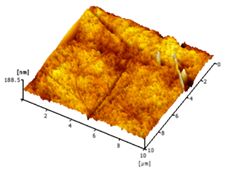 | 6.21 |
| 0.25 Cu2O/PVDF DLHF membrane | 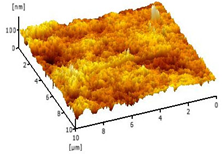 | 7.13 |
| 0.50 Cu2O/PVDF DLHF membrane | 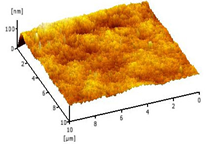 | 7.51 |
| 0.75 Cu2O/PVDF DLHF membrane | 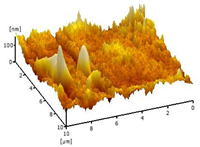 | 32.64 |
| Photocatalyst | UV/Visible Light | BPA Conc. | Removal (%) | References |
|---|---|---|---|---|
| N-doped TiO2/PVDF DLHF membrane | Visible light | 5 mg/L | 81.6% | [67] |
| Ag@TiO2 single layer hollow fiber membrane | Visible light | 10 mg/L | 90.51% | [68] |
| ZnO | UV light | 25 mg/L | 98% | [70] |
| TiO2 | UV light | 25 mg/L | 65% | [70] |
| SnO2 | UV light | 25 mg/L | 48% | [70] |
| CuNPs@CALB-3 | UV light | 45 mg/L | 95% | [8] |
| WO3@MoS2/Ag hollow nanotubes | Visible light | 10 mg/L | 92.51% | [71] |
| WO3 | Visible light | 10 mg/L | 10.55% | [71] |
| Visible light/Cu2O/H2O2 | Visible light | 10 mg/L | 100% | [72] |
| Cu2O/PVDF DLHF membrane | Visible light | 10 mg/L | 75% | This research |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Noor, S.H.; Othman, M.H.D.; Khongnakorn, W.; Sinsamphanh, O.; Abdullah, H.; Puteh, M.H.; Kurniawan, T.A.; Zakria, H.S.; El-badawy, T.; Ismail, A.F.; et al. Bisphenol A Removal Using Visible Light Driven Cu2O/PVDF Photocatalytic Dual Layer Hollow Fiber Membrane. Membranes 2022, 12, 208. https://doi.org/10.3390/membranes12020208
Mohamed Noor SH, Othman MHD, Khongnakorn W, Sinsamphanh O, Abdullah H, Puteh MH, Kurniawan TA, Zakria HS, El-badawy T, Ismail AF, et al. Bisphenol A Removal Using Visible Light Driven Cu2O/PVDF Photocatalytic Dual Layer Hollow Fiber Membrane. Membranes. 2022; 12(2):208. https://doi.org/10.3390/membranes12020208
Chicago/Turabian StyleMohamed Noor, Siti Hawa, Mohd Hafiz Dzarfan Othman, Watsa Khongnakorn, Oulavanh Sinsamphanh, Huda Abdullah, Mohd Hafiz Puteh, Tonni Agustiono Kurniawan, Hazirah Syahirah Zakria, Tijjani El-badawy, Ahmad Fauzi Ismail, and et al. 2022. "Bisphenol A Removal Using Visible Light Driven Cu2O/PVDF Photocatalytic Dual Layer Hollow Fiber Membrane" Membranes 12, no. 2: 208. https://doi.org/10.3390/membranes12020208
APA StyleMohamed Noor, S. H., Othman, M. H. D., Khongnakorn, W., Sinsamphanh, O., Abdullah, H., Puteh, M. H., Kurniawan, T. A., Zakria, H. S., El-badawy, T., Ismail, A. F., Rahman, M. A., & Jaafar, J. (2022). Bisphenol A Removal Using Visible Light Driven Cu2O/PVDF Photocatalytic Dual Layer Hollow Fiber Membrane. Membranes, 12(2), 208. https://doi.org/10.3390/membranes12020208











