Effects of Processing Conditions and Plasticizing-Reinforcing Modification on the Crystallization and Physical Properties of PLA Films
Abstract
:1. Introduction
2. Experimental
2.1. Materials
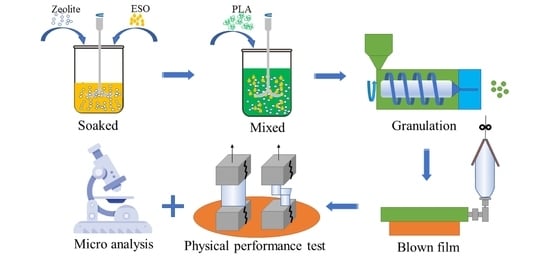
2.2. Manufacturing Methods
2.3. Performance Testing and Characterization Methods
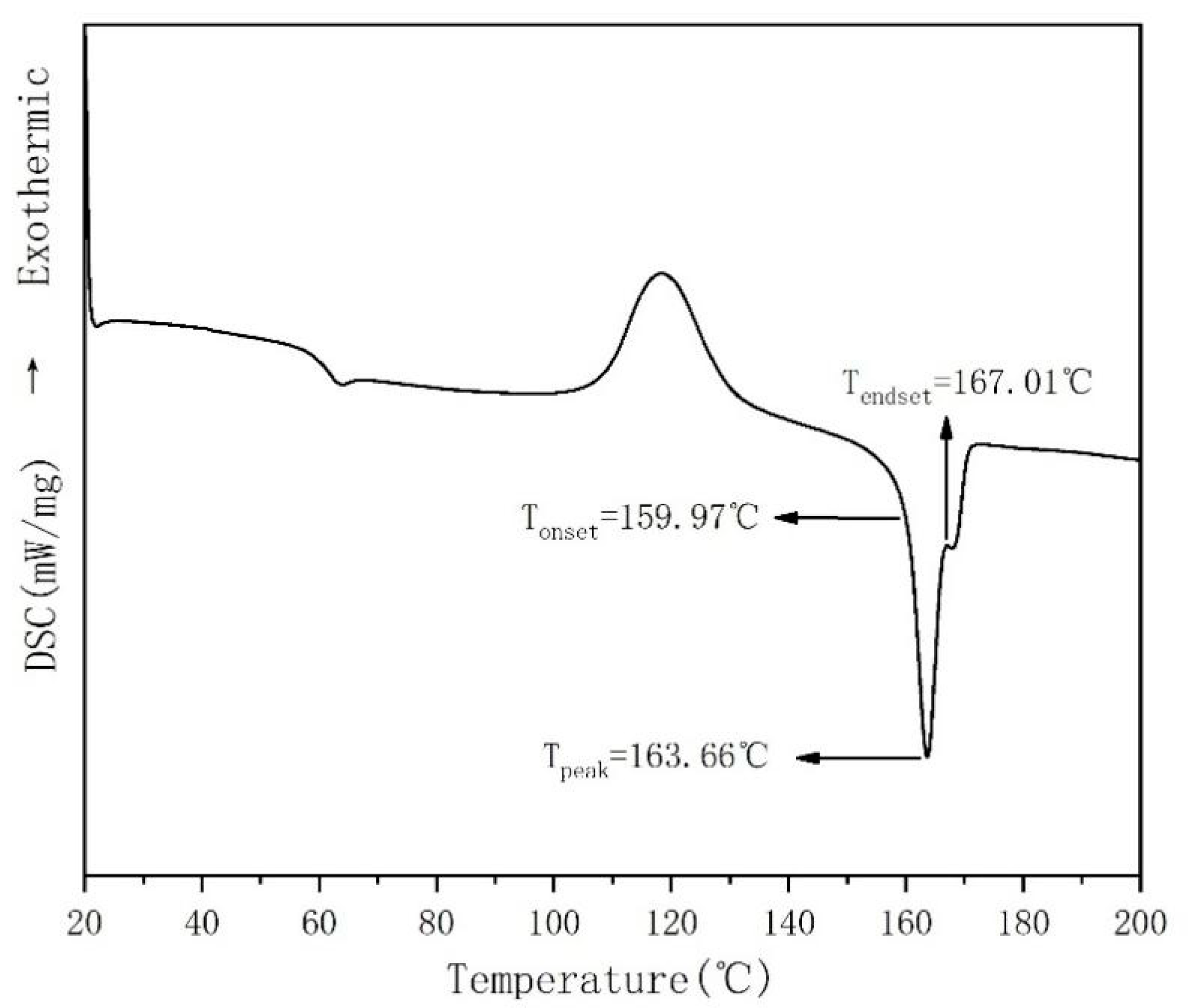
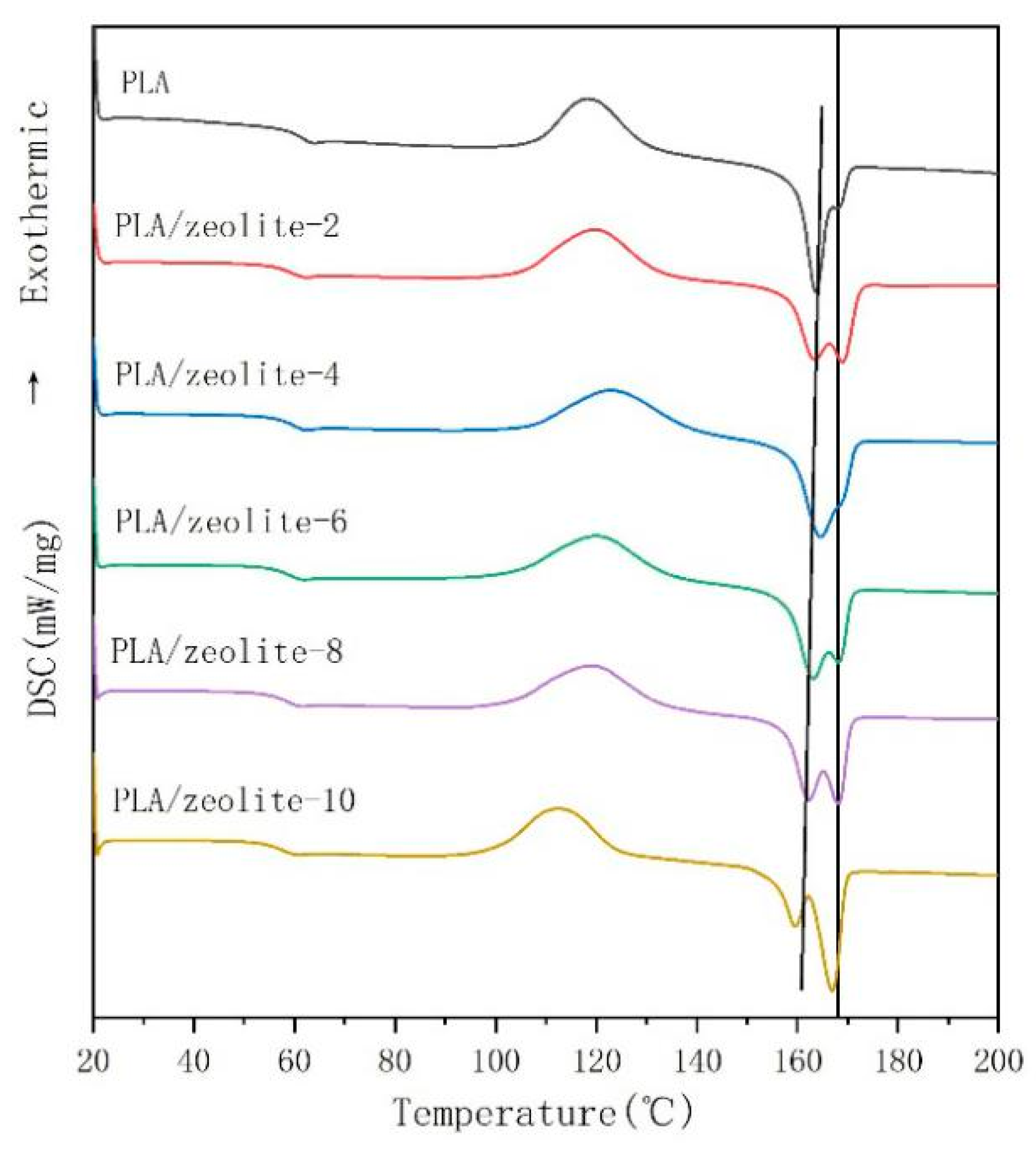
2.3.1. Analysis of Differential Scanning Calorimetry (DSC)
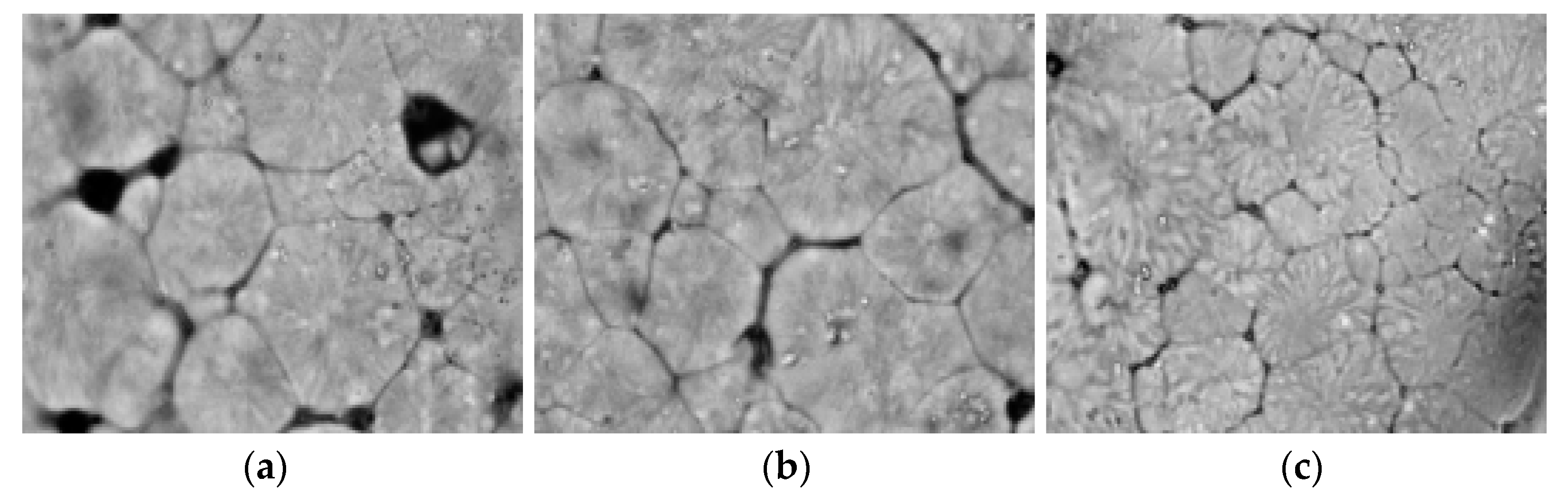
2.3.2. Analysis of Polarizing Microscopy
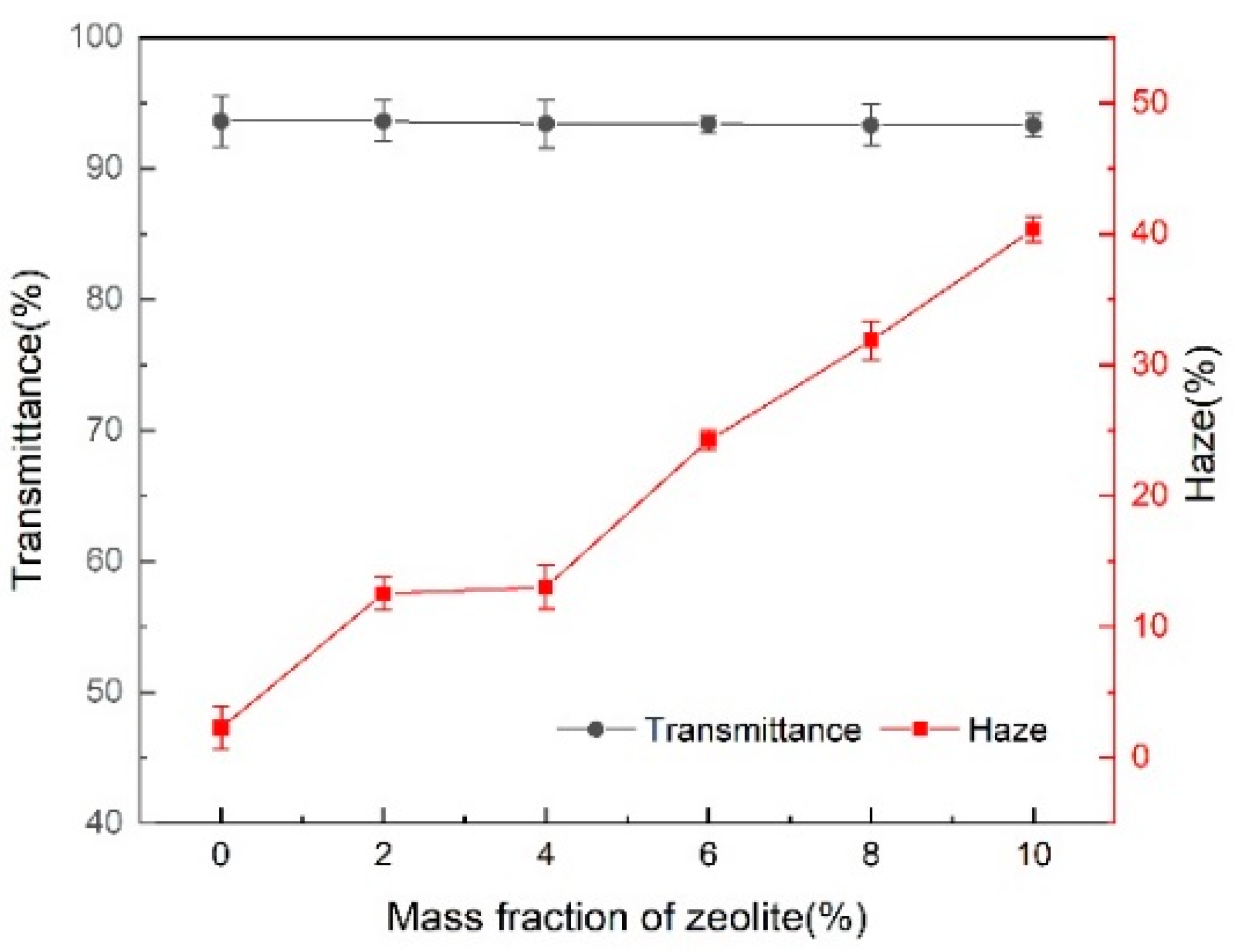
2.3.3. Transparency Test
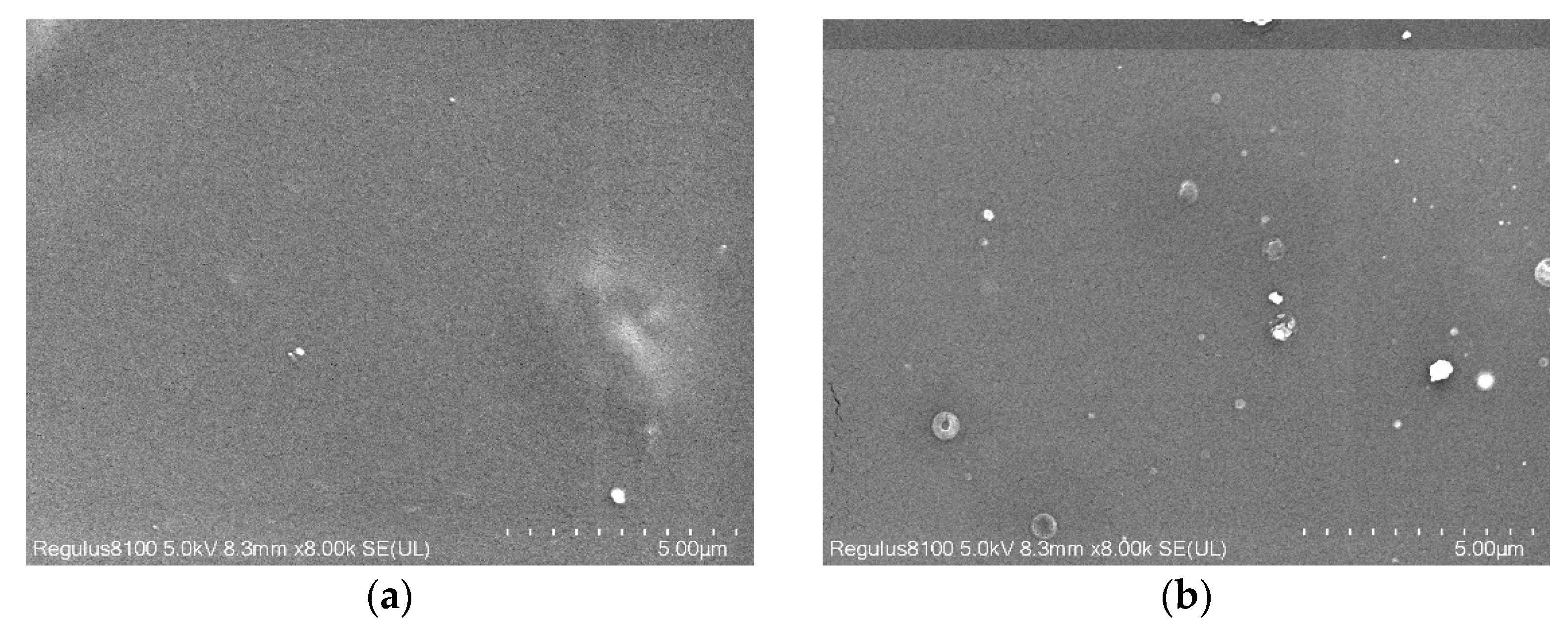
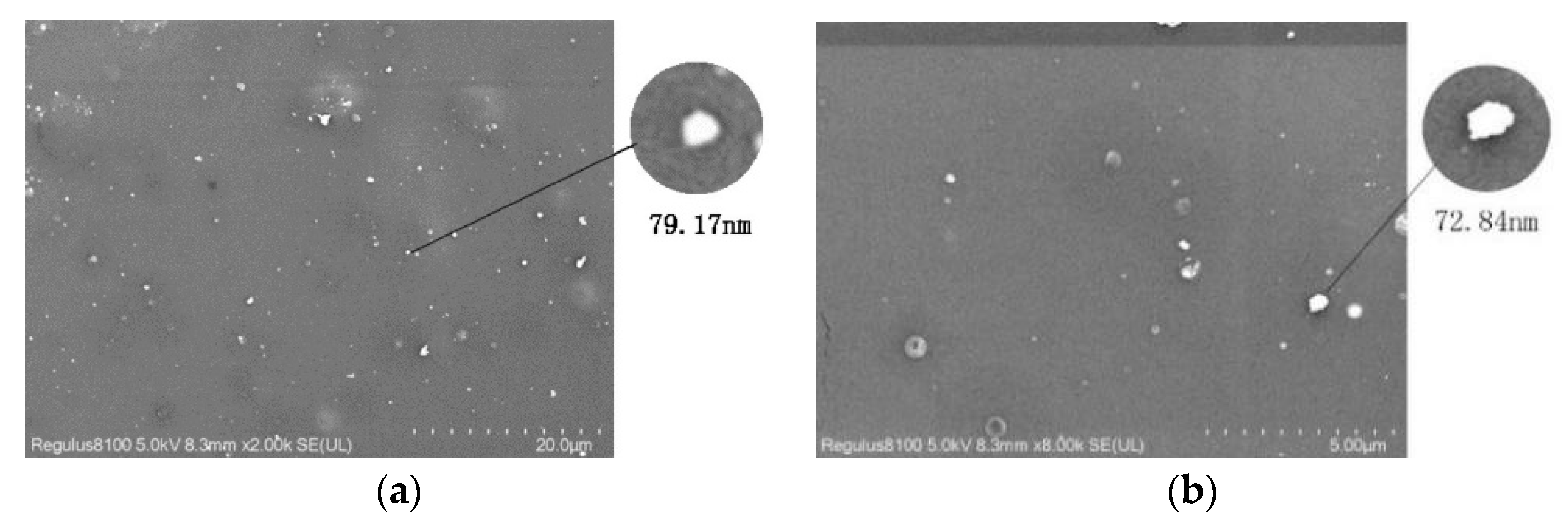
2.3.4. Analysis of Scanning Electron Microscopy (SEM)
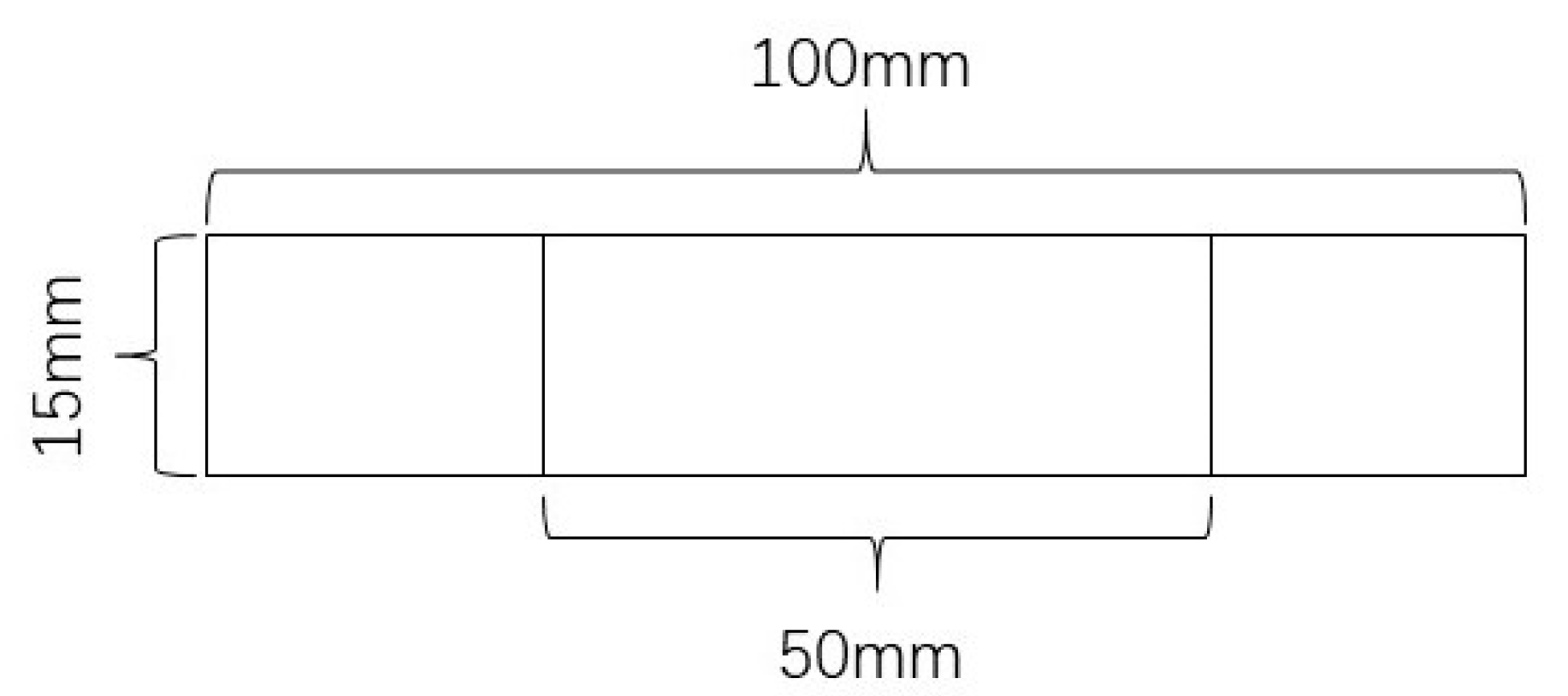
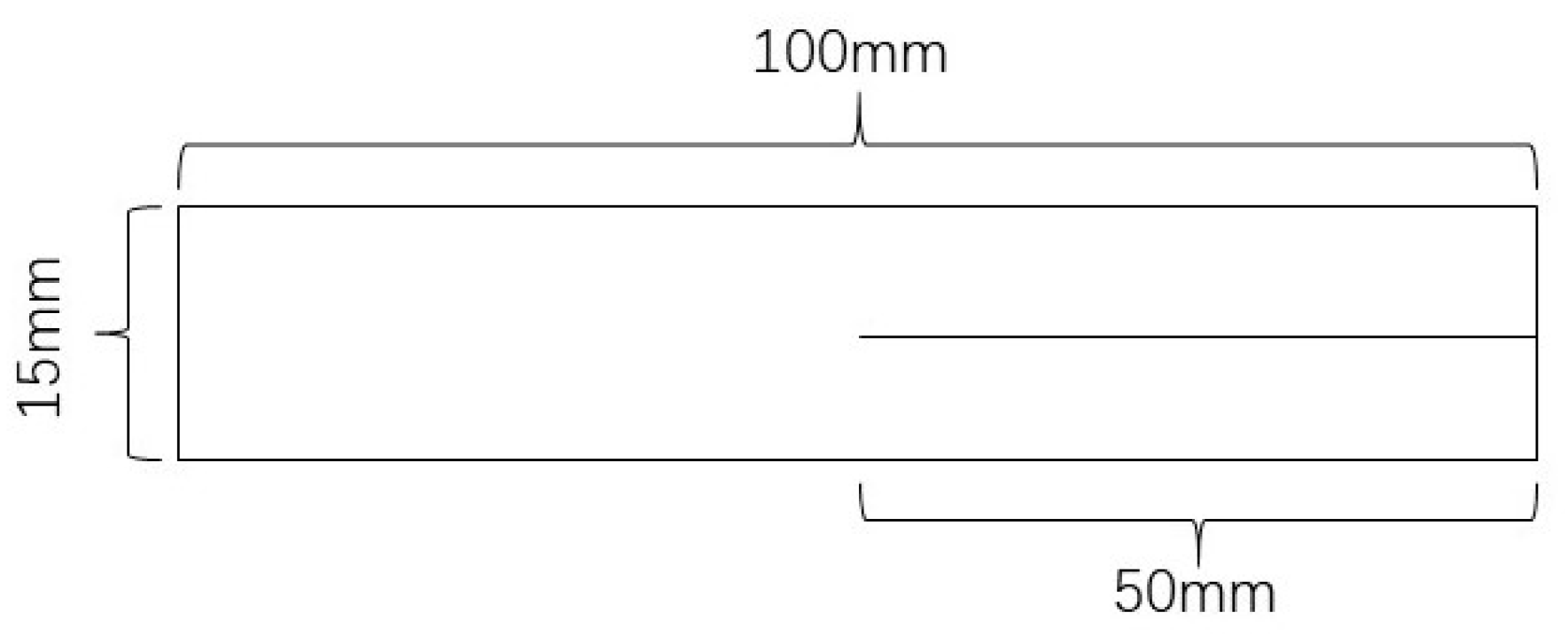
2.3.5. Film Mechanical Properties Test
3. Results and Discussion
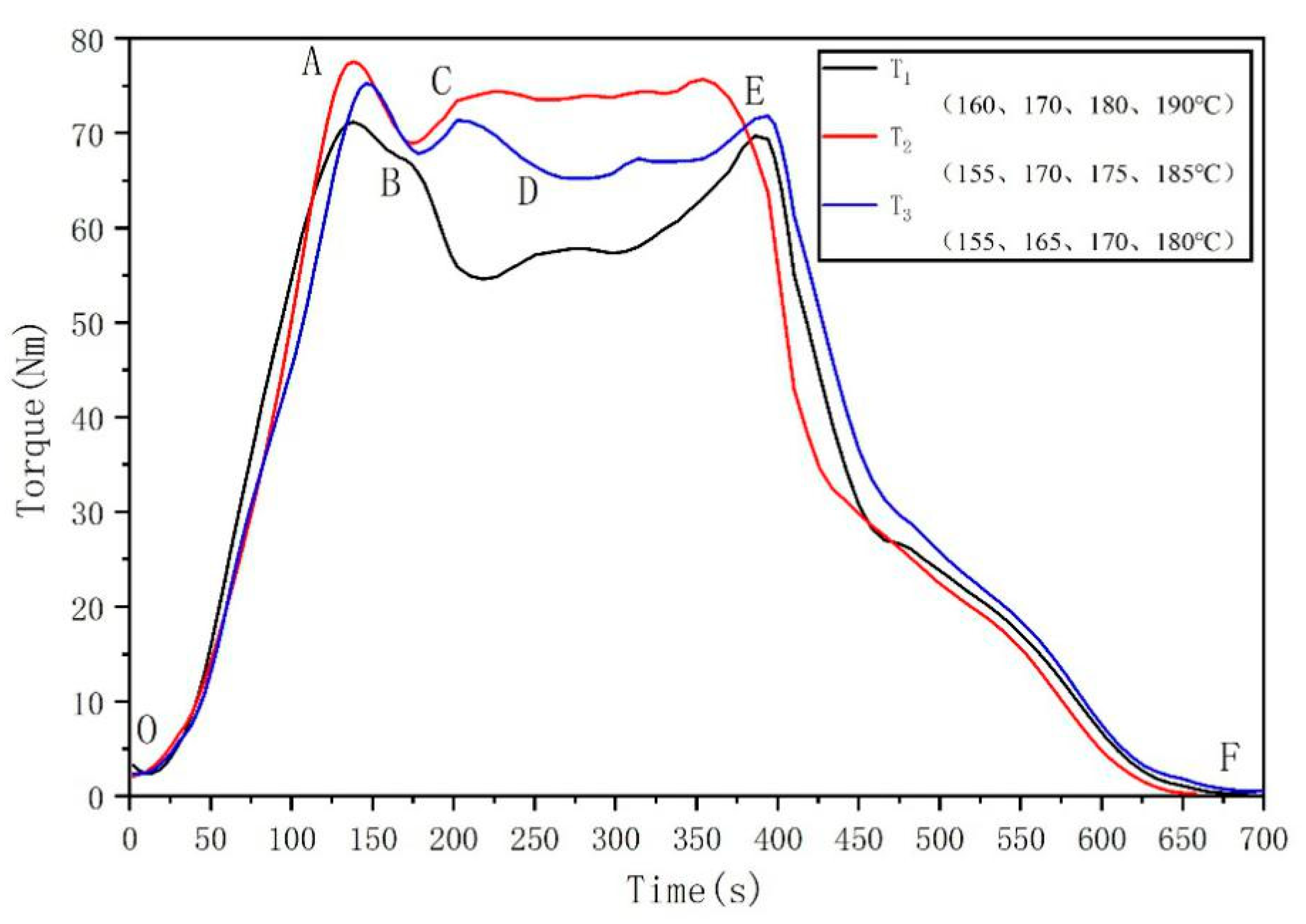
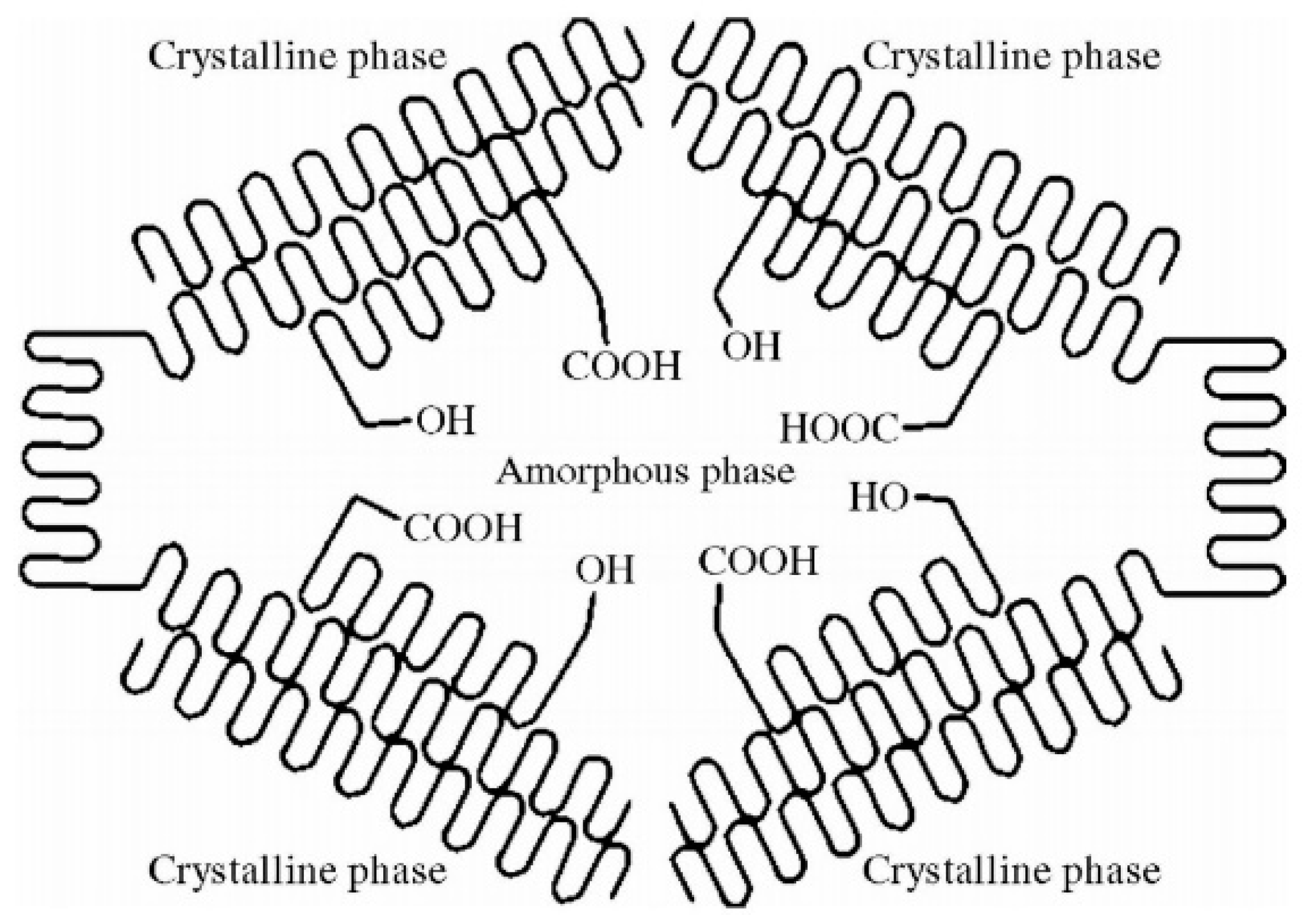
3.1. Thermodynamic Properties and Processing Conditions of Polylactic Acid Resin
3.2. Effect of Zeolite Addition on the Crystallinity and Transparency of the PLA Film
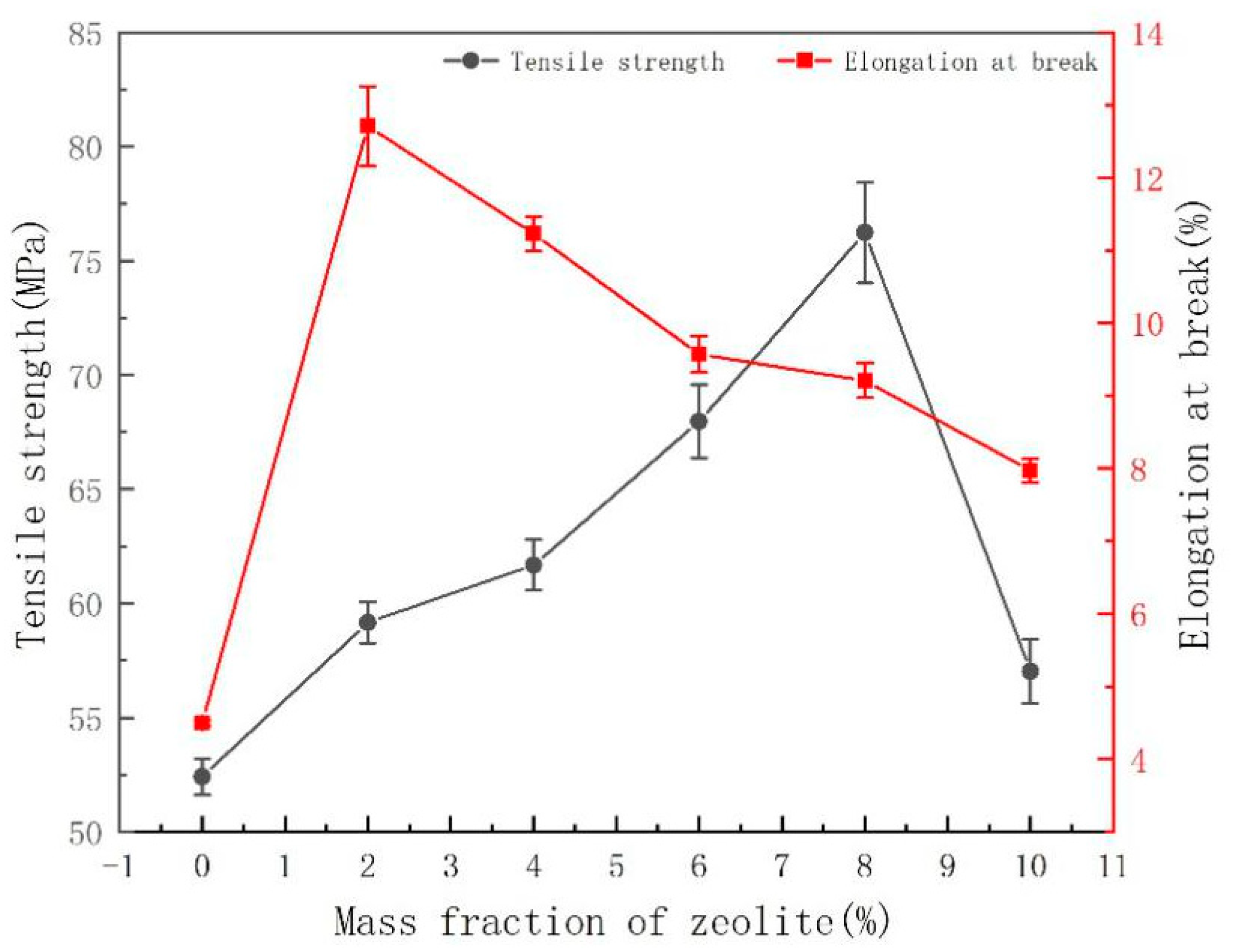
3.3. Effect of ESO and Zeolite Addition on the Structure and Physical Properties of PLA Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Y.; Shi, J.; Mao, L.; Wang, Z.; Zhang, L. Improvement of Compatibility and Mechanical Performances of PLA/PBAT Composites with Epoxidized Soybean Oil as Compatibilizer. Ind. Eng. Chem. Res. 2020, 59, 21779–21790. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Chaiwong, C.; Rachtanapun, P.; Wongchaiya, P.; Auras, R.; Boonyawan, D. Effect of plasma treatment on hydrophobicity and barrier property of polylactic acid. Surf. Coat. Technol. 2010, 204, 2933–2939. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Gross, R.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vink, E.; Davies, S. Life Cycle Inventory and Impact Assessment Data for 2014 IngeoTM Polylactide Production. Ind. Biotechnol. 2015, 11, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Gorrasi, G.; Vittoria, V.; Murariu, M.; Ferreira, A.D.S.; Alexandre, M.; Dubois, P. Effect of Filler Content and Size on Transport Properties of Water Vapor in PLA/Calcium Sulfate Composites. Biomacromolecules 2008, 9, 984–990. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Fischer, W.; Epple, M. Controlled Release of Goserelin from Microporous Polyglycolide and Polylactide. Macromol. Biosci. 2005, 5, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Zhang, K.; Misra, M.; Mohanty, A.K. Overcoming the Fundamental Challenges in Improving the Impact Strength and Crystallinity of PLA Biocomposites: Influence of Nucleating Agent and Mold Temperature. ACS Appl. Mater. Interfaces 2015, 7, 11203–11214. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I. Aliphatic Polyesters: Synthesis, Properties and Applications. In Degradable Aliphatic Polyesters; Springer: Berlin, Germany, 2001; Volume 157, pp. 1–40. [Google Scholar]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Bai, H.; Huang, C.; Xiu, H.; Zhang, Q.; Deng, H.; Wang, K.; Chen, F.; Fu, Q. Significantly Improving Oxygen Barrier Properties of Polylactide via Constructing Parallel-Aligned Shish-Kebab-Like Crystals with Well-Interlocked Boundaries. Biomacromolecules 2014, 15, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Keating, M.Y.; Gardner, K. Material Design in Poly(Lactic Acid) Systems: Block Copolymers, Star Homo and Copolymers, and Stereocomplexes. J. Macromol. Sci. Part A 1996, 33, 1497–1530. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Ouchi, T.; Ohya, Y. Design of Lactide Copolymers as Biomaterials. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 453–462. [Google Scholar] [CrossRef]
- Vainionpää, S.; Rokkanen, P.; Törmälä, P. Surgical applications of biodegradable polymers in human tissues. Prog. Polym. Sci. 1989, 14, 679–716. [Google Scholar] [CrossRef]
- Varma, I.K.; Albertsson, A.-C.; Rajkhowa, R.; Srivastava, R.K. Enzyme catalyzed synthesis of polyesters. Prog. Polym. Sci. 2005, 30, 949–981. [Google Scholar] [CrossRef]
- Gupta, A.; Katiyar, V. Cellulose Functionalized High Molecular Weight Stereocomplex Polylactic Acid Biocomposite Films with Improved Gas Barrier, Thermomechanical Properties. ACS Sustain. Chem. Eng. 2017, 5, 6835–6844. [Google Scholar] [CrossRef]
- Singh, A.A.; Genovese, M.E.; Mancini, G.; Marini, L.; Athanassiou, A. Green Processing Route for Polylactic Acid–Cellulose Fiber Biocomposites. ACS Sustain. Chem. Eng. 2020, 8, 4128–4136. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Szabo, P.; Daugaard, A.E.; Giacinti Baschetti, M. Effect of Crystallinity on Water Vapor Sorption, Diffusion, and Permeation of PLA-Based Nanocomposites. ACS Omega 2020, 5, 15362–15369. [Google Scholar] [CrossRef]
- Gigante, V.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rubber Toughening of Polylactic Acid (PLA) with Poly(butylene adipate-co-terephthalate) (PBAT): Mechanical Properties, Fracture Mechanics and Analysis of Ductile-to-Brittle Behavior while Varying Temperature and Test Speed. Eur. Polym. J. 2019, 115, 125–137. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Weng, Y.-X. Improvement of the Gas Barrier Properties of PLA/OMMT Films by Regulating the Interlayer Spacing of OMMT and the Crystallinity of PLA. ACS Omega 2020, 5, 18675–18684. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Yao, C.; Hongmei, W.; Wu, L. Progress in Modification of Poly(lactic acid) by Citrate Ester and Epoxy Soybean Oil Plasticizer. Eng. Plast. Appl. 2020, 48, 164–169. [Google Scholar]
- Urayama, H.; Moon, S.-I.; Kimura, Y. Microstructure and Thermal Properties of Polylactides with Different L- and D-Unit Sequences: Importance of the Helical Nature of the L-Sequenced Segments. Macromol. Mater. Eng. 2003, 288, 137–143. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Crystallization from the melt of poly(lactide)s with different optical purities and their blends. Macromol. Chem. Phys. 1996, 197, 3483–3499. [Google Scholar] [CrossRef]
- Dorgan, J.; Janzen, J.; Clayton, M.; Hait, S.; Knauss, D. Melt rheology of variable L-content poly(lactic acid). J. Rheol. 2005, 49, 607–619. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Li, Y.; Luo, C.; Yang, C.; Shi, W.; Li, L. Covalent Immobilization of Polypeptides on Polylactic Acid Films and Their Application to Fresh Beef Preservation. J. Agric. Food Chem. 2020, 68, 10532–10541. [Google Scholar] [CrossRef]
- Liu, M.; Lyu, S.; Peng, L.; Cai, L.; Huang, Z.; Lyu, J. Improvement of Toughness and Mechanical Properties of Furfurylated Wood by Biosourced Epoxidized Soybean Oil. ACS Sustain. Chem. Eng. 2021, 9, 8142–8155. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Tsubakihara, S.; Iura, K.; Ono, Y.; Dan, Y.; Takahashi, K. Crystallization behavior of poly(l-lactic acid). Polymer 2006, 47, 7554–7563. [Google Scholar] [CrossRef]
- Lin, L.; Deng, C.; Lin, G.-P.; Wang, Y.-Z. Super Toughened and High Heat-Resistant Poly(Lactic Acid) (PLA)-Based Blends by Enhancing Interfacial Bonding and PLA Phase Crystallization. Ind. Eng. Chem. Res. 2015, 54, 5643–5655. [Google Scholar] [CrossRef]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Arnoult, M.; Dargent, E.; Mano, J.F. Mobile amorphous phase fragility in semi-crystalline polymers: Comparison of PET and PLLA. Polymer 2007, 48, 1012–1019. [Google Scholar] [CrossRef]
- Takahashi, K.; Sawai, D.; Yokoyama, T.; Kanamoto, T.; Hyon, S.H. Crystal transformation from the a- to the b-form upon tensile drawing of poly(l-lactic acid). Polymer 2004, 45, 4969–4976. [Google Scholar] [CrossRef]
- Sawai, D.; Yokoyama, T.; Kanamoto, T.; Sungil, M.; Hyon, S.-H.; Myasnikova, L.P. Crystal Transformation and Development of Tensile Properties upon Drawing of Poly(L-lactic acid) by Solid-State Coextrusion: Effects of Molecular Weight. Macromol. Symp. 2006, 242, 93–103. [Google Scholar] [CrossRef]
- Eling, B.; Gogolewski, S.; Pennings, A.J. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer 1982, 23, 1587–1593. [Google Scholar] [CrossRef]
- Puiggali, J.; Ikada, Y.; Tsuji, H.; Cartier, L.; Okihara, T.; Lotz, B. The frustrated structure of poly(l-lactide). Polymer 2000, 41, 8921–8930. [Google Scholar] [CrossRef]
- Brant, D.A.; Tonelli, A.E.; Flory, P.J. The Configurational Statistics of Random Poly(lactic acid) Chains. II. Theory. Macromolecules 1969, 2, 228–235. [Google Scholar] [CrossRef]
- Leenslag, J.W.; Pennings, A.J. High-strength poly(l-lactide) fibres by a dry-spinning/hot-drawing process. Polymer 1987, 28, 1695–1702. [Google Scholar] [CrossRef]
- Sawai, D.; Takahashi, K.; Sasashige, A.; Kanamoto, T. Preparation of Oriented β-Form Poly(l-lactic acid) by Solid-State Coextrusion: Effect of Extrusion Variables. Macromolecules 2003, 36, 3601–3605. [Google Scholar] [CrossRef]
- Qiu-en, L. Research Progress of Biodegradable Materials and Analysis of Domestic and Foreign Industry Status. J. Hunan Packag. 2021, 36, 31–34. [Google Scholar]
- Chang-jiao, S.; Hai-xin, C.; Yan, W.; Zhang-hua, Z.; Xiang, Z.; Bo, C. Studies on Applications of Nanomaterial and Nanotechnology in Agriculture. J. Agric. Sci. Technol. 2016, 18, 18–25. [Google Scholar]

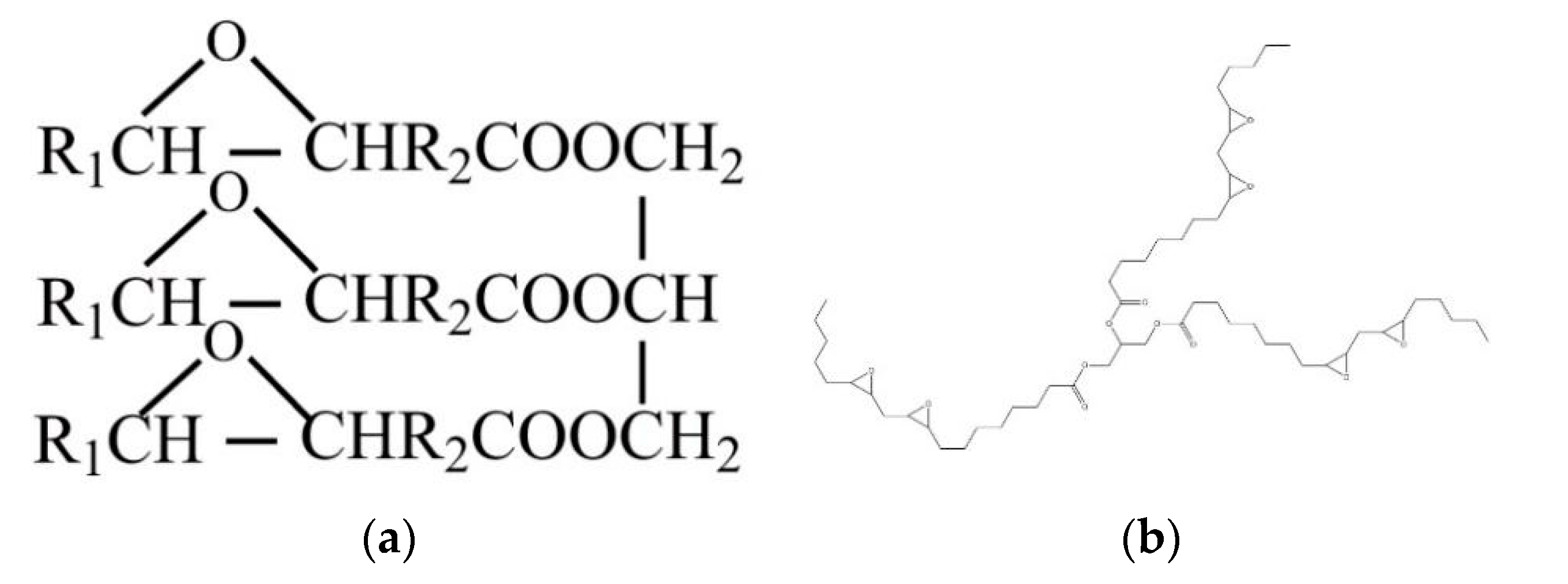

| Samples | Mass Fraction of PLA (wt%) | Mass Fraction of ESO (wt%) | Mass Fraction of Zeolite (wt%) |
|---|---|---|---|
| PLA resin | 100 | 0 | 0 |
| PLA/zeolite-2 | 95 | 3 | 2 |
| PLA/zeolite-4 | 93 | 3 | 4 |
| PLA/zeolite-6 | 91 | 3 | 6 |
| PLA/zeolite-8 | 89 | 3 | 8 |
| PLA/zeolite-10 | 87 | 3 | 10 |
| Samples | Tg (℃) | Tc (℃) | β-Tm (℃) | α-Tm (℃) | ∆T (℃) | β-Xc/% | α-Xc/% | Xc/% |
|---|---|---|---|---|---|---|---|---|
| PLA | 59.50 | 118.28 | 163.66 | 168.11 | 4.45 | 28.33 | 6.30 | 34.63 |
| PLA/zeolite-2 | 57.79 | 119.80 | 163.41 | 169.07 | 5.56 | 20.21 | 16.29 | 36.50 |
| PLA/zeolite-4 | 58.27 | 123.10 | 164.52 | 168.78 | 4.26 | 30.26 | 10.09 | 40.35 |
| PLA/zeolite-6 | 57.41 | 120.27 | 163.09 | 168.11 | 5.02 | 24.49 | 13.97 | 38.50 |
| PLA/zeolite-8 | 56.94 | 119.11 | 162.28 | 168.11 | 5.83 | 22.87 | 17.57 | 40.44 |
| PLA/zeolite-10 | 56.12 | 112.95 | 159.50 | 166.93 | 7.43 | 15.44 | 28.79 | 44.24 |
| Sample | Average Diameter/μm |
|---|---|
| PLA/zeolite-4 | 152.48 |
| PLA/zeolite-6 | 135.74 |
| PLA/zeolite-8 | 96.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, B.; Qin, Y.; Guo, H. Effects of Processing Conditions and Plasticizing-Reinforcing Modification on the Crystallization and Physical Properties of PLA Films. Membranes 2021, 11, 640. https://doi.org/10.3390/membranes11080640
Wang S, Liu B, Qin Y, Guo H. Effects of Processing Conditions and Plasticizing-Reinforcing Modification on the Crystallization and Physical Properties of PLA Films. Membranes. 2021; 11(8):640. https://doi.org/10.3390/membranes11080640
Chicago/Turabian StyleWang, Shuo, Baodong Liu, Yingying Qin, and Hongge Guo. 2021. "Effects of Processing Conditions and Plasticizing-Reinforcing Modification on the Crystallization and Physical Properties of PLA Films" Membranes 11, no. 8: 640. https://doi.org/10.3390/membranes11080640
APA StyleWang, S., Liu, B., Qin, Y., & Guo, H. (2021). Effects of Processing Conditions and Plasticizing-Reinforcing Modification on the Crystallization and Physical Properties of PLA Films. Membranes, 11(8), 640. https://doi.org/10.3390/membranes11080640