1. Introduction
In a year, almost 1000–3000 m
3 of effluent gets discharged from the textiles industry per day and approximately 15% of the dyes get washed out due to inefficient dyeing process during manufacturing and processing operations [
1,
2]. These industrial effluents are comprised of water, dyes and chemicals, some of which are non-biodegradable and synthetic in nature. Dyes have at least one chromophore (color bearing group) and auxochromes (color inducers) which contribute towards coloring, decorative, aesthetic and artistic effects. Due to these effects, the use of dyes became important and one of the main attractions that enhance the commercial value of a particular fabric. However, dyes themselves are toxic, have poor biodegradability, high color depth, are able to resist some action of oxidizing agents and can interfere with light transmission, all of which pose a serious threat to the ecosystem [
3,
4]. The most commonly used dye in the wood, cotton and textiles industries is methylene blue (MB) [
5]. MB belongs to a class of synthetic cationic thiazine dyes. Even at low concentrations, MB can produce a slightly toxic effect that can result in nausea, vomiting, Heinz body formation and methemoglobinemia [
6,
7,
8]. Hence, it is vital to treat this dye in wastewater in an economical and efficient method.
Intensive reviews in removing heavy metals and dyes were reported by previous studies [
9,
10,
11]. However, among all treatment methods of dye removal such as adsorption, coagulation, chemical precipitation, flocculation and membrane filtration [
12], there has been little discussion on the combination of the membrane and adsorption methods. A membrane is a physical separation that forms a barrier for matter transport by allowing only certain particles, smaller than the pore size, to pass through [
13]. In the case of textile wastewater, the membrane method is only able to separate the suspended solid, and not the dyes, because most of the dyes are water soluble [
14]. Thus, the most suitable and simple method to remove suspended solid matters and dyes is through using the membrane-adsorption technique which involves dye molecules to adsorb to the membrane surface while the solid suspension is separated.
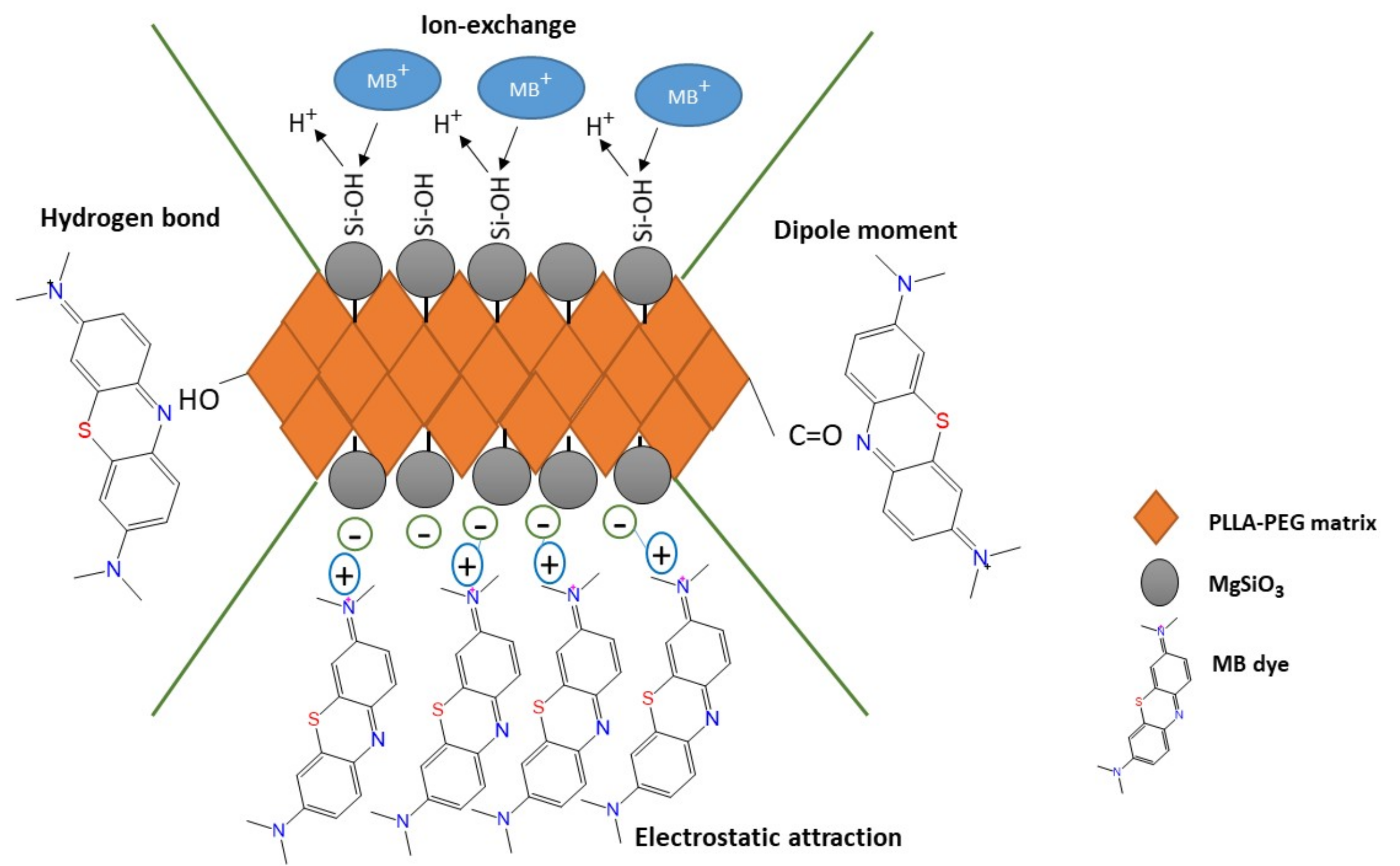
Recently, the incorporation of magnesium silicate (MgSiO
3) into membranes and composites received enormous attention from researchers across the world. MgSiO3 helps in enhancing the adsorption capacity of membranes with its own properties, namely fast rate of adsorption and high adsorption capacity in addition to improving the physical characteristics of membranes. It was revealed that pure MgSiO
3 had outstanding adsorption capacities of 244, 107.8 and 418.4 mg/g for Rhodamine B, Cadmium and MB removal, respectively [
15,
16]. The impregnation of MgSiO
3 into palm-shell waste powdered activated carbon increased the lead removal from 391.3 mg/g to 419.9 mg/g [
17]. Furthermore, MgSiO
3 has a negatively charged surface that potentially can adsorb cationic dyes due to their large surface areas and richness in silicates groups. This surface charge plays an important role by attracting the cationic dye to the surface during the adsorption process [
18,
19].
Traditionally, the influence of parameters in the adsorption performance was evaluated one factor at time while keeping other factors at a constant level. This specific technique is known as one-variable-at-a-time [
20]. However, this technique has a few major drawbacks which are that it fails to include the complete interaction effects between the parameters as well as requires a large number of experiments. With the use of response surface methodology (RSM), this problem can be resolved by designing a set of experiments. RSM is a collection of mathematical and statistical techniques that are suitable for analyzing the contribution of parameters by determining the interaction between the parameters and modeling the system [
21]. The most common and efficient designs used in RSM modeling are Box–Behnken designs (BBD) and central composite design (CCD) [
22]. In this study, CCD was chosen because it gives higher prediction of the response while saving time and costs by reducing the number of trials. This design was also suitable for use for at least two or more parameters involved in the experiment.
Poly(Lactic Acid) (PLA) was chosen as the membrane matrix due to its eco-friendly polymer, thermoplastic nature and high strength. Unfortunately, its major drawbacks are that it is hydrophobic and brittle and has poor toughness [
23]. Therefore, an excellent plasticizer such as poly(ethylene glycol) (PEG) is needed to improve the matrix membrane, as PEG is a hydrophilic polymer, non-toxic and has good biocompatibility. In addition, PLA co-polymerized with PEG can enhance their membrane’s properties by increasing the hydrophilicity, thermal stability and mechanical strength [
24]. Herein, we have successfully fabricated a PLA-PEG membrane and PLA-PEG with magnesium silicate (MgSiO
3) (PLA-PEG/MgSiO
3) to evaluate its dual functions which act as filter and adsorbent. The adsorption performance towards MB dye was systematically investigated with the aid of RSM. RSM was used to determine the optimization condition of MB removal efficiency in synthetic wastewater which included mass of membranes and initial concentration of MB solution. Then, the effect of pH solution and ionic strength as well as adsorption kinetics and adsorption isotherms were distinctly evaluated to determine the adsorption behavior and mechanism involved during the adsorption process. Due to the good mechanical properties of the membrane, it was possible to carry out the reusability study of the PLA-PEG/MgSiO
3 membrane accordingly.
2. Materials and Methods
2.1. Materials
Poly(lactic acid) (PLA) was acquired from Shenzhen Esun Industrial Co., Ltd. (Shenzen, China) and magnesium silicate was purchased from Dallas Group of America Inc. (Whitehouse, NJ, USA). Other chemicals used were poly(ethylene glycol) (PEG, Sigma Aldrich, Saint Louis, MO, USA) with an average molecular weight of 10,000, tin(II) 2-ethylhexanoate (Sn(oct)2, 95.0%, Sigma Aldrich), 1,6-hexamethylene diisocyanate (HMDI, 98.0%, Sigma Aldrich), dichloromethane (DCM, 98.0%, Sigma Aldrich), and tetrahydrofuran (THF, 99.8%, Sigma Aldrich). All chemicals and reagents were used without further purification. Magnesium silicate gel was sieved to the size of 0–45 μm prior to usage.
2.2. Preparation of Membranes
PLA (5 g) pellets were dissolved in a round bottom flask containing 30 mL of DCM for 2 h. In the meantime, 0.6 g of magnesium silicate (MgSiO3) was stirred with 30 mL THF in a beaker. After a few hours, 5 g of PEG was added into the flask containing PLA solution, followed by 36 mg of Sn (Oct)2 and 0.7 g of HMDI. The mixture was stirred for an additional 2 h at 45 °C. Then, the MgSiO3/THF mixture was added into the flask containing PLA-PEG copolymer solution and stirred overnight at 45 °C. PLA-PEG/MgSiO3 solution was cast onto a glass plate using a casting knife. The solvent was evaporated for 200 s before being immersed in distilled waste and the off-white membrane formed was carefully peeled off. The experiment was repeated in the absence of MgSiO3.
2.3. Characterization of Membranes
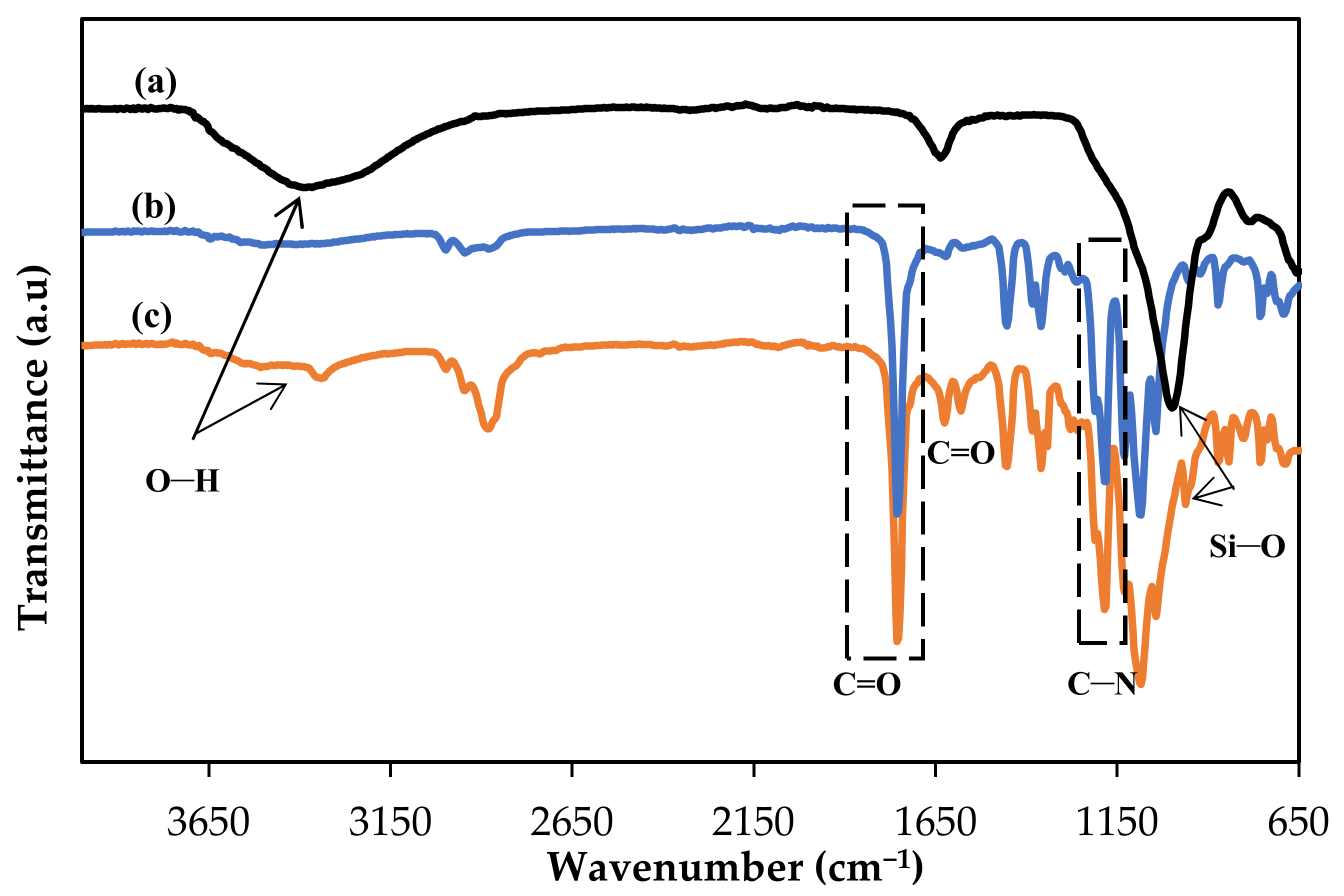
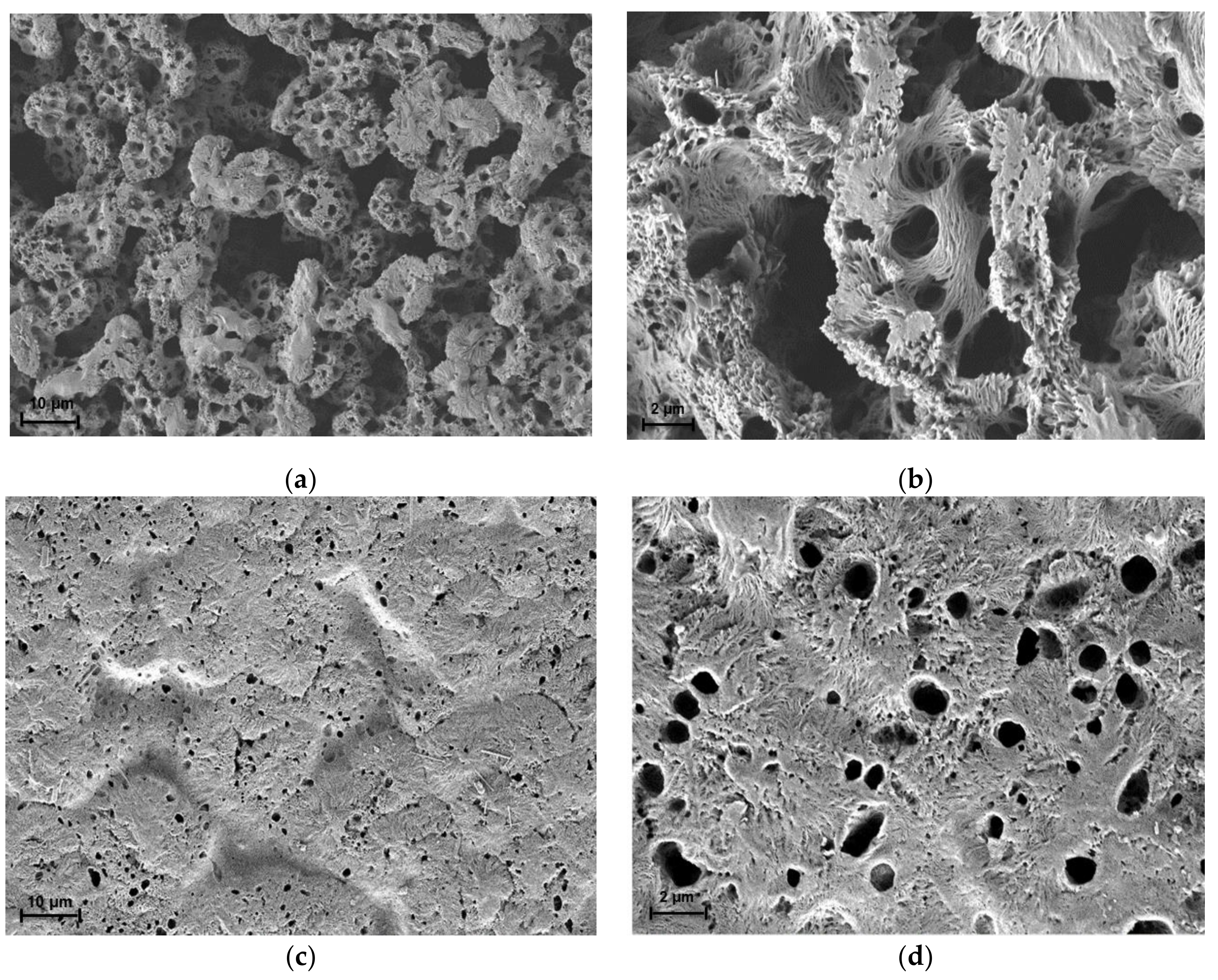
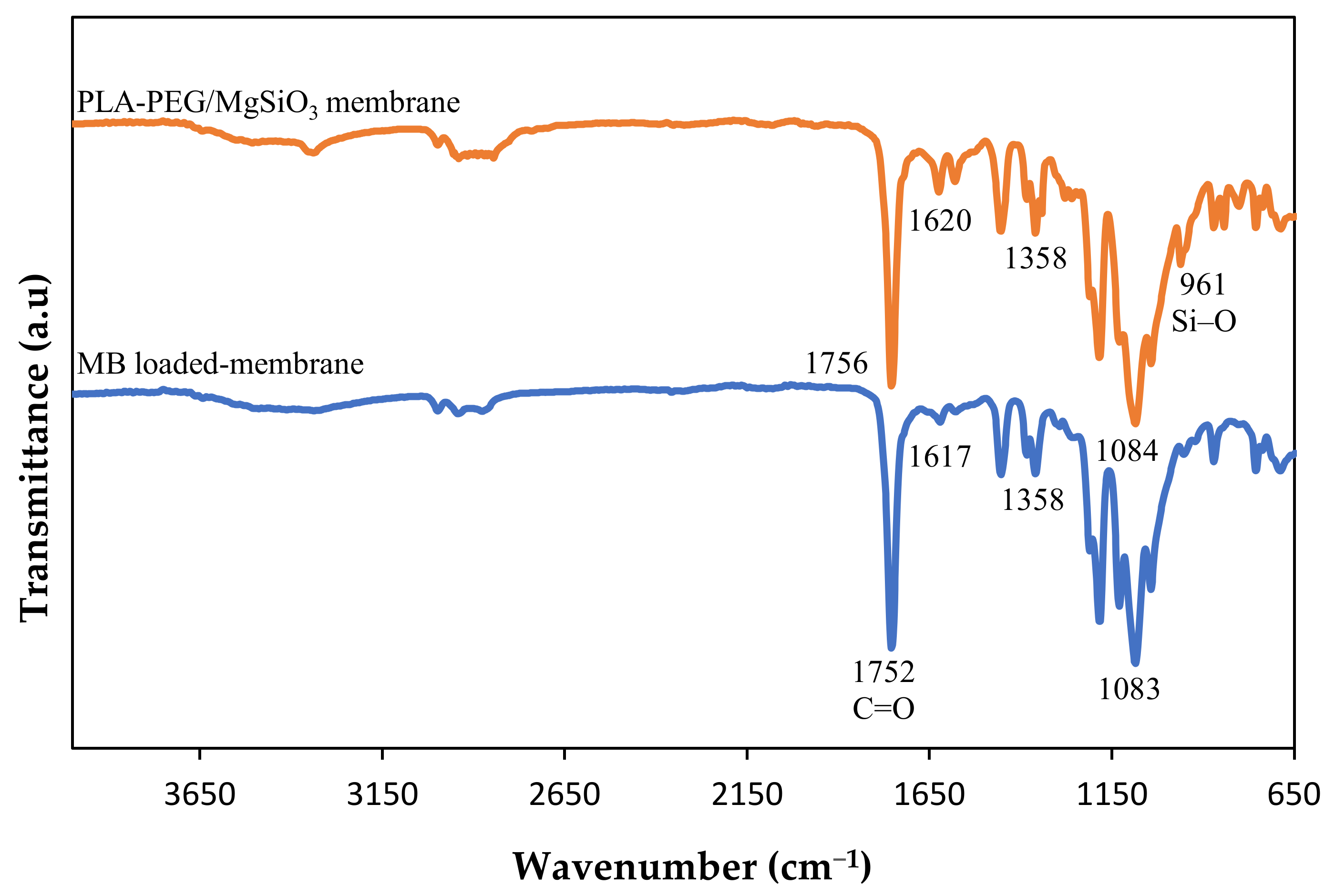
Fourier-Transform infrared (FTIR) was used to determine the presence of functional groups in the membranes in the range of 650–4000 cm−1. This analysis was run using Perkin Elmer (Waltham, MA, USA) model Spectrum 400, in ATR-FTIR mode. Tensile tests were performed according to the standard ASTM D882 by using an Instron (Norwood, MA, USA) model 5566 universal tensile machine. Several samples of each membrane were tested, and the average values were taken from eight readings. The membranes’ surface morphologies were investigated using scanning electron microscopy (Zeiss/SUPRA 55VP, Oberkochen, Germany) using gold coating. The water contact angle analysis of the membranes was conducted using a drop shape analyzer (Krüss GmbH, Hamburg, Germany, model FM40Mk2). Four microliters of deionized water were dropped on the membrane surfaces and 5 spots of each membrane were taken every 10 ms for 1 min. The average values were calculated.
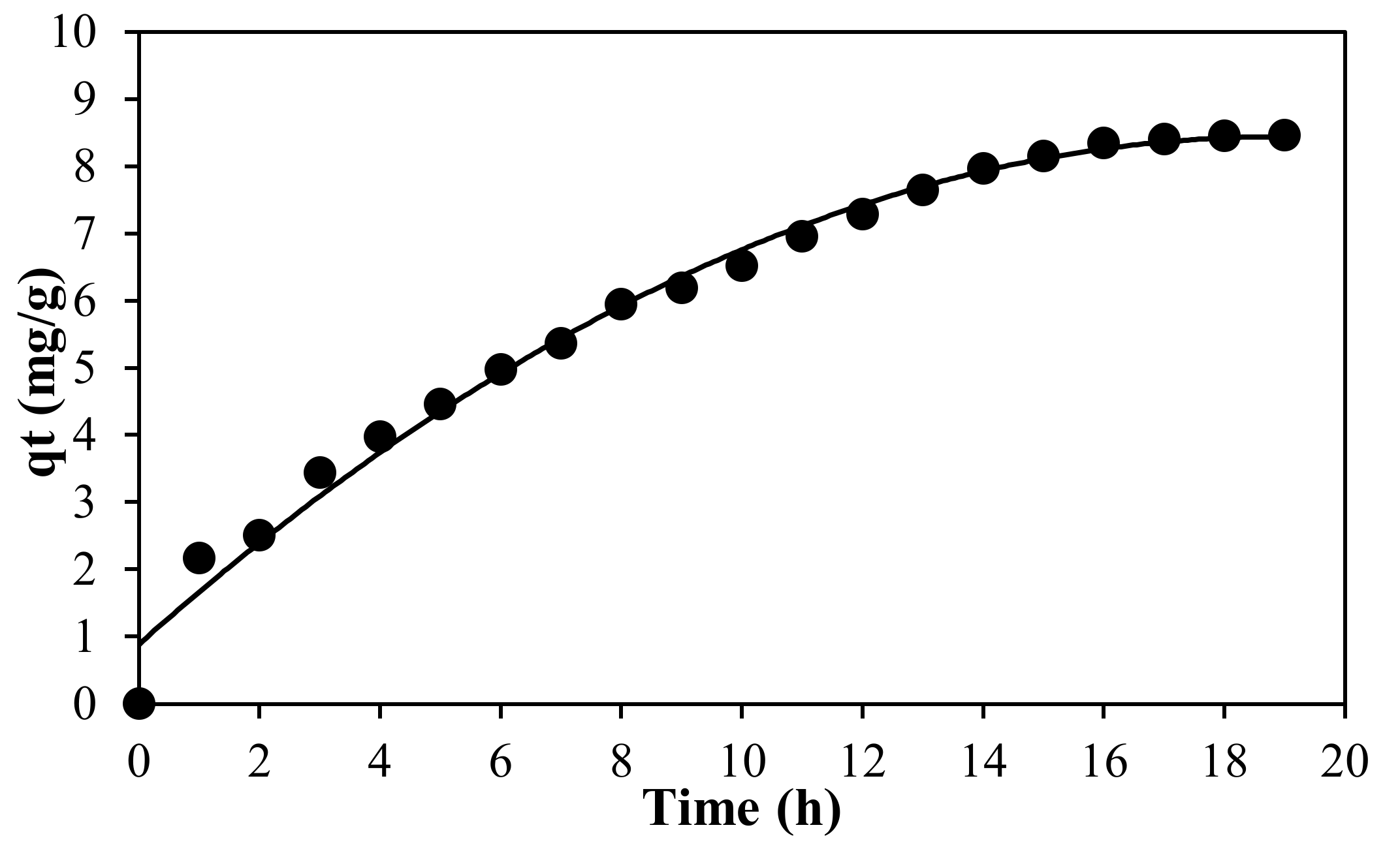
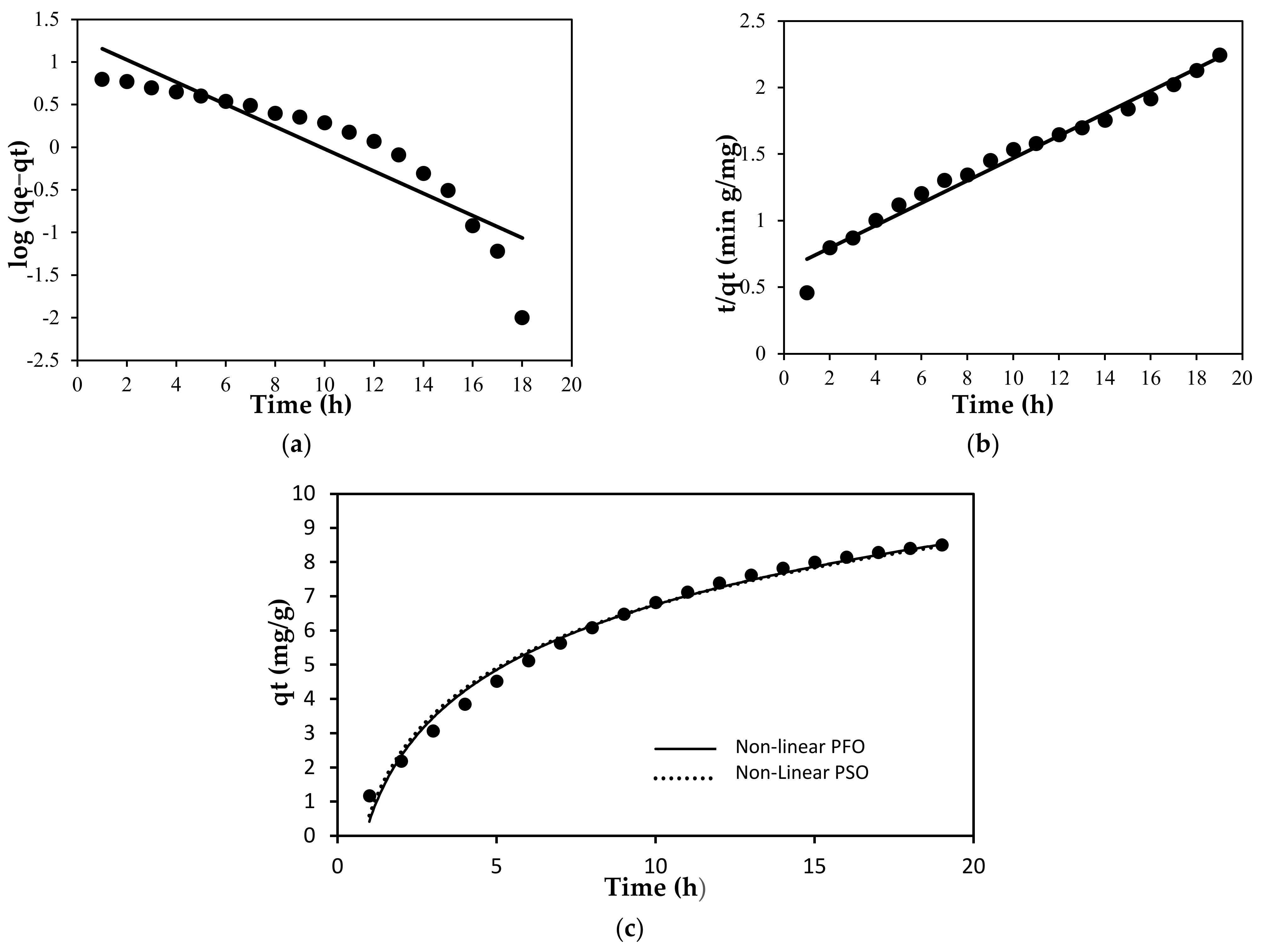
2.4. Adsorption Studies
Each part of the designed experiment was carried out in 50 mL of MB solution (using distilled water, pH = 6.65) before being left at room temperature for 24 h. The equilibrium concentration of MB solution was measured using UV-Visible Spectrometer (Shimadzu Europe/UV-1650 PC, Shimadzu Europe, Duisburg, Germany) at the λ
max value at 664 nm. The amount of the MB adsorbed onto PLA-PEG and PLA-PEG/MgSiO
3 membranes was determined by the difference between the initial and equilibrium concentration of MB solution. The adsorption performance or removal percentage of MB on membranes was calculated using the following equation:
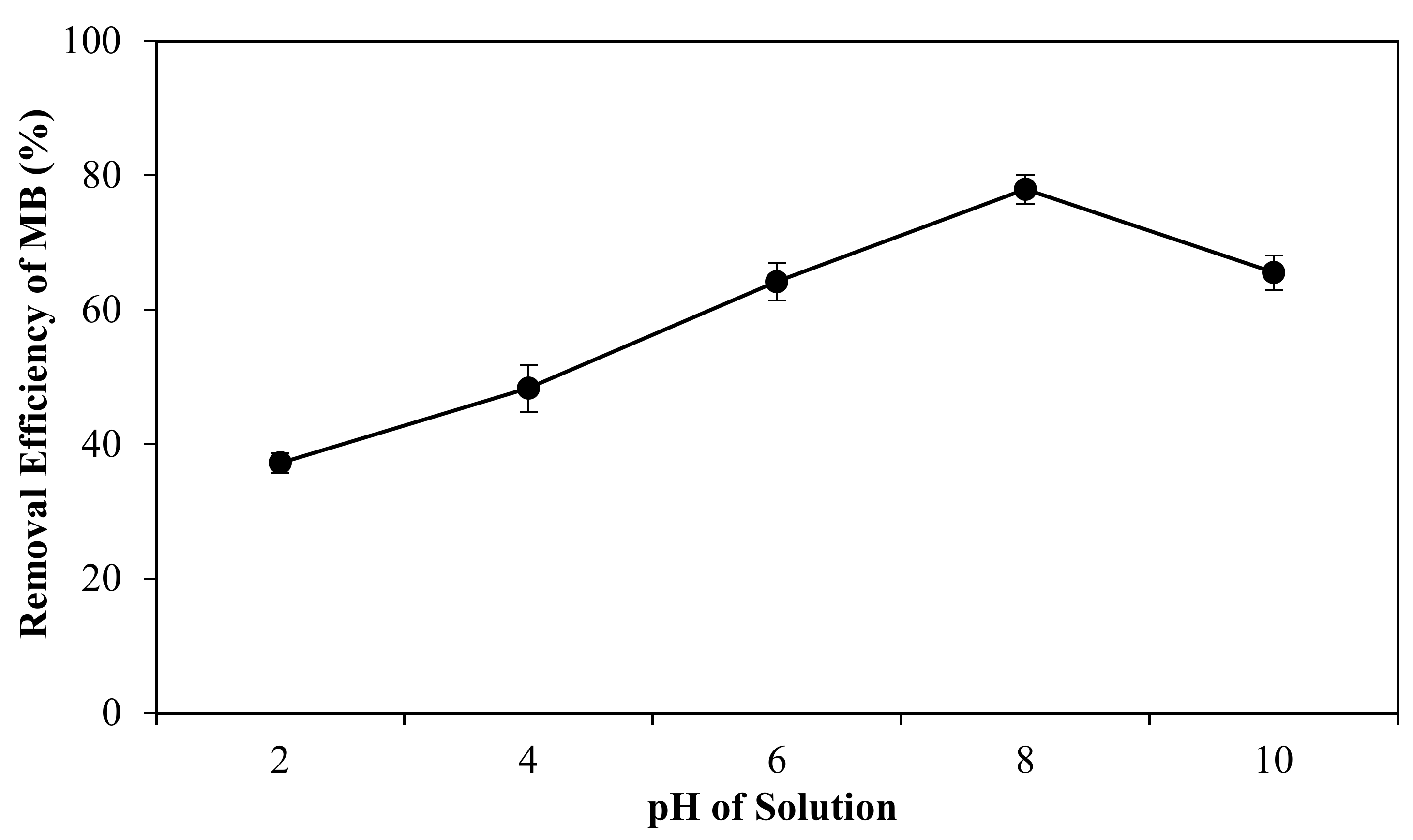
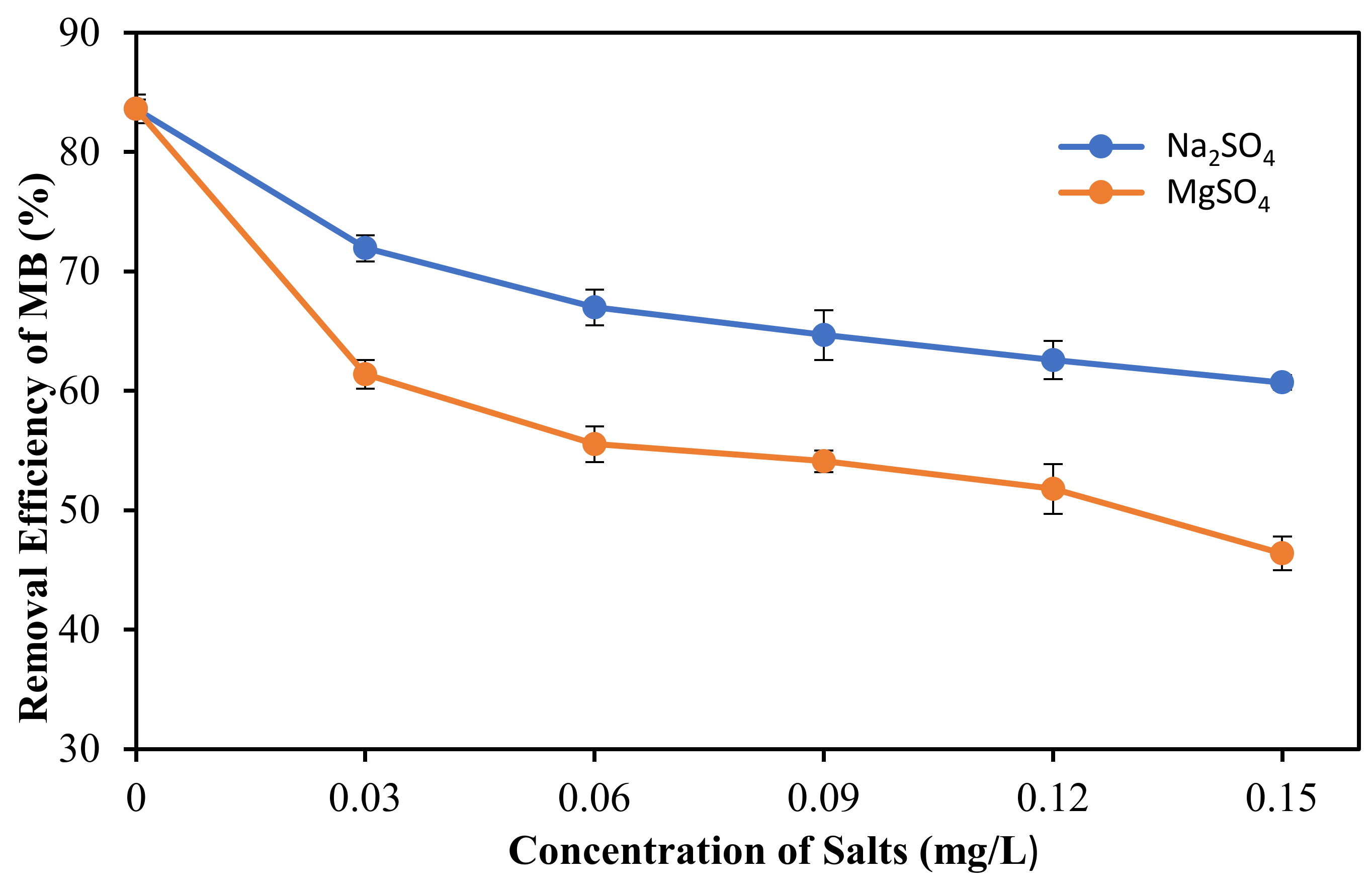
where Co and Ce are the initial and equilibrium concentrations of solutions (mg/L), respectively. The effect of pH solution and ionic strength on adsorption performance was also examined by a fixed amount of 3 mg of porous film and 5.5 ppm of initial concentration of MB under the same conditions and the percentage removal was also determined by using Equation (1).
2.5. Response Surface Methodology
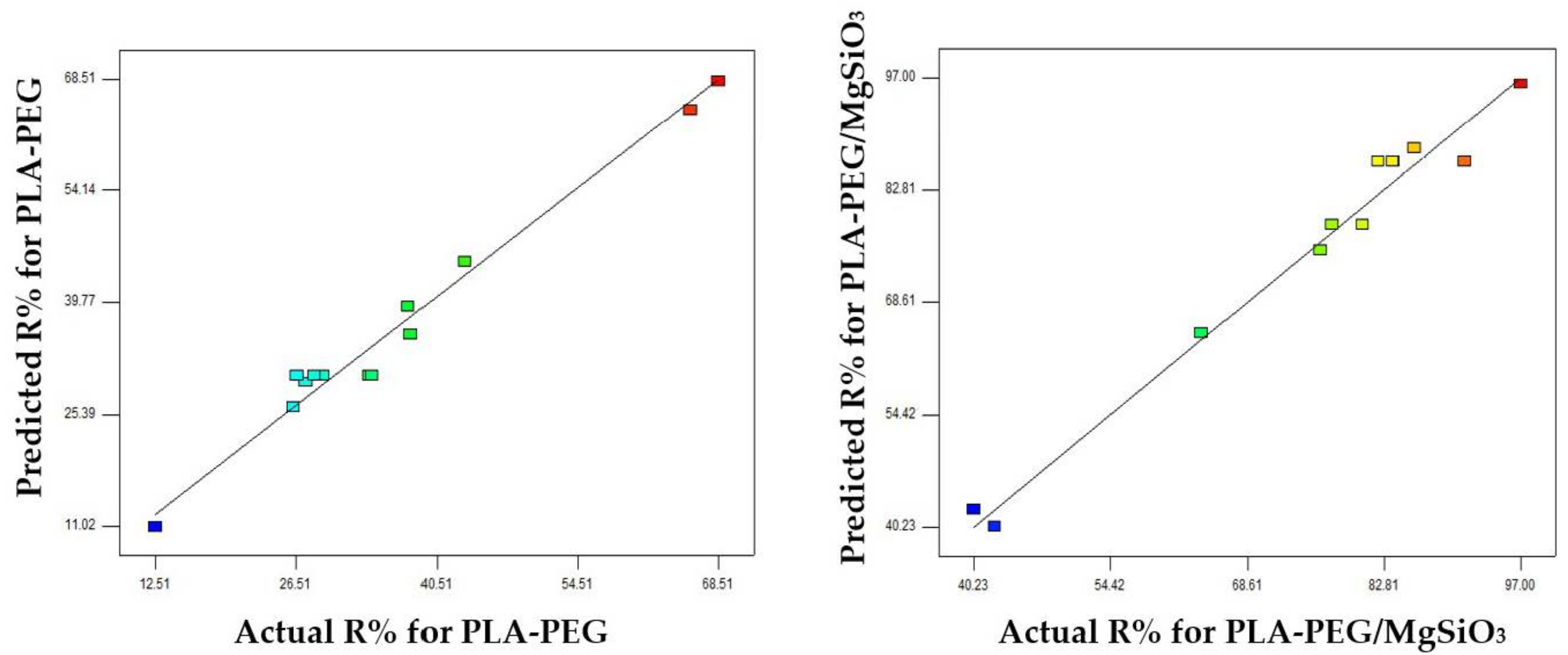
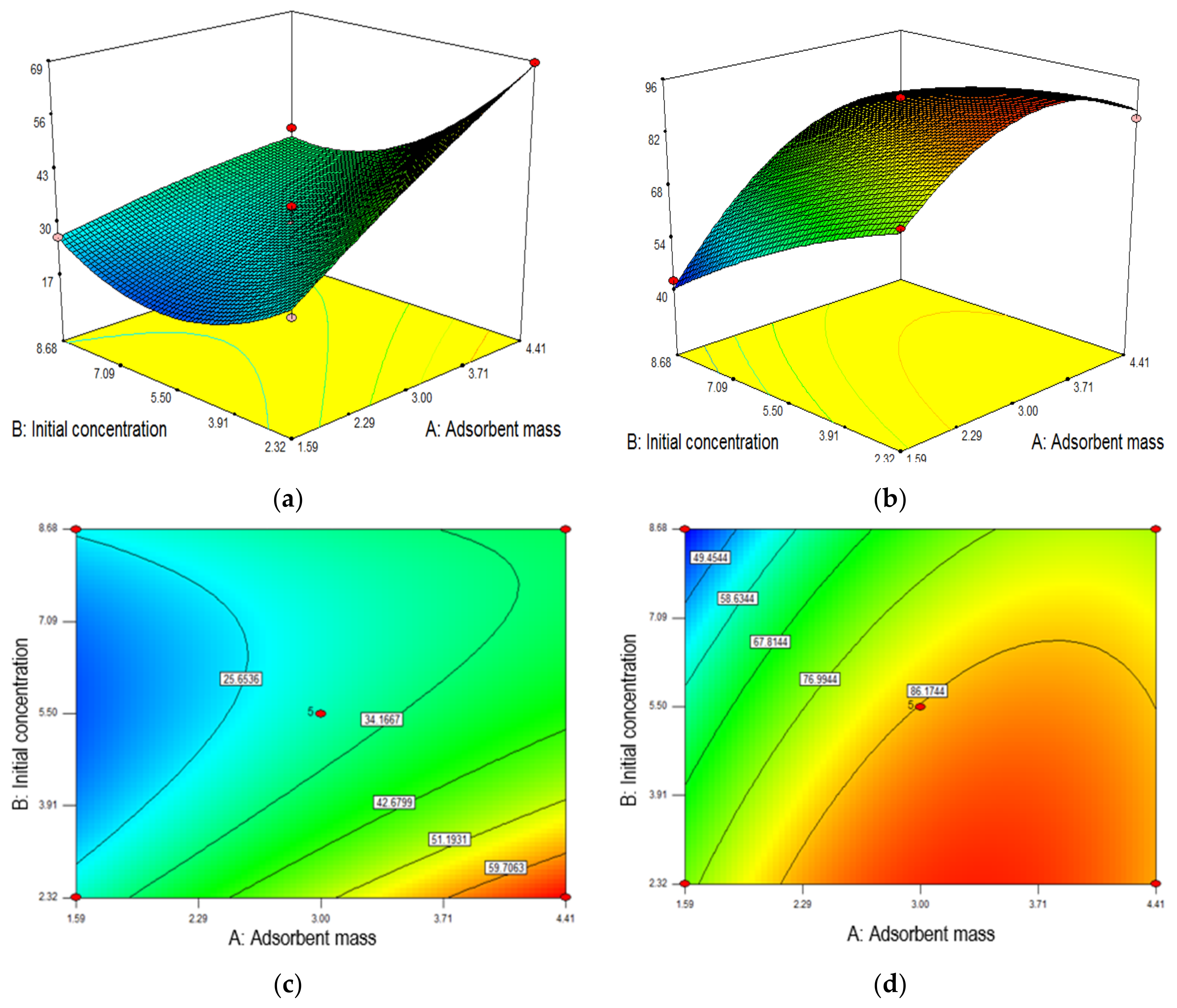
Adsorption performance was evaluated by 13 batch experiments designed by central composite design (CCRD) of RSM using Design Expert 7.15 software (Stat Ease Inc., Minneapolis, MN, USA). It is to investigate the effect of mass of membranes (1–5 mg) and initial concentration of methylene blue solution (1–10 ppm). All parameters in CCD were studied in five levels as shown in
Table 1 which includes coded –α, −1, 0, +1 and +α. The level coded of α was made by the choice to enable the rotatable design of CCRD. Moreover, CCD design is useful in predicting the linear and quadratic interaction effect for the parameters. It is also said to be more accurate in determining the optimum condition over other factorial designs because of the axial central point data. By using 2 parameters, one set of 13 experimental tests were given with 2
2 factorial points, the 4 axial points, and the 5 central points which can be expressed as follows:
where k is the number of factors, and C
o is the number of central points [
25]. Data from these 5 central points are used to determine the reliability of the data and calculate the experimental error [
26]. In addition, this model is further improved as compared to Box–Behnken model due to more data points used for optimization calculation.
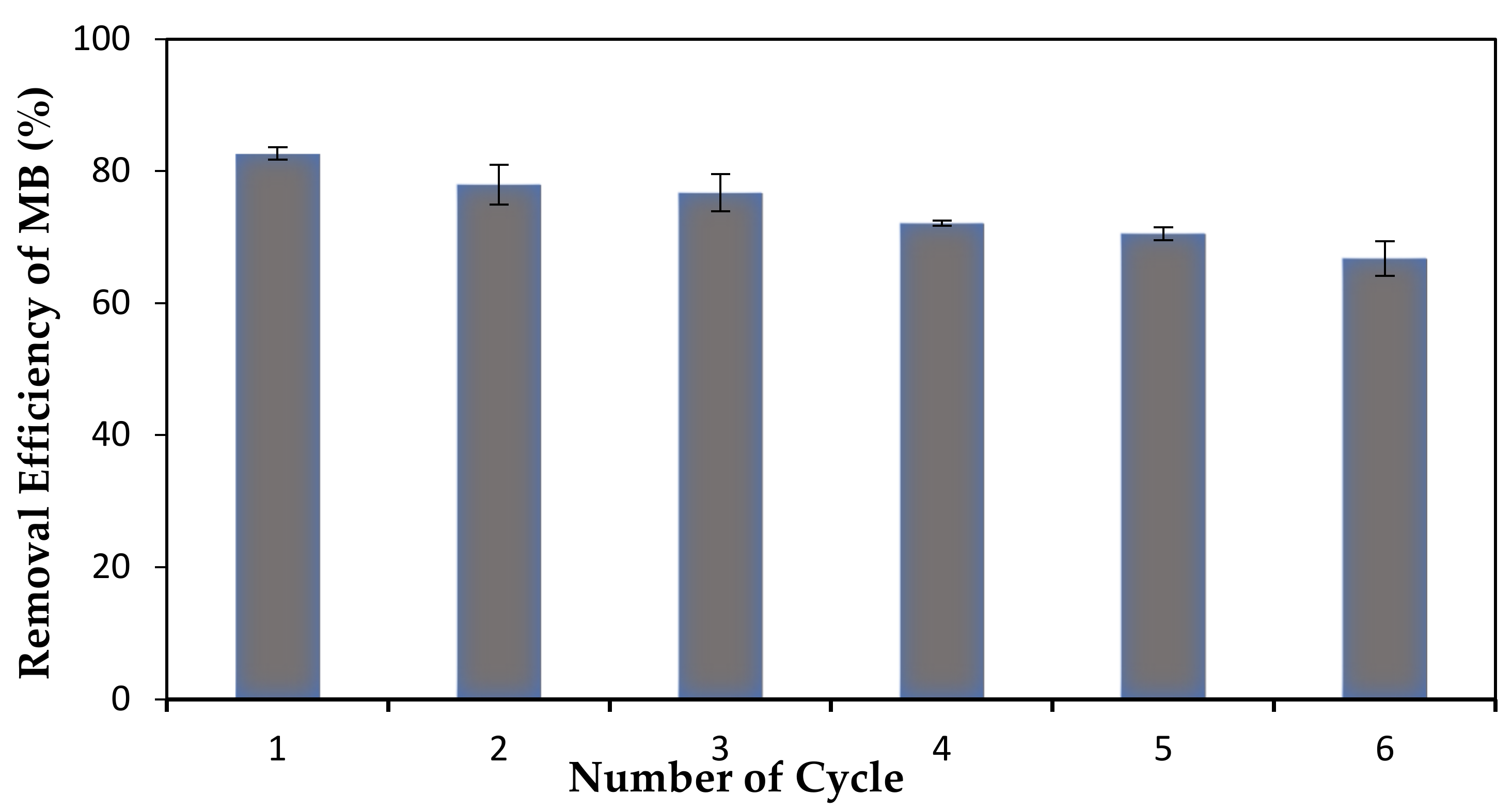
2.6. Reusability and Regeneration Study
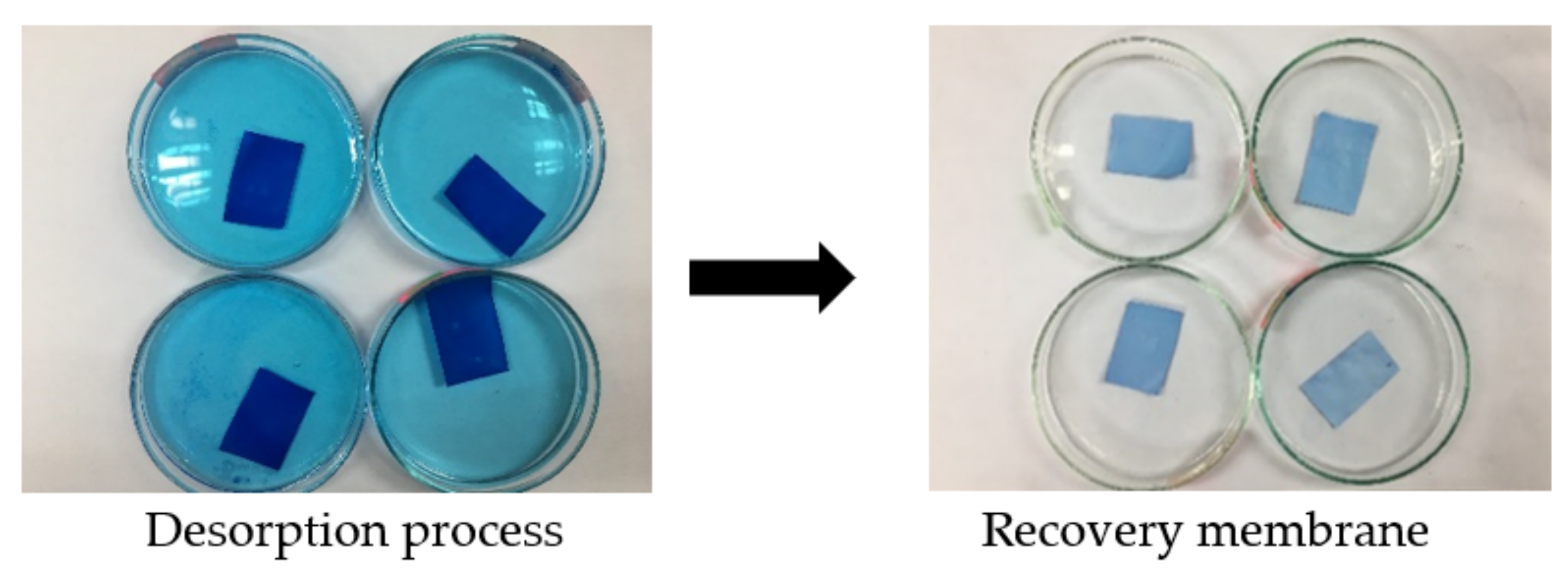
The reusability and regeneration study was carried out by using methanol as desorbing agent through adsorption-desorption method (
Figure 1). After the adsorption process took place, MB-loaded membrane was dried in the desiccator for 24 h. Then, the membrane was immersed in 50 mL of methanol solution for another 48 h. This cycle was repeated upon membrane recovery. After the required time of the experiments, the amount of dye adsorbed for every cycle was measured by using UV-Visible Spectrometer.