Combined Nanofiltration and Thermocatalysis for the Simultaneous Degradation of Micropollutants, Fouling Mitigation and Water Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cerium-Doped Strontium Ferrate Perovskite Synthesis
2.2. Membrane Fabrication
2.3. SEM
2.4. Effluent Sampling
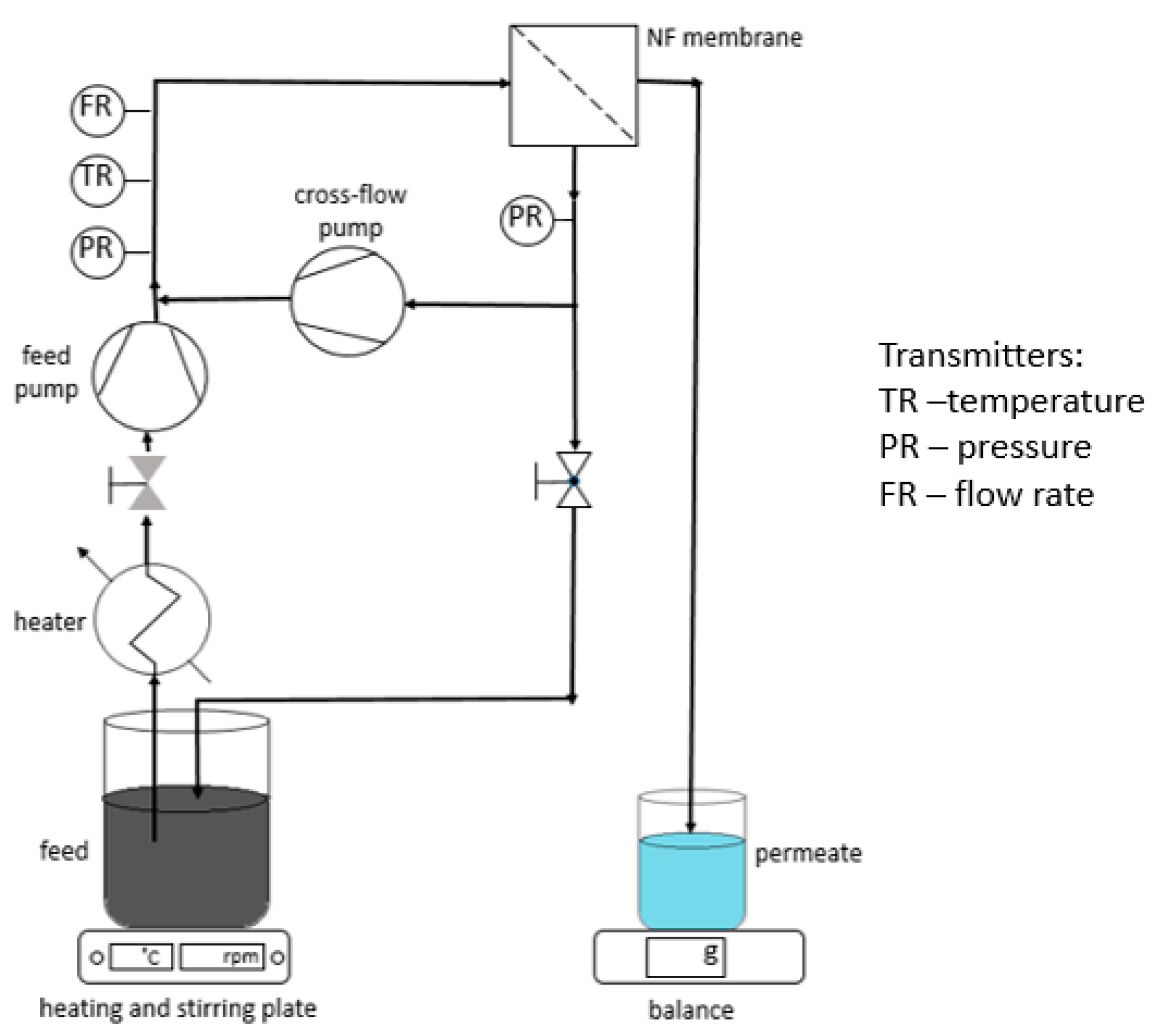
2.5. Nanofiltration Apparatus
2.6. COD Analysis
2.7. Determination of BPA Concentration in Water Samples
3. Results
3.1. SEM of Membrane
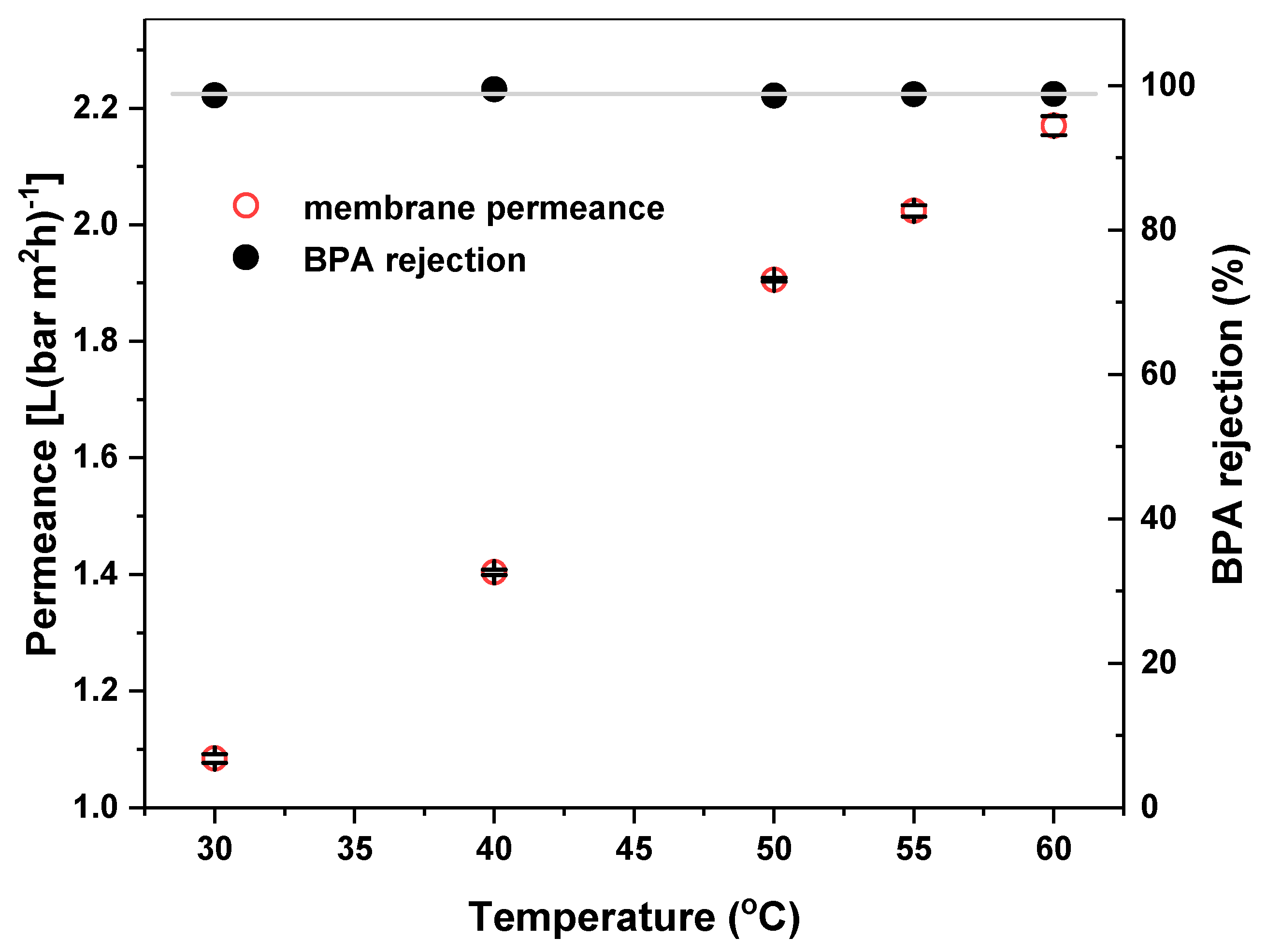
3.2. BPA Rejection
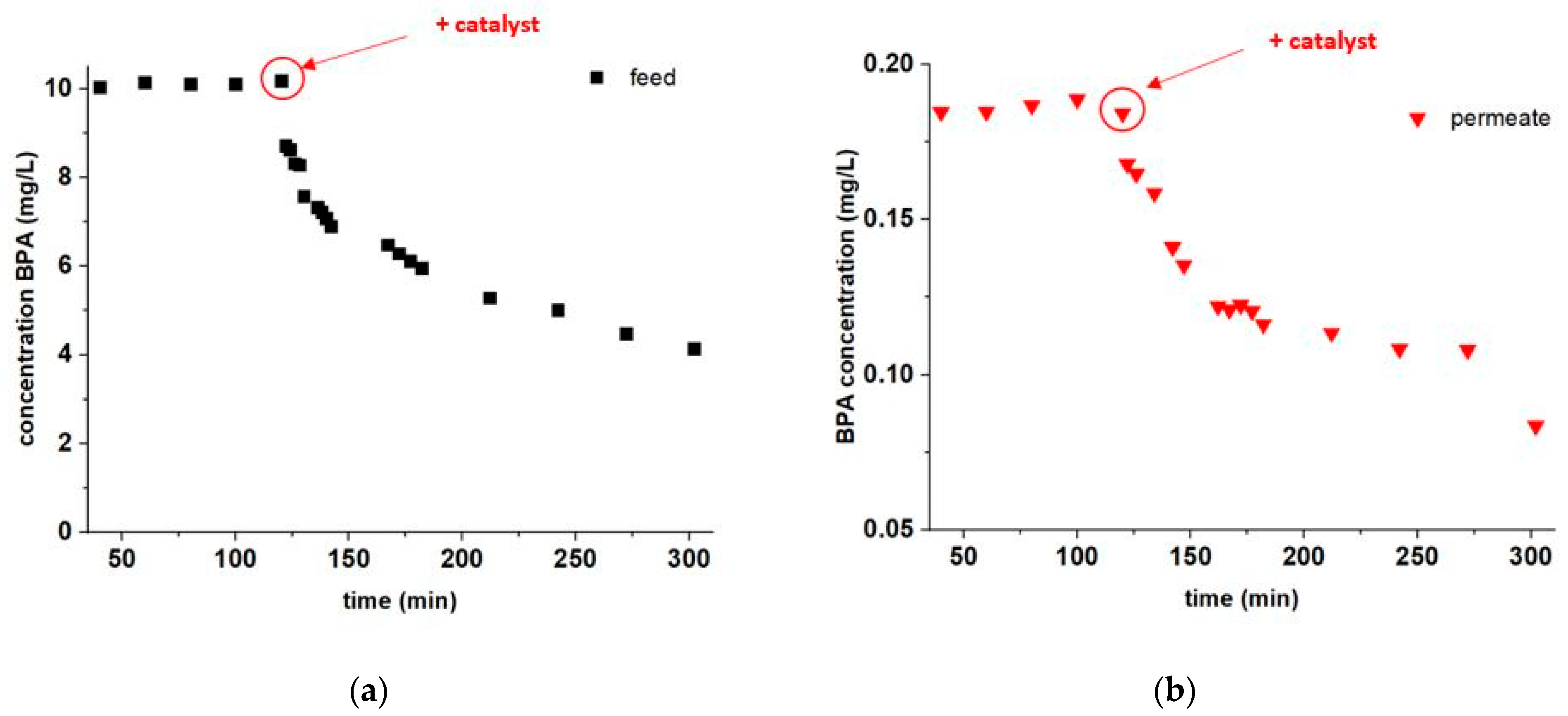
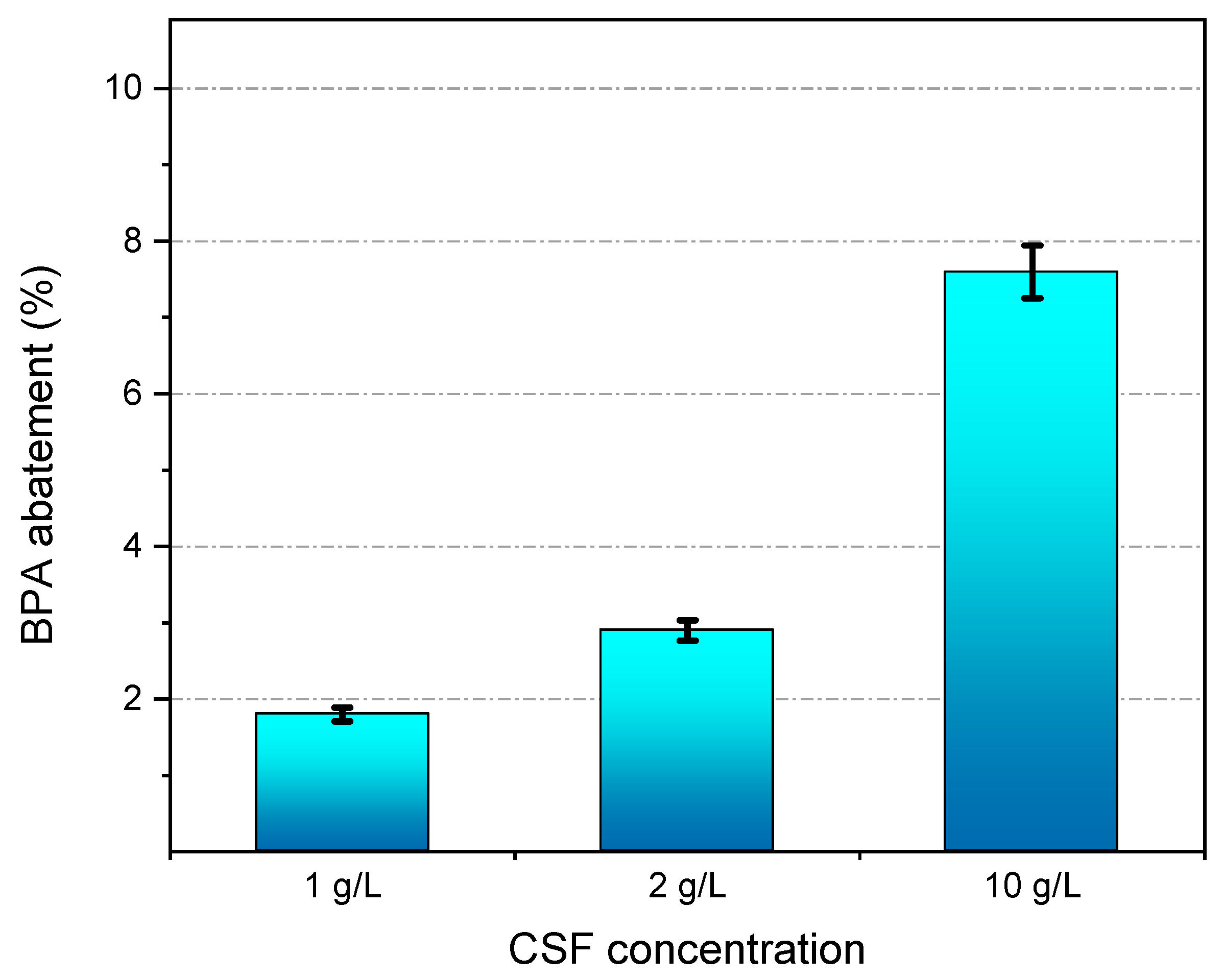
3.3. BPA Abatement during Filtration
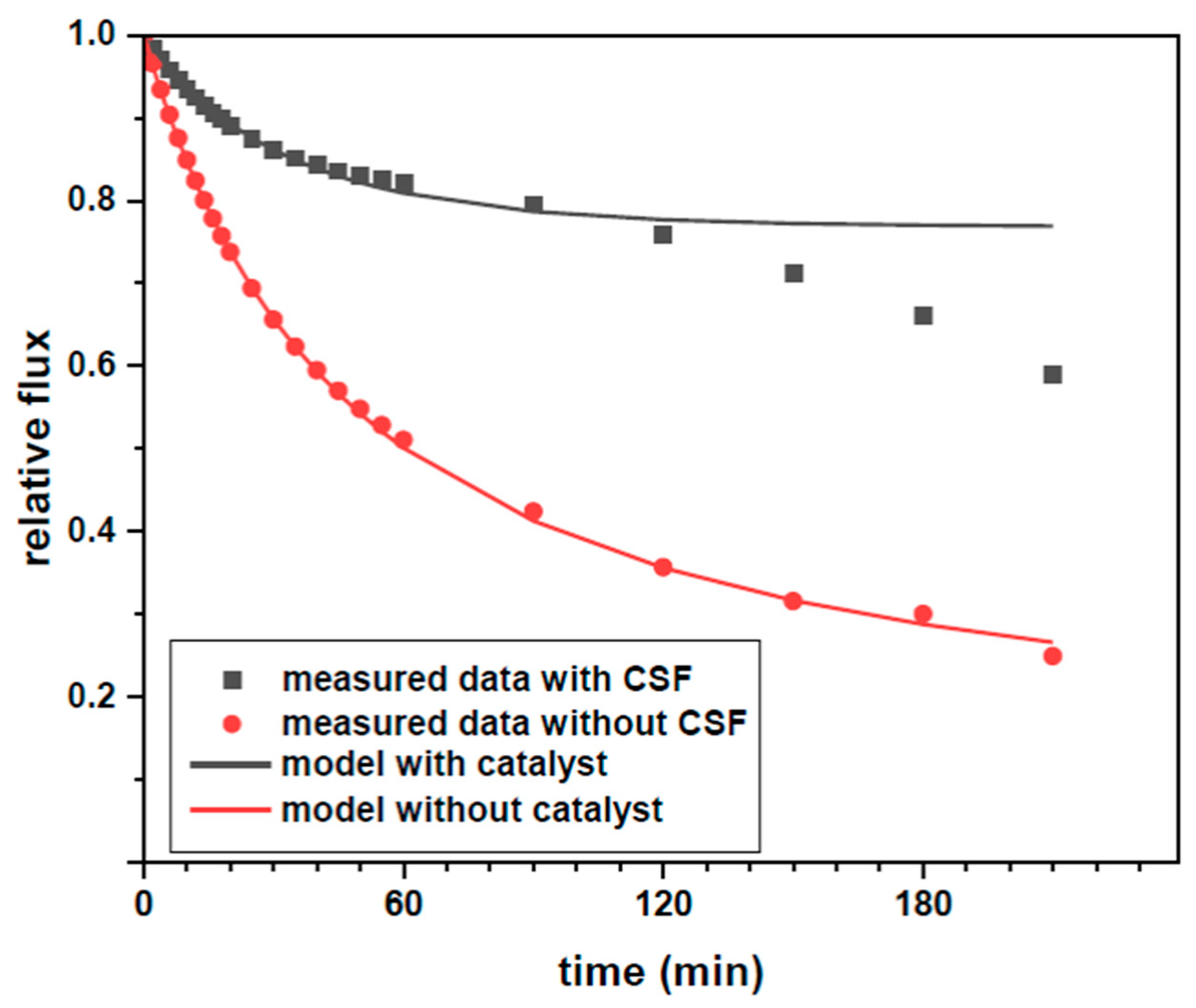
3.4. Fouling Mitigation
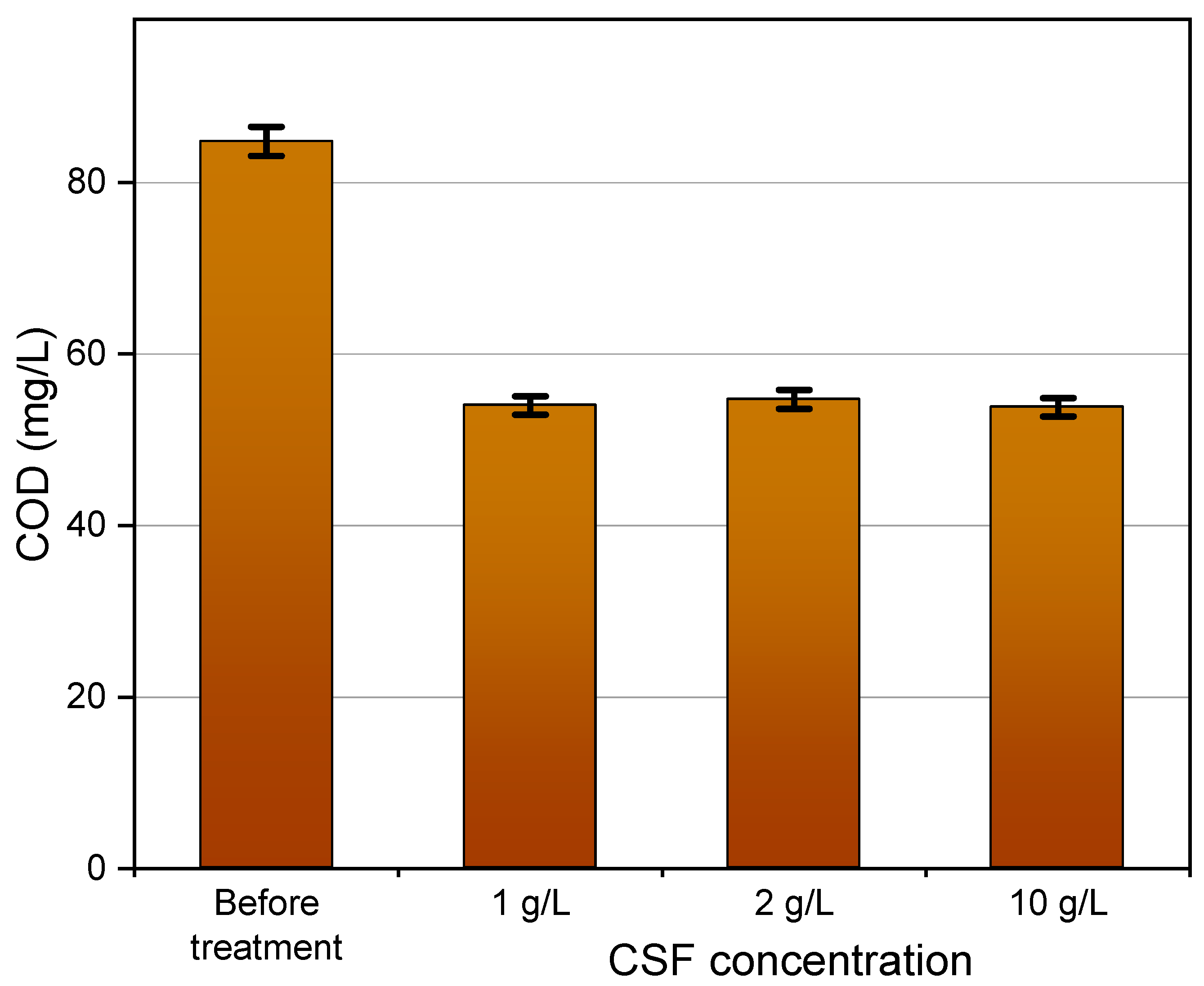
3.5. Thermocatalytic Treatment of the NF Concentrate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valbonesi, P.; Pro, M.; Vasumini, I.; Fabbri, E. Science of the Total Environment Contaminants of emerging concern in drinking water: Quality assessment by combining chemical and biological analysis. Sci. Total Environ. 2021, 758, 143624. [Google Scholar] [CrossRef] [PubMed]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ku, J.; Wang, L. Thermal catalysis under dark ambient conditions in environmental remediation: Fundamental principles, development, and challenges. Chin. J. Catal. 2019, 40, 1117–1134. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Anderson, M.G.; Mcdonnell, J.; Ximing, C.; Cline, S.A.; Balance, W.W.; Rockstrom, J.; Daily, G.C.; Ehrlich, P.R.; Reidy, C.A.; Dynesius, M.; et al. The Challenge of Micropollutants in Aquatic Systems. Science 2006, 313, 1072–1077. [Google Scholar]
- Xu, R.; Qin, W.; Tian, Z.; He, Y.; Wang, X.; Wen, X. Enhanced micropollutants removal by nanofiltration and their environmental risks in wastewater reclamation: A pilot-scale study. Sci. Total Environ. 2020, 744, 140954. [Google Scholar] [CrossRef] [PubMed]
- Escalona, I.; Fortuny, A.; Stüber, F.; Bengoa, C.; Fabregat, A.; Font, J. Fenton coupled with nanofiltration for elimination of Bisphenol A. Desalination 2014, 345, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef]
- Wang, P.; Wang, F.; Jiang, H.; Zhang, Y.; Zhao, M.; Xiong, R.; Ma, J. Strong improvement of nanofiltration performance on micropollutant removal and reduction of membrane fouling by hydrolyzed-aluminum nanoparticles. Water Res. 2020, 175, 115649. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Farsi, A.; Malvache, C.; de Bartolis, O.; Magnacca, G.; Kristensen, P.K.; Christensen, M.L.; Boffa, V. Design and fabrication of silica-based nanofiltration membranes for water desalination and detoxification. Microporous Mesoporous Mater. 2017, 237, 117–126. [Google Scholar] [CrossRef]
- García, N.; Purcell-milton, F.; Gun, Y.K. Recent progress and future prospects in development of advanced materials for nano fi ltration. Mater. Today Commun. 2020, 23, 100888. [Google Scholar] [CrossRef]
- Chon, K.; Cho, J. Fouling behavior of dissolved organic matter in nanofiltration membranes from a pilot-scale drinking water treatment plant: An autopsy study. Chem. Eng. J. 2016, 295, 268–277. [Google Scholar] [CrossRef]
- Ma, X.; Janowska, K.; Boffa, V.; Fabbri, D.; Magnacca, G.; Calza, P.; Yue, Y. Surfactant-assisted fabrication of alumina-doped amorphous silica nanofiltration membranes with enhanced water purification performances. Nanomaterials 2019, 9, 1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuru, T. Inorganic porous membranes for liquid phase separation. Sep. Purif. Methods 2001, 30, 191–220. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Zhang, H.; Zhang, Z.; Ding, J.; Lu, J. Comparing the performance of various nanofiltration membranes in advanced oxidation-nanofiltration treatment of reverse osmosis concentrates. Environ. Sci. Pollut. Res. 2019, 26, 17472–17481. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.E.J.I.N. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Arca-Ramos, A.; Eibes, G.; Feijoo, G.; Lema, J.M.; Moreira, M.T. Potentiality of a ceramic membrane reactor for the laccase-catalyzed removal of bisphenol A from secondary effluents. Appl. Microbiol. Biotechnol. 2015, 99, 9299–9308. [Google Scholar] [CrossRef]
- Zielińska, M.; Cydzik-Kwiatkowska, A.; Bułkowska, K.; Bernat, K.; Wojnowska-Baryła, I. Treatment of Bisphenol A-Containing Effluents from Aerobic Granular Sludge Reactors with the Use of Microfiltration and Ultrafiltration Ceramic Membranes. Water. Air Soil Pollut. 2017, 228, 282. [Google Scholar] [CrossRef] [Green Version]
- Janowska, K.; Boffa, V.; Jørgensen, M.K.; Quist-Jensen, C.A.; Hubac, F.; Deganello, F.; Coelho, F.E.B.; Magnacca, G. Thermocatalytic membrane distillation for clean water production. NPJ Clean Water 2020, 3, 1–7. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surfaces A Physicochem. Eng. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Deganello, F.; Tyagi, A.K. Solution combustion synthesis, energy and environment: Best parameters for better materials. Prog. Cryst. Growth Charact. Mater. 2018, 64, 23–61. [Google Scholar] [CrossRef]
- Farsi, A.; Boffa, V.; Qureshi, H.F.; Nijmeijer, A.; Winnubst, L.; Christensen, M.L. Modeling water flux and salt rejection of mesoporous γ-alumina and microporous organosilica membranes. J. Membr. Sci. 2014, 470, 307–315. [Google Scholar] [CrossRef]
- Tsuru, T.; Izumi, S.; Yoshioka, T.; Asaeda, M. Temperature Effect on Transport Performance by Inorganic Nanofiltration Membranes. AIChE J. 2000, 46, 565–574. [Google Scholar] [CrossRef]
- Mora, F.; Karla, P.; Quezada, C.; Herrera, C.; Cassano, A. Impact of Membrane Pore Size on the Clarification Performance of Grape Marc Extract by Microfiltration. Membranes 2019, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Pérez, J.A.S.; Sánchez-Moreno, R.; Malato, S. Is the combination of nanofiltration membranes and AOPs for removing microcontaminants cost effective in real municipal wastewater effluents? Environ. Sci. Water Res. Technol. 2016, 2, 511–520. [Google Scholar] [CrossRef]
- Oller, I.; Miralles-cuevas, S.; Agüera, A.; Malato, S. Monitoring and Removal of Organic Micro-pollutants by Combining Membrane Technologies with Advanced Oxidation Processes. Curr. Org. Chem. 2018, 22, 1103–1119. [Google Scholar] [CrossRef]

| Parameter | Unit | Value |
|---|---|---|
| pH (22.0 °C) | 7.48 ± 0.01 | |
| Conductivity (22.5 °C) | mS/cm | 1.15 ± 0.01 |
| COD | mg/L | 33.4 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowska, K.; Ma, X.; Boffa, V.; Jørgensen, M.K.; Candelario, V.M. Combined Nanofiltration and Thermocatalysis for the Simultaneous Degradation of Micropollutants, Fouling Mitigation and Water Purification. Membranes 2021, 11, 639. https://doi.org/10.3390/membranes11080639
Janowska K, Ma X, Boffa V, Jørgensen MK, Candelario VM. Combined Nanofiltration and Thermocatalysis for the Simultaneous Degradation of Micropollutants, Fouling Mitigation and Water Purification. Membranes. 2021; 11(8):639. https://doi.org/10.3390/membranes11080639
Chicago/Turabian StyleJanowska, Katarzyna, Xianzheng Ma, Vittorio Boffa, Mads Koustrup Jørgensen, and Victor M. Candelario. 2021. "Combined Nanofiltration and Thermocatalysis for the Simultaneous Degradation of Micropollutants, Fouling Mitigation and Water Purification" Membranes 11, no. 8: 639. https://doi.org/10.3390/membranes11080639
APA StyleJanowska, K., Ma, X., Boffa, V., Jørgensen, M. K., & Candelario, V. M. (2021). Combined Nanofiltration and Thermocatalysis for the Simultaneous Degradation of Micropollutants, Fouling Mitigation and Water Purification. Membranes, 11(8), 639. https://doi.org/10.3390/membranes11080639









