Effects of Benzalkonium Chloride Contents on Structures, Properties, and Ultrafiltration Performances of Chitosan-Based Nanocomposite Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Chitosan/PEG/CNT/BKC Nanocomposite Membranes
2.3. Characterization of Nanocomposite Membranes
2.4. Performances of Nanocomposite Membranes
3. Results
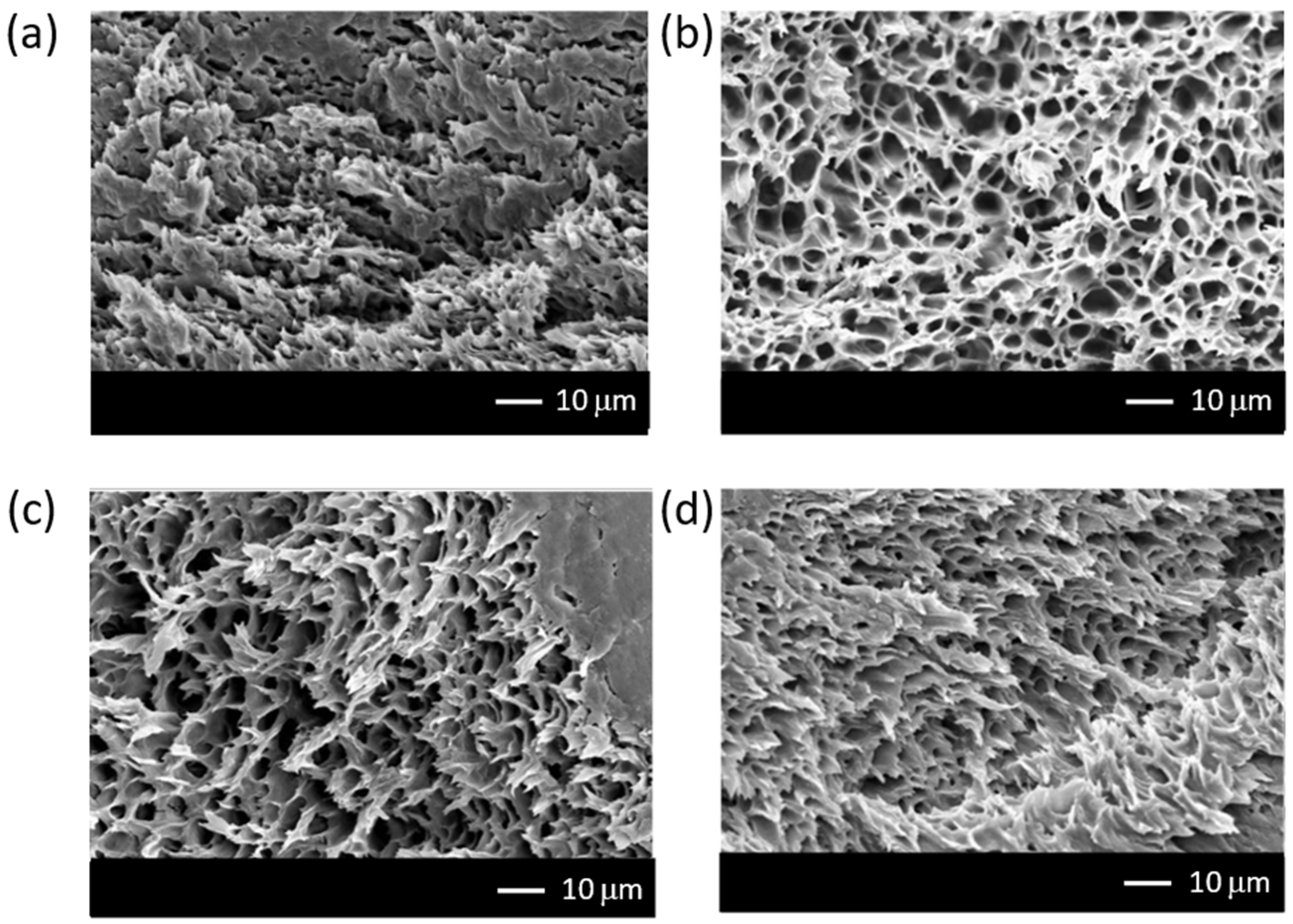
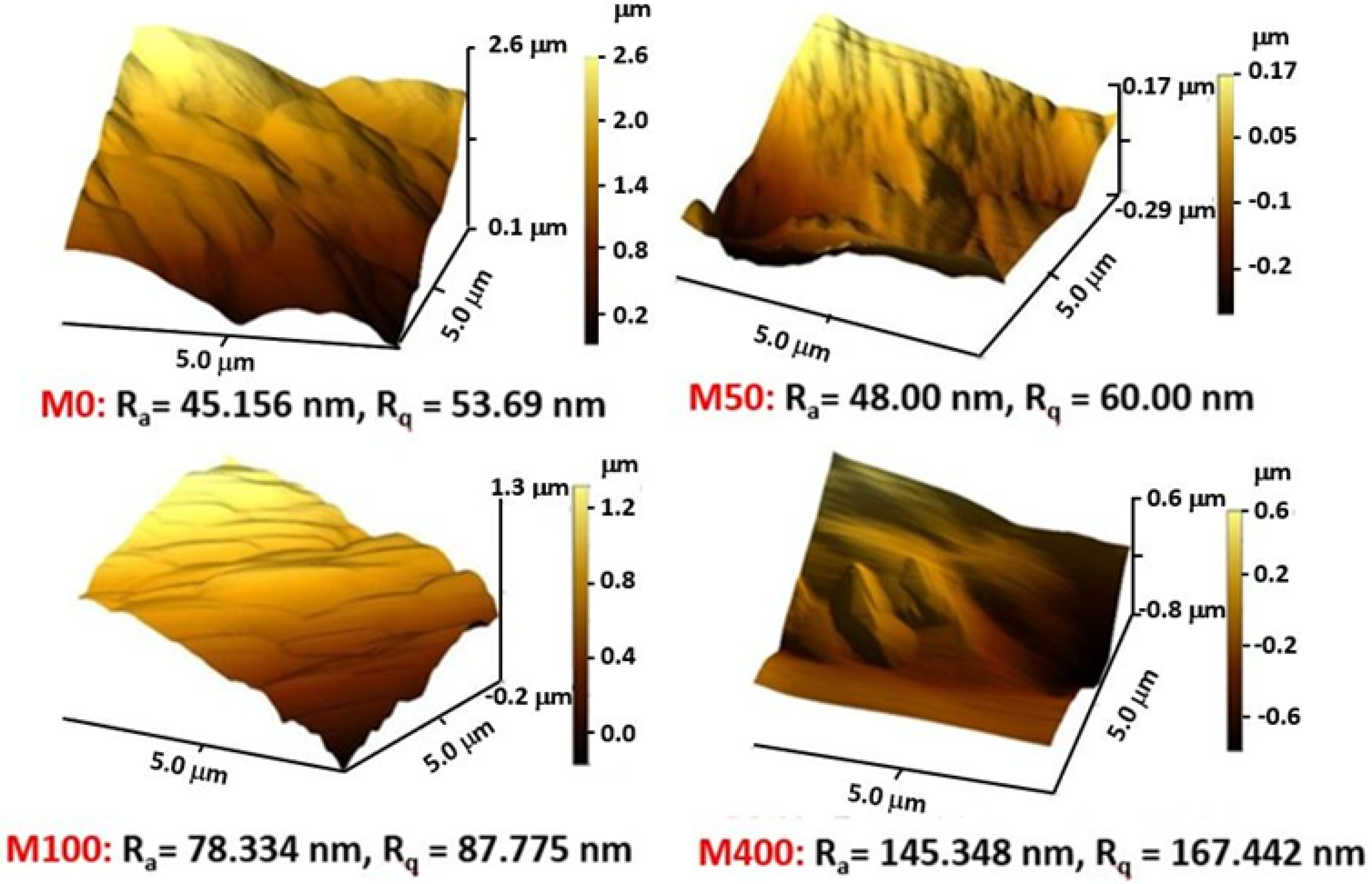
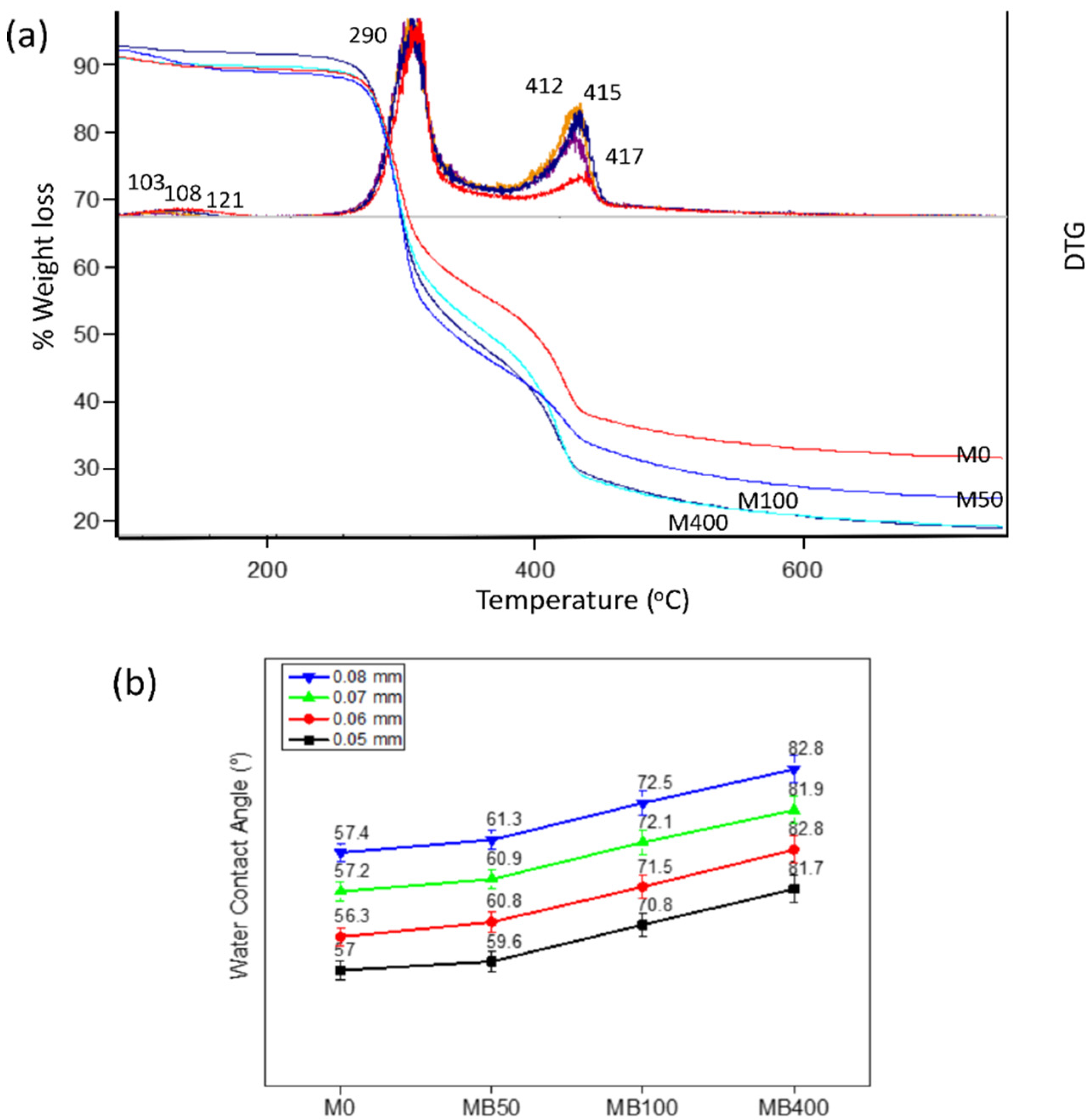
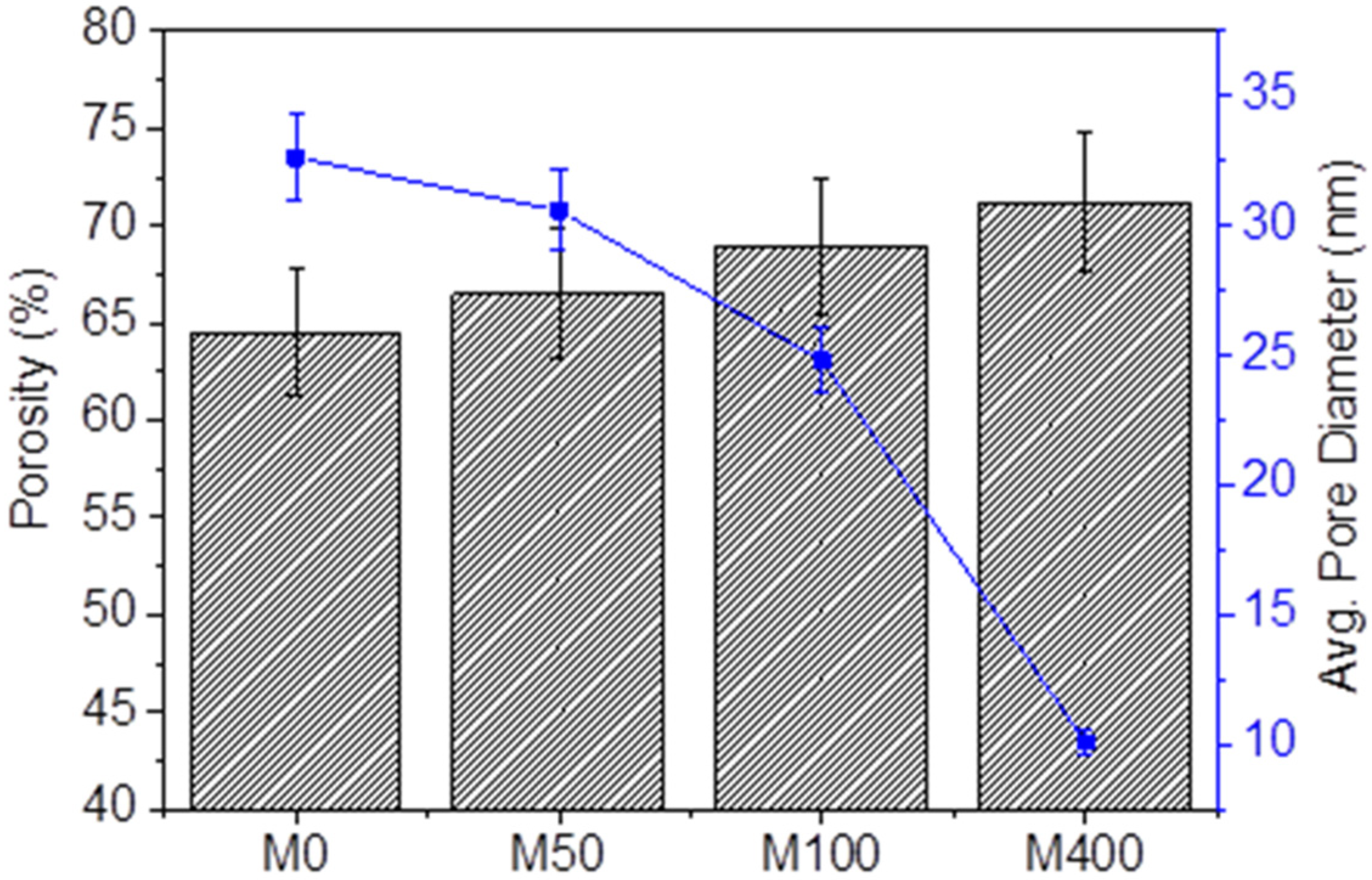
3.1. Characterization of Nanocomposite Membranes
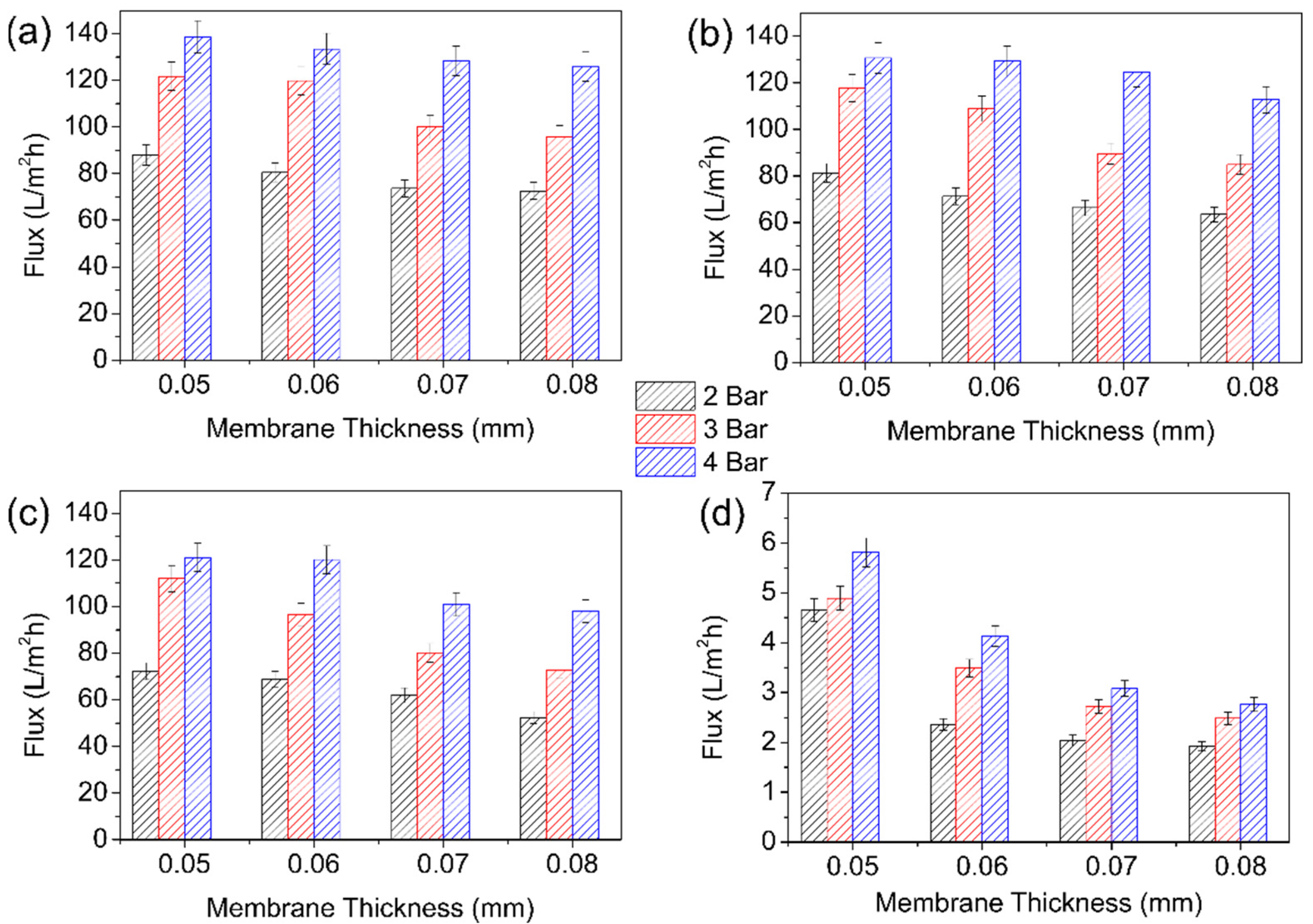
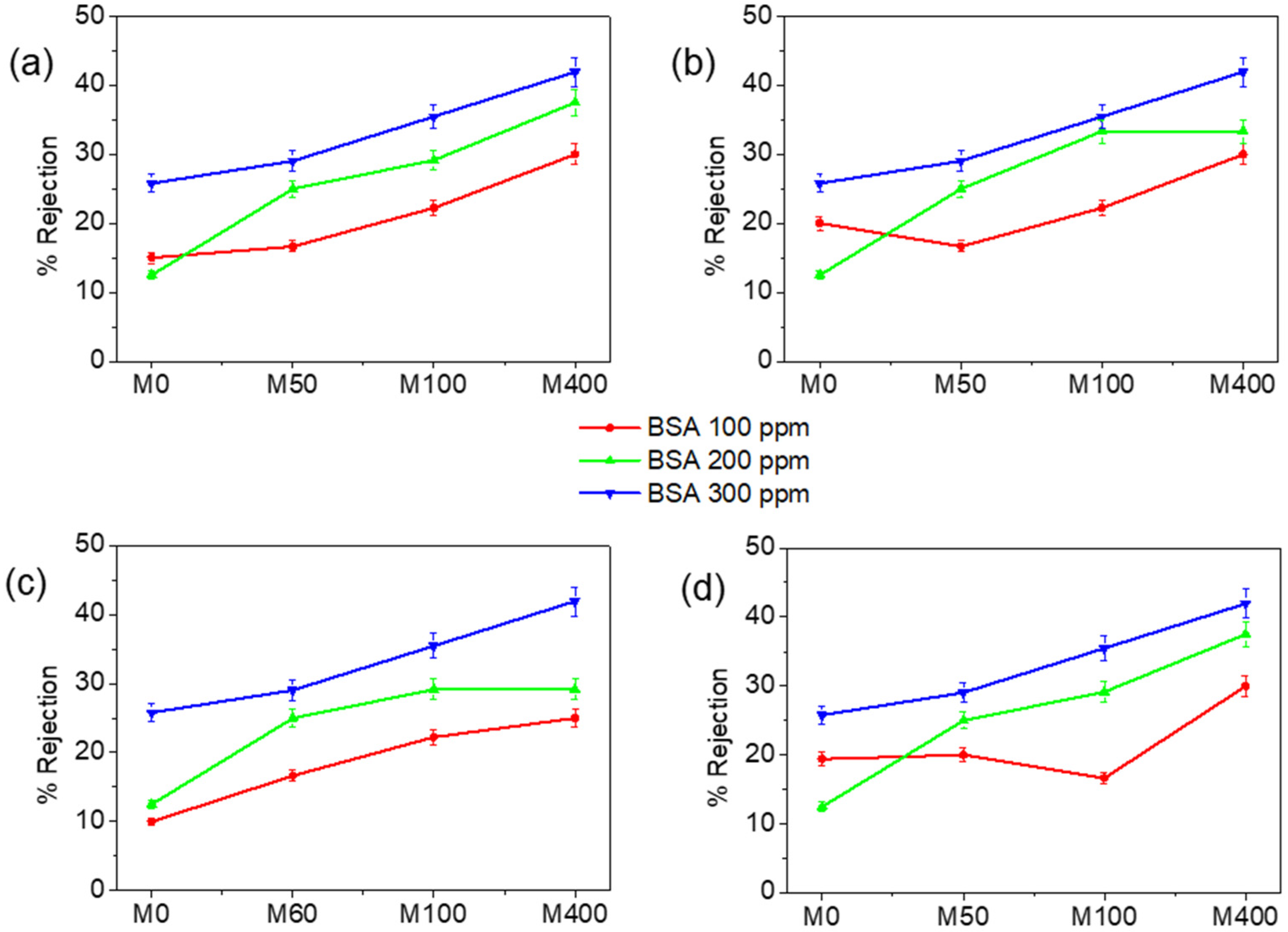
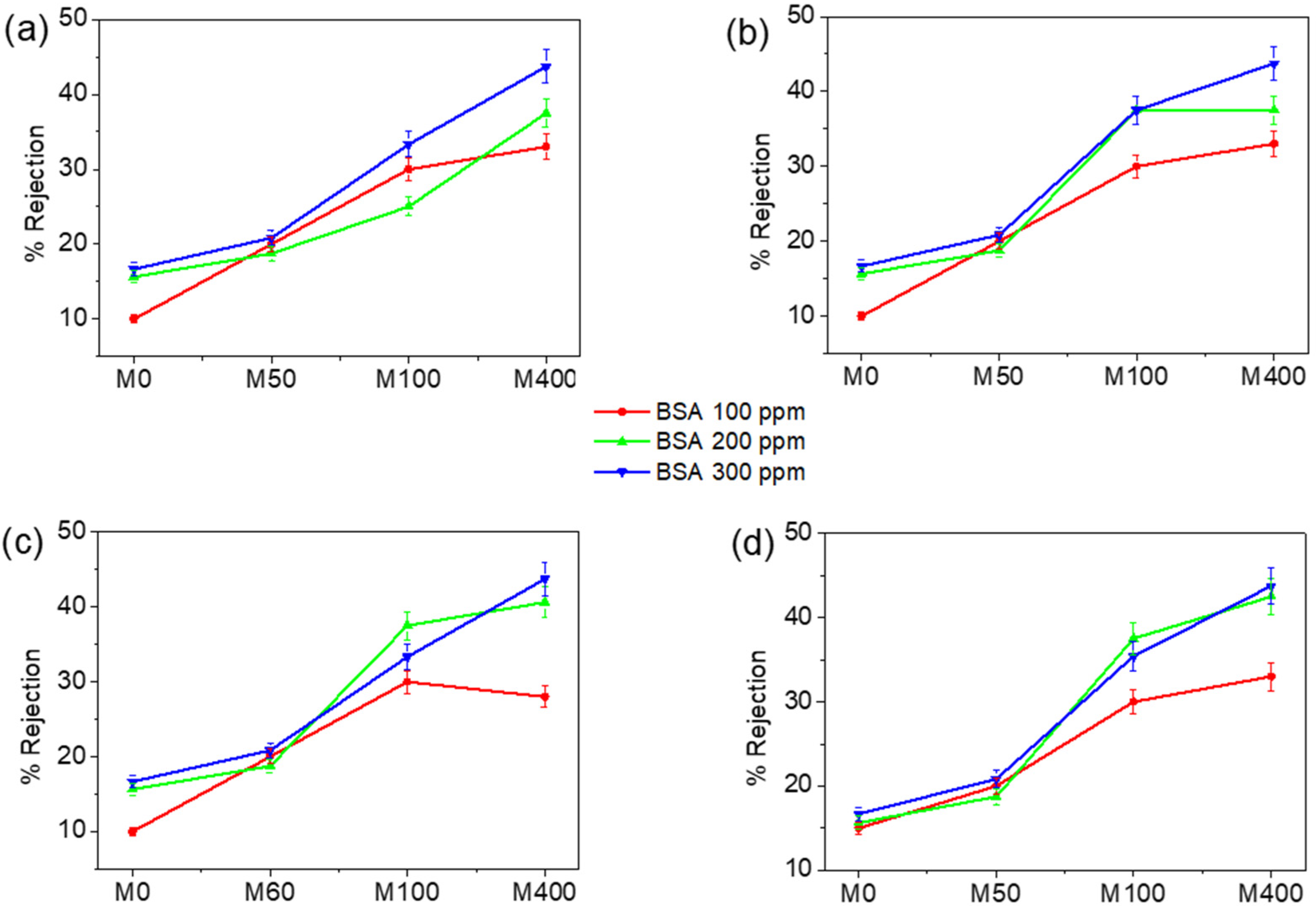
3.2. Membrane Performance Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, A.K.; Tortajada, C. Water crisis and water wars: Myths and realities. Int. J. Water Resour. Dev. 2019, 35, 727–731. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Saha, B.; Saha, B.; Uddin, M.S.; Panna, C.H.; Bhattacharya, P.; Saha, R. Groundwater governance in Bangladesh: Established practices and recent trends. Groundw. Sustain. Dev. 2019, 8, 69–81. [Google Scholar] [CrossRef]
- Lee, S. Performance Comparison of Spiral-Wound and Plate-and-Frame Forward Osmosis Membrane Module. Membranes 2020, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Kukučka, M.; Kukučka Stojanović, N. Intrinsic Dependence of Groundwater Cation Hydraulic and Concentration Features on Negatively Charged Thin Composite Nanofiltration Membrane Rejection and Permeation Behavior. Membranes 2022, 12, 79. [Google Scholar] [CrossRef]
- Guo, Y.; Bai, L.; Tang, X.; Huang, Q.; Xie, B.; Wang, T.; Wang, J.; Li, G.; Liang, H. Coupling continuous sand filtration to ultrafiltration for drinking water treatment: Improved performance and membrane fouling control. J. Membr. Sci. 2018, 567, 18–27. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Behboudi, A.; Jafarzadeh, Y.; Yegani, R. Enhancement of antifouling and antibacterial properties of PVC hollow fiber ultrafiltration membranes using pristine and modified silver nanoparticles. J. Environ. Chem. Eng. 2018, 6, 1764–1773. [Google Scholar] [CrossRef]
- Zhu, M.-M.; Fang, Y.; Chen, Y.-C.; Lei, Y.-Q.; Fang, L.-F.; Zhu, B.-K.; Matsuyama, H. Antifouling and antibacterial behavior of membranes containing quaternary ammonium and zwitterionic polymers. J. Colloid Interface Sci. 2021, 584, 225–235. [Google Scholar] [CrossRef]
- Gafri, H.F.; Zuki, F.M.; Aroua, M.K.; Bello, M.M. Enhancing the Anti-biofouling Properties of Polyethersulfone Membrane Using Chitosan-Powder Activated Carbon Composite. J. Polym. Environ. 2019, 27, 2156–2166. [Google Scholar] [CrossRef]
- Lin, W.; Li, M.; Xiao, K.; Huang, X. The role shifting of organic, inorganic and biological foulants along different positions of a two-stage nanofiltration process. J. Membr. Sci. 2020, 602, 117979. [Google Scholar] [CrossRef]
- Tibi, F.; Charfi, A.; Cho, J.; Kim, J. Effect of interactions between ammonium and organic fouling simulated by sodium alginate on performance of direct contact membrane distillation. Sep. Purif. Technol. 2021, 278, 119551. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bucs, S.S.; Farhat, N.; Kruithof, J.C.; Picioreanu, C.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Review on strategies for biofouling mitigation in spiral wound membrane systems. Desalination 2018, 434, 189–197. [Google Scholar] [CrossRef]
- Abushaban, A.; Salinas-Rodriguez, S.G.; Mangal, M.N.; Mondal, S.; Goueli, S.A.; Knezev, A.; Vrouwenvelder, J.S.; Schippers, J.C.; Kennedy, M.D. ATP measurement in seawater reverse osmosis systems: Eliminating seawater matrix effects using a filtration-based method. Desalination 2019, 453, 1–9. [Google Scholar] [CrossRef]
- Hou, S.; Xing, J.; Dong, X.; Zheng, J.; Li, S. Integrated antimicrobial and antifouling ultrafiltration membrane by surface grafting PEO and N-chloramine functional groups. J. Colloid Interface Sci. 2017, 500, 333–340. [Google Scholar] [CrossRef]
- Birsan, I.-G.; Pintilie, S.C.; Pintilie, L.G.; Lazar, A.L.; Circiumaru, A.; Balta, S. New Understanding of the Difference in Filtration Performance between Anatase and Rutile TiO2 Nanoparticles through Blending into Ultrafiltration PSF Membranes. Membranes 2021, 11, 841. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Kulsum, C.; Dara, F.; Nurhayati, M.; Nashrah, N.; Fatimah, S.; Pratiwi, A.; Hendrawan, H.; Nasir, M.; Ko, Y.G.; et al. Toughened chitosan-based composite membranes with antibiofouling and antibacterial properties via incorporation of benzalkonium chloride. RSC Adv. 2021, 11, 16814–16822. [Google Scholar] [CrossRef]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Kampf, G. Adaptive microbial response to low-level benzalkonium chloride exposure. J. Hosp. Infect. 2018, 100, e1–e22. [Google Scholar] [CrossRef]
- Bondurant, S.; McKinney, T.; Bondurant, L.; Fitzpatrick, L. Evaluation of a benzalkonium chloride hand sanitizer in reducing transient Staphylococcus aureus bacterial skin contamination in health care workers. Am. J. Infect. Control 2020, 48, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Fazlara, A.; Ekhtelat, M. The disinfectant effects of benzalkonium chloride on some important foodborne pathogens. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 23–29. [Google Scholar]
- Chen, M.; Zhang, X.; Wang, Z.; Wang, L.; Wu, Z. QAC modified PVDF membranes: Antibiofouling performance, mechanisms, and effects on microbial communities in an MBR treating municipal wastewater. Water Res. 2017, 120, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Pant, J.; Gao, J.; Goudie, M.J.; Hopkins, S.P.; Locklin, J.; Handa, H. A multi-defense strategy: Enhancing bactericidal activity of a medical grade polymer with a nitric oxide donor and surface-immobilized quaternary ammonium compound. Acta Biomater. 2017, 58, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ping, M.; Zhang, X.; Liu, M.; Wu, Z.; Wang, Z. Surface modification of polyvinylidene fluoride membrane by atom-transfer radical-polymerization of quaternary ammonium compound for mitigating biofouling. J. Membr. Sci. 2019, 570, 286–293. [Google Scholar] [CrossRef]
- Ali, N.; Khan, A.; Malik, S.; Badshah, S.; Bilal, M.; Iqbal, H.M.N. Chitosan-based green sorbent material for cations removal from an aqueous environment. J. Environ. Chem. Eng. 2020, 8, 104064. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ooi, C.W.; Song, C.P.; Wang, C.-Y.; Liu, B.-L.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial efficacy of quaternized chitosan/poly (vinyl alcohol) nanofiber membrane crosslinked with blocked diisocyanate. Carbohydr. Polym. 2021, 262, 117910. [Google Scholar] [CrossRef]
- Nayab, S.S.; Abbas, M.A.; Mushtaq, S.; Khan Niazi, B.; Batool, M.; Shehnaz, G.; Ahmad, N.; Ahmad, N.M. Anti-Foulant Ultrafiltration Polymer Composite Membranes Incorporated with Composite Activated Carbon/Chitosan and Activated Carbon/Thiolated Chitosan with Enhanced Hydrophilicity. Membranes 2021, 11, 827. [Google Scholar] [CrossRef]
- Kamrani, M.; Akbari, A.; Yunessnia lehi, A. Chitosan-modified acrylic nanofiltration membrane for efficient removal of pharmaceutical compounds. Carbohydr. Polym. 2018, 6, 583–587. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Hendrawan, H.; Primastari, D.R.; Agiawati, R. Effect of MWCNT Filler on Properties and Flux of Chitosan/PEG based Nanocomposites Membranes. MATEC Web Conf. 2018, 156, 04001. [Google Scholar] [CrossRef][Green Version]
- Khoerunnisa, F.; Hendrawan, H.; Sonjaya, Y.; Putri, A. Synthesis and characterization of composites filtration membranes based on chitosan-poly(ethylene glycol). In Proceedings of the AIP Conference Proceedings, Semarang, Indonesia, 29–30 December 2015. [Google Scholar]
- Wang, S.-Y.; Fang, L.-F.; Cheng, L.; Jeon, S.; Kato, N.; Matsuyama, H. Novel ultrafiltration membranes with excellent antifouling properties and chlorine resistance using a poly(vinyl chloride)-based copolymer. J. Membr. Sci. 2018, 549, 101–110. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Rahmah, W.; Seng Ooi, B.; Dwihermiati, E.; Nashrah, N.; Fatimah, S.; Ko, Y.G.; Ng, E.-P. Chitosan/PEG/MWCNT/Iodine composite membrane with enhanced antibacterial properties for dye wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103686. [Google Scholar] [CrossRef]
- Chen, F.; Shi, X.; Chen, X.; Chen, W. An iron (II) phthalocyanine/poly(vinylidene fluoride) composite membrane with antifouling property and catalytic self-cleaning function for high-efficiency oil/water separation. J. Membr. Sci. 2018, 552, 295–304. [Google Scholar] [CrossRef]
- Hosseinifard, S.M.; Aroon, M.A.; Dahrazma, B. Application of PVDF/HDTMA-modified clinoptilolite nanocomposite membranes in removal of reactive dye from aqueous solution. Sep. Purif. Technol. 2020, 251, 117294. [Google Scholar] [CrossRef]
- Davenport, D.M.; Ritt, C.L.; Verbeke, R.; Dickmann, M.; Egger, W.; Vankelecom, I.F.J.; Elimelech, M. Thin film composite membrane compaction in high-pressure reverse osmosis. J. Membr. Sci. 2020, 610, 118268. [Google Scholar] [CrossRef]
- Rezania, J.; Vatanpour, V.; Shockravi, A.; Ehsani, M. Preparation of novel carboxylated thin-film composite polyamide-polyester nanofiltration membranes with enhanced antifouling property and water flux. React. Funct. Polym. 2018, 131, 123–133. [Google Scholar] [CrossRef]
- Kallem, P.; Ibrahim, Y.; Hasan, S.W.; Show, P.L.; Banat, F. Fabrication of novel polyethersulfone (PES) hybrid ultrafiltration membranes with superior permeability and antifouling properties using environmentally friendly sulfonated functionalized polydopamine nanofillers. Sep. Purif. Technol. 2021, 261, 118311. [Google Scholar] [CrossRef]
- Vahdatifar, S.; Ali Khodadadi, A.; Mortazavi, Y.; Greenlee, L.F. Functionalized open-ended vertically aligned carbon nanotube composite membranes with high salt rejection and enhanced slip flow for desalination. Sep. Purif. Technol. 2021, 279, 119773. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes 2021, 12, 14. [Google Scholar] [CrossRef]
- Saraswathi, M.S.A.; Divya, K.; Selvapandian, P.; Mohan, D.; Rana, D.; Nagendran, A. Permeation and antifouling performance of poly (ether imide) composite ultrafiltration membranes customized with manganese dioxide nanospheres. Mater. Chem. Phys. 2019, 231, 159–167. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, X.; Ni, L.; Tang, Z.; Zhang, Y.; Zhang, Y.; Zhang, W. Antibacterial cellulose membrane via one-step covalent immobilization of ammonium/amine groups. Desalination 2015, 359, 156–166. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Li, J.; Du, N.; Li, D.; Li, F.; Man, J. Application status and technical analysis of chitosan-based medical dressings: A review. RSC Adv. 2020, 10, 34308–34322. [Google Scholar] [CrossRef]
- Katsumata, K.; Saito, T.; Yu, F.; Nakamura, N.; Inoue, Y. The toughening effect of a small amount of poly(ɛ-caprolactone) on the mechanical properties of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/PCL blend. Polym. J. 2011, 43, 484–492. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Non-isothermal kinetics of thermal degradation of chitosan. Chem. Cent. J. 2012, 6, 81. [Google Scholar] [CrossRef]
- López, F.A.; Mercê, A.L.R.; Alguacil, F.J.; López-Delgado, A. A kinetic study on the thermal behaviour of chitosan. J. Therm. Anal. Calorim. 2008, 91, 633–639. [Google Scholar] [CrossRef]
- Sun, S.; An, Q.; Li, X.; Qian, L.; He, B.; Xiao, H. Synergistic effects of chitosan–guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour. Technol. 2010, 101, 5693–5700. [Google Scholar] [CrossRef]
- Harney, M.B.; Pant, R.R.; Fulmer, P.A.; Wynne, J.H. Surface Self-Concentrating Amphiphilic Quaternary Ammonium Biocides as Coating Additives. ACS Appl. Mater. Interfaces 2009, 1, 39–41. [Google Scholar] [CrossRef]
- Fukuda, M.; Yoshimoto, H.; Saomoto, H.; Sakai, K. Validity of Three-Dimensional Tortuous Pore Structure and Fouling of Hemoconcentration Capillary Membrane Using the Tortuous Pore Diffusion Model and Scanning Probe Microscopy. Membranes 2020, 10, 315. [Google Scholar] [CrossRef]
- Kim, T.-N.; Lee, J.; Choi, J.-H.; Ahn, J.-H.; Yang, E.; Hwang, M.-H.; Chae, K.-J. Tunable Atomic Level Surface Functionalization of a Multi-Layered Graphene Oxide Membrane to Break the Permeability-Selectivity Trade-off in Salt Removal of Brackish Water. Sep. Purif. Technol. 2021, 274, 119047. [Google Scholar] [CrossRef]
- Mao, H.; Zhou, S.; Shi, S.; Xue, A.; Li, M.; Cai, J.; Zhao, Y.; Xing, W. Anti-Fouling and Easy-Cleaning PVDF Membranes Blended with Hydrophilic Thermo-Responsive Nanofibers for Efficient Biological Wastewater Treatment. Sep. Purif. Technol. 2022, 281, 119881. [Google Scholar] [CrossRef]
- Mah, S.-K.; Chang, C.C.H.; Wu, T.Y.; Chai, S.-P. The Study of Reverse Osmosis on Glycerin Solution Filtration: Dead-End and Crossflow Filtrations, Transport Mechanism, Rejection and Permeability Investigations. Desalination 2014, 352, 66–81. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Wang, H. Interplay of the Factors Affecting Water Flux and Salt Rejection in Membrane Distillation: A State-of-the-Art Critical Review. Water 2020, 12, 2841. [Google Scholar] [CrossRef]
- Striemer, C.C.; Gaborski, T.R.; McGrath, J.L.; Fauchet, P.M. Charge- and Size-Based Separation of Macromolecules Using Ultrathin Silicon Membranes. Nature 2007, 445, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Ohanessian, K.; Monnot, M.; Moulin, P.; Ferrasse, J.-H.; Barca, C.; Soric, A.; Boutin, O. Dead-End and Crossflow Ultrafiltration Process Modelling: Application on Chemical Mechanical Polishing Wastewaters. Chem. Eng. Res. Des. 2020, 158, 164–176. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoerunnisa, F.; Nurhayati, M.; Annisa, N.A.A.; Fatimah, S.; Nashrah, N.; Hendrawan, H.; Ko, Y.-G.; Ng, E.-P.; Opaprakasit, P. Effects of Benzalkonium Chloride Contents on Structures, Properties, and Ultrafiltration Performances of Chitosan-Based Nanocomposite Membranes. Membranes 2022, 12, 268. https://doi.org/10.3390/membranes12030268
Khoerunnisa F, Nurhayati M, Annisa NAA, Fatimah S, Nashrah N, Hendrawan H, Ko Y-G, Ng E-P, Opaprakasit P. Effects of Benzalkonium Chloride Contents on Structures, Properties, and Ultrafiltration Performances of Chitosan-Based Nanocomposite Membranes. Membranes. 2022; 12(3):268. https://doi.org/10.3390/membranes12030268
Chicago/Turabian StyleKhoerunnisa, Fitri, Mita Nurhayati, Noor Azmi Aulia Annisa, Siti Fatimah, Nisa Nashrah, Hendrawan Hendrawan, Young-Gun Ko, Eng-Poh Ng, and Pakorn Opaprakasit. 2022. "Effects of Benzalkonium Chloride Contents on Structures, Properties, and Ultrafiltration Performances of Chitosan-Based Nanocomposite Membranes" Membranes 12, no. 3: 268. https://doi.org/10.3390/membranes12030268
APA StyleKhoerunnisa, F., Nurhayati, M., Annisa, N. A. A., Fatimah, S., Nashrah, N., Hendrawan, H., Ko, Y.-G., Ng, E.-P., & Opaprakasit, P. (2022). Effects of Benzalkonium Chloride Contents on Structures, Properties, and Ultrafiltration Performances of Chitosan-Based Nanocomposite Membranes. Membranes, 12(3), 268. https://doi.org/10.3390/membranes12030268









