Silica Fouling in Reverse Osmosis Systems–Operando Small-Angle Neutron Scattering Studies
Abstract
1. Introduction
2. Experimental
2.1. Silica Synthesis and Characterization
2.2. Instrumentation
2.3. Experimental Procedure
3. Theory: Stability of Aqueous Silica Dispersions (DLVO Theory)
4. SANS Results and Interpretation
4.1. Aqueous Silica-130Å Dispersions of 1.2 mM Ionic Strength and Concentration of 1 vol% (BW30LE RO Membrane)
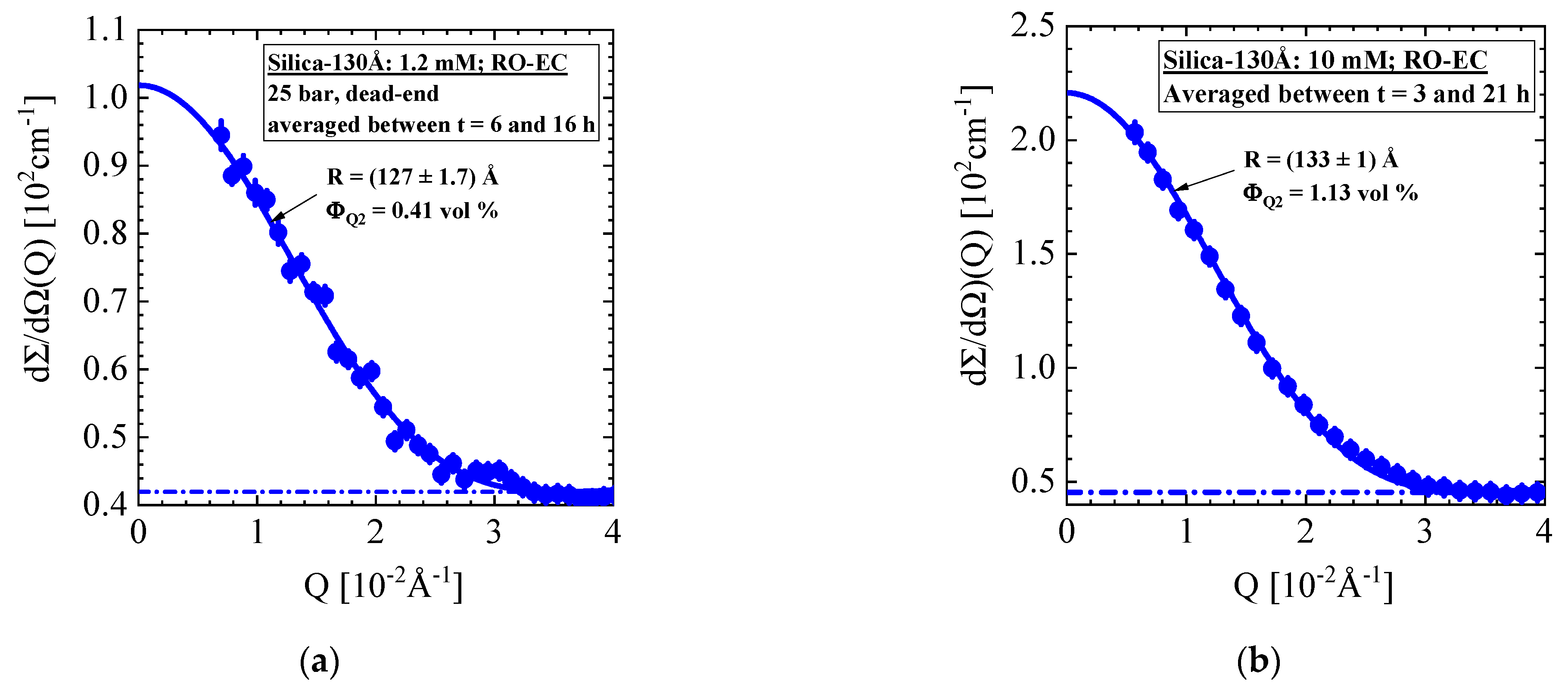
4.2. Aqueous Silica-130Å Dispersions of 10 mM Ionic Strength and Concentration of 1 vol% (RO Membrane BW30LE)
4.3. Aqueous Silica-130Å Dispersions of 10 mM Ionic Strength and Nominal Concentration of 4.74 vol% (RO98 pHt-Alfa Laval Membrane)
4.4. Silica-250Å Colloids of 10 mM Ionic Strength and Nominal Volume Fraction of 0.4 vol% (BW30LE Membrane)
4.5. Aqueous Silica-150Å Dispersion of 10 mM Ionic Strength and Nominal 1.09 vol% Volume Fraction (RO98 pHt-Alfa Laval Membrane)
5. Discussion and Summary
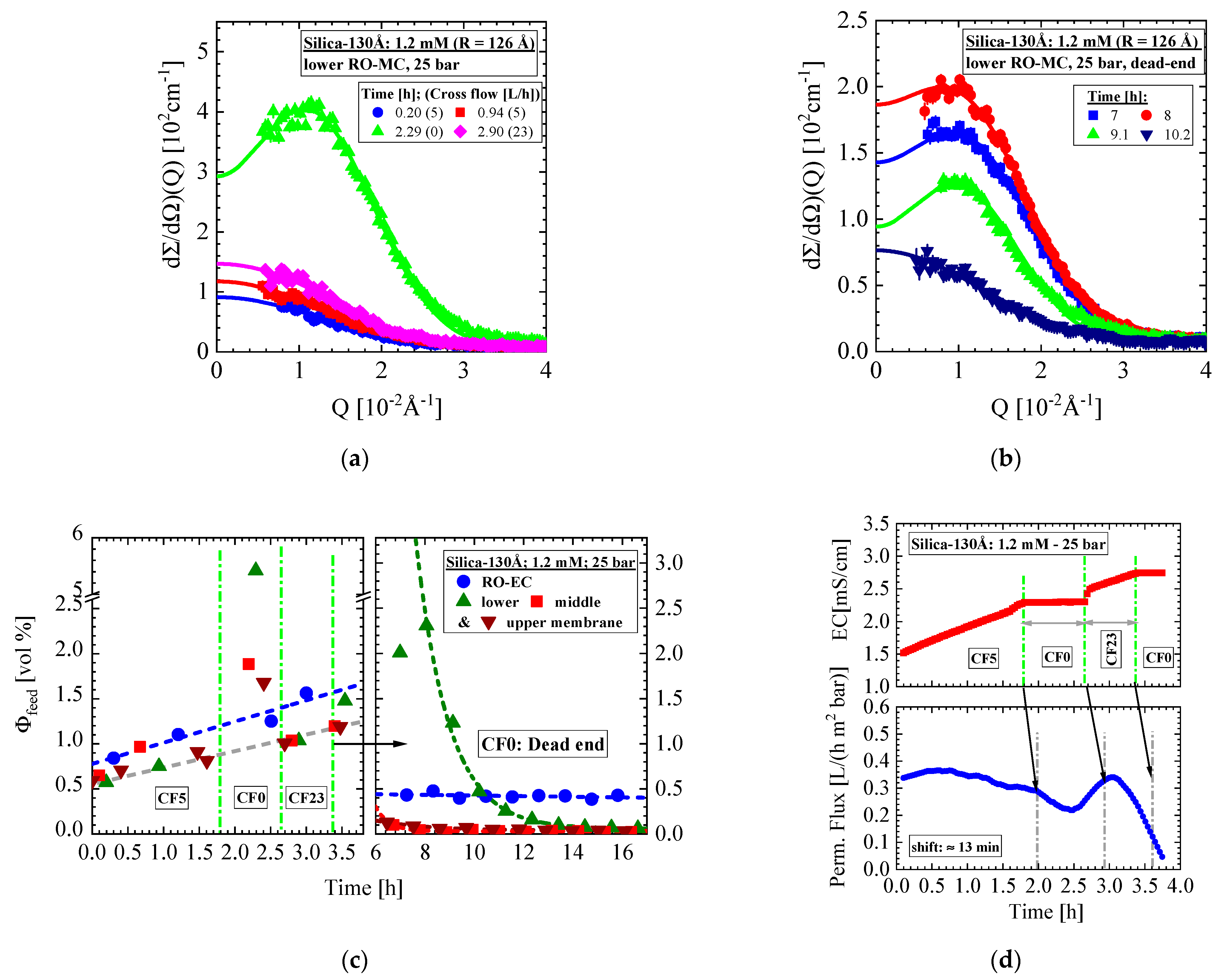
- Silica-130Å dispersion in Figure 4 (1 vol%, 1.2 mM, BW30LE membrane): Gas-like silica dispersion, no cake formation, and negligible concentration polarization at finite cross-flow of 5 and 23 L/h at 25 bar. Change to liquid-like dispersion (scattering shows interparticle interference peak) at 25 bar and dead-end condition due to large increase in concentration polarization but no cake formation.
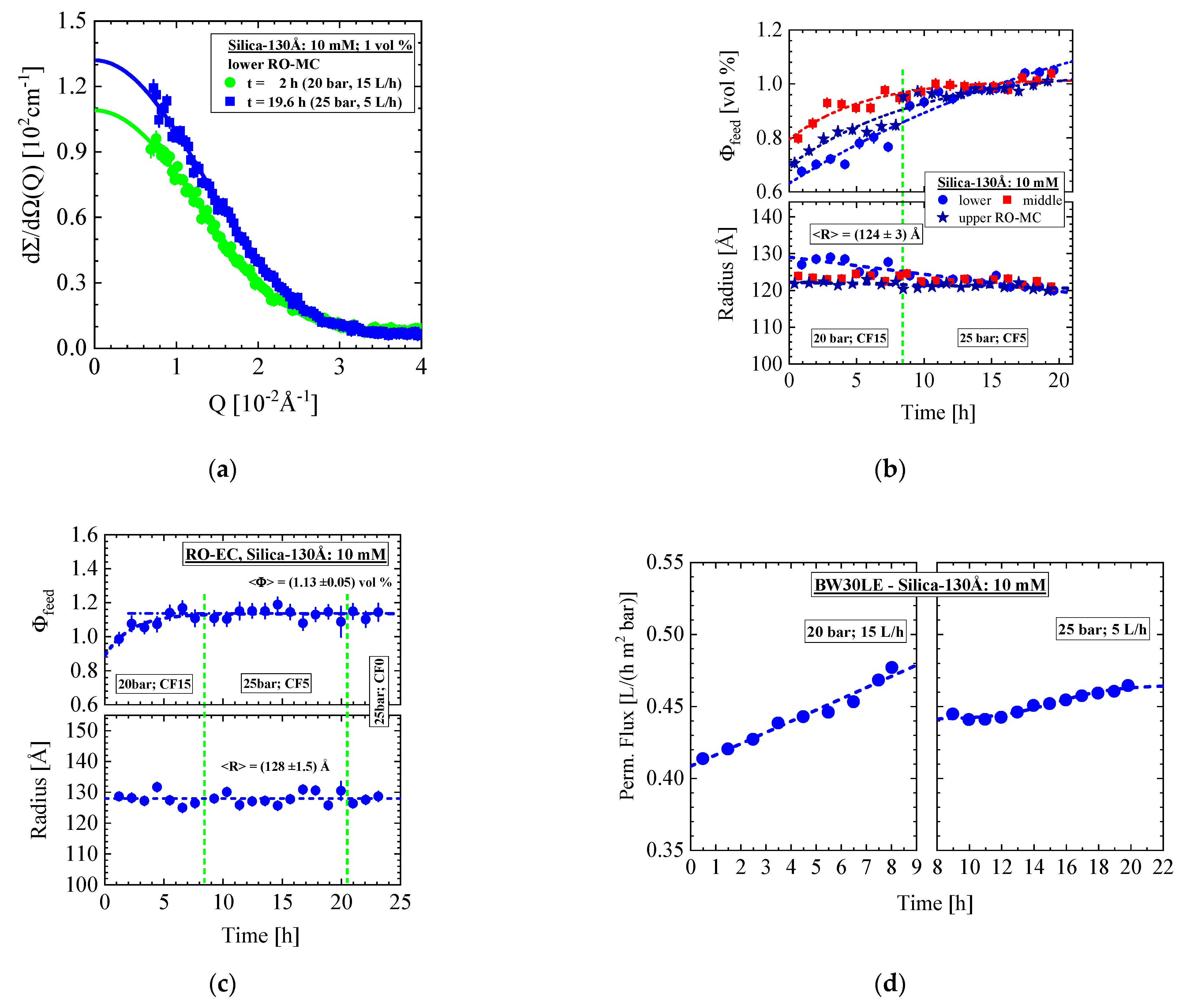
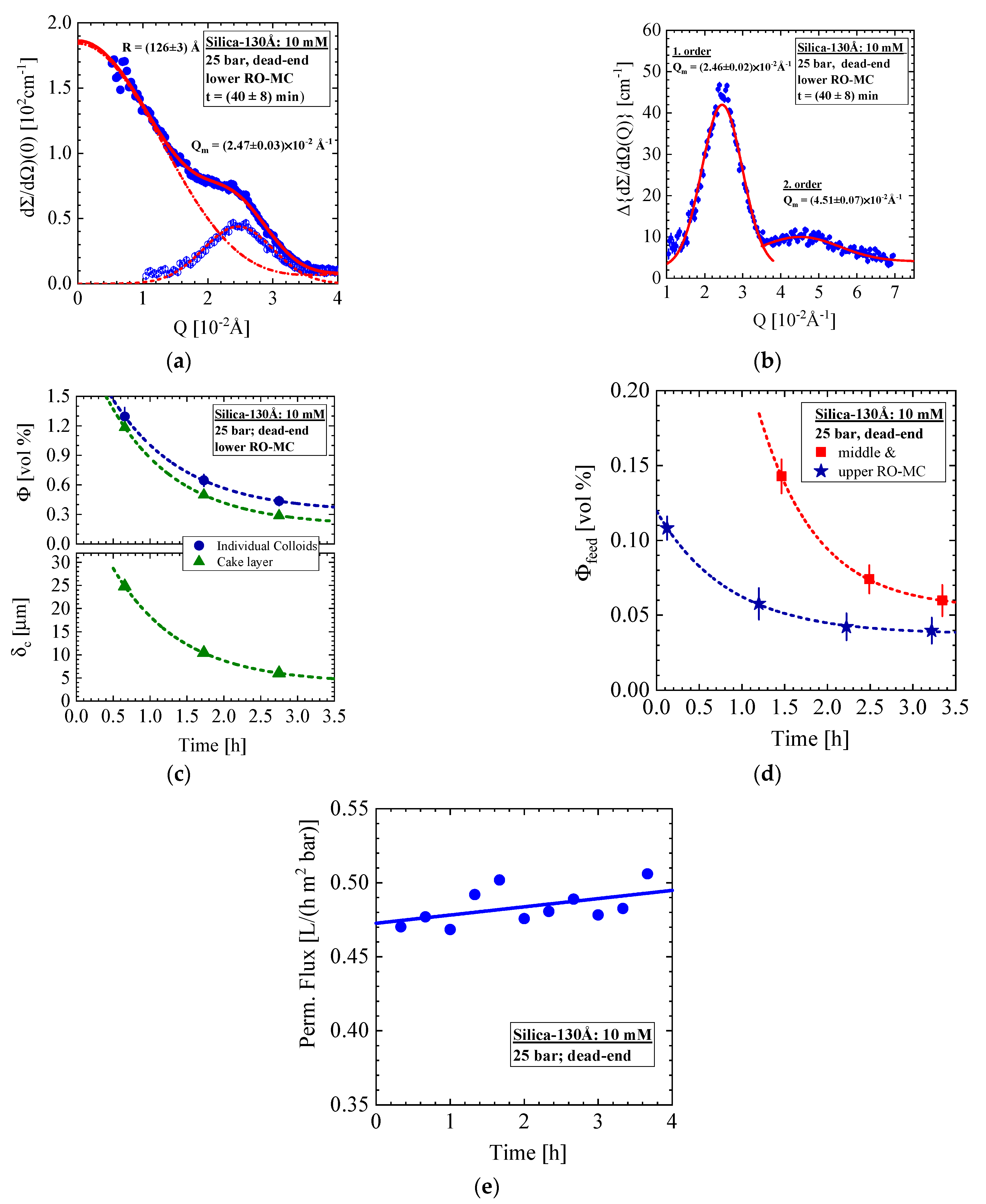
- Silica-130Å dispersion in Figure 5 and Figure 6 (1 vol%, 10 mM, BW30LE membrane): Gas-like silica dispersion showing no cake formation or concentration polarization at finite cross-flow of 15 and 5 L/h at, respectively, 20 and 25 bar. Cake layer but no concentration polarization is formed at 25 bar and dead-end condition. The cake layer disappears for the most part within 3 hours, first in the upper window followed by the middle and lower ones. This observation allowed us to interpret the cake disappearance as a cooperative particle flow driven by gravity.
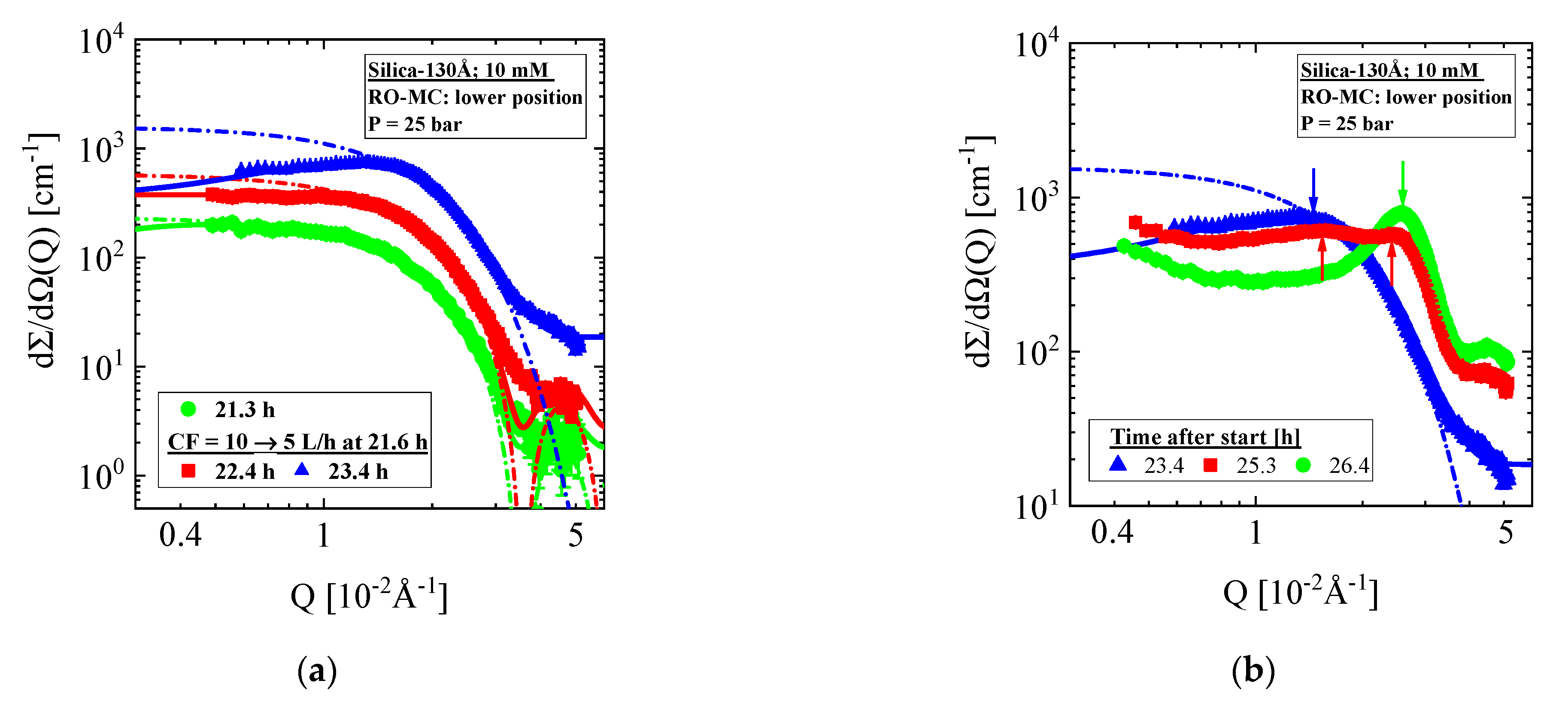
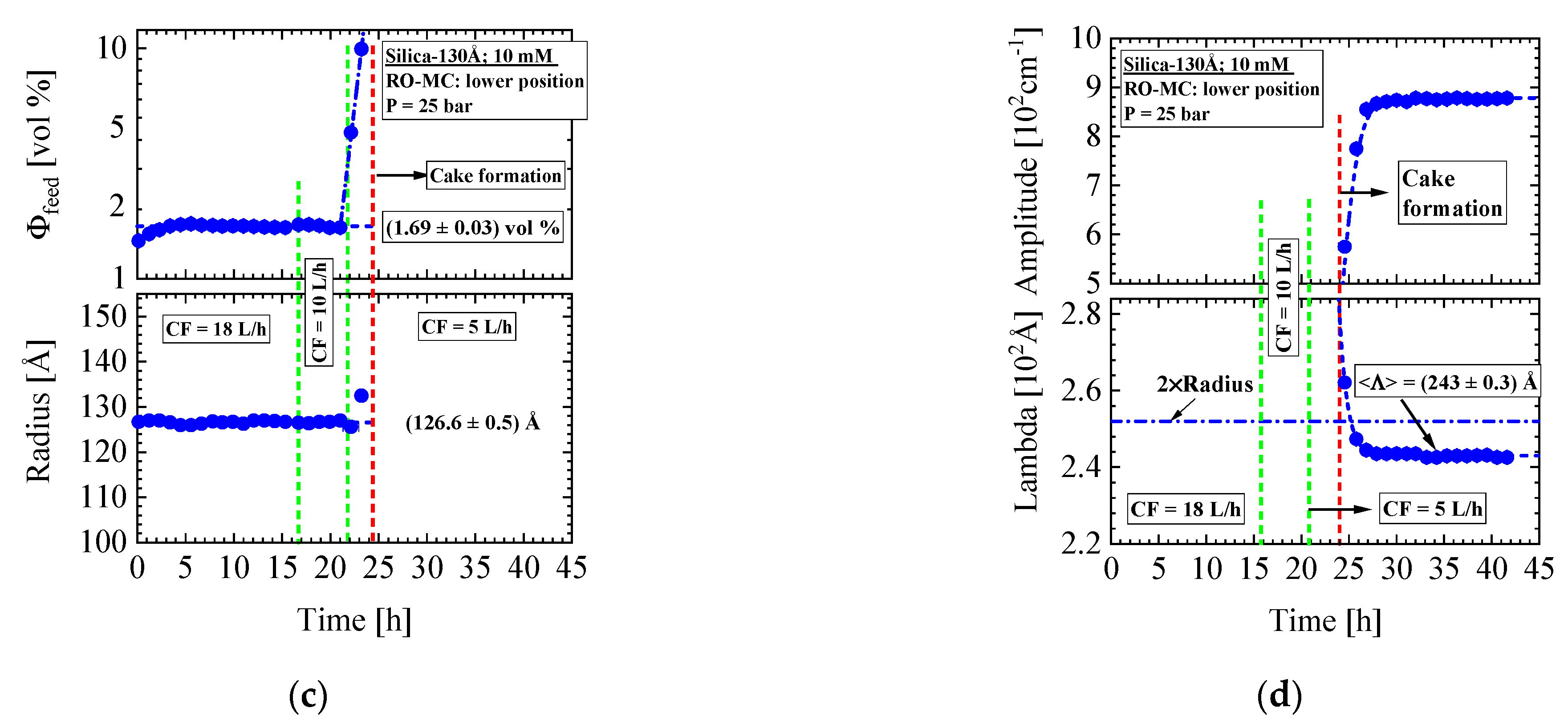
- Silica-130Å dispersion in Figure 7 (1 vol%, 10 mM, BW30LE membrane): Gas-like silica dispersion with no cake layer formation and concentration polarization at 18 and 10 L/h cross-flow. Switching from 10 to 5 L/h cross-flow changes the feed at the lower part of the membrane to a liquid-like dispersion due to strong increase in concentration polarization, which transforms within 5 hours to an irreversible cake layer filling the whole channel. This process indicated a first order liquid–solid phase transition. Meanwhile, the feed in the RO-EC cell showed only a slight increase in silica concentration.
- Silica-130Å dispersion in Figure 8 (4.74 vol%, 10 mM, RO98 pHt membrane): This liquid-like silica dispersion shows concentration polarization about twice as large as concentration averaged over the channel height in comparison with the feed in the RO-EC cell, and no cake layer formation. This experiment was always performed at finite cross-flow.
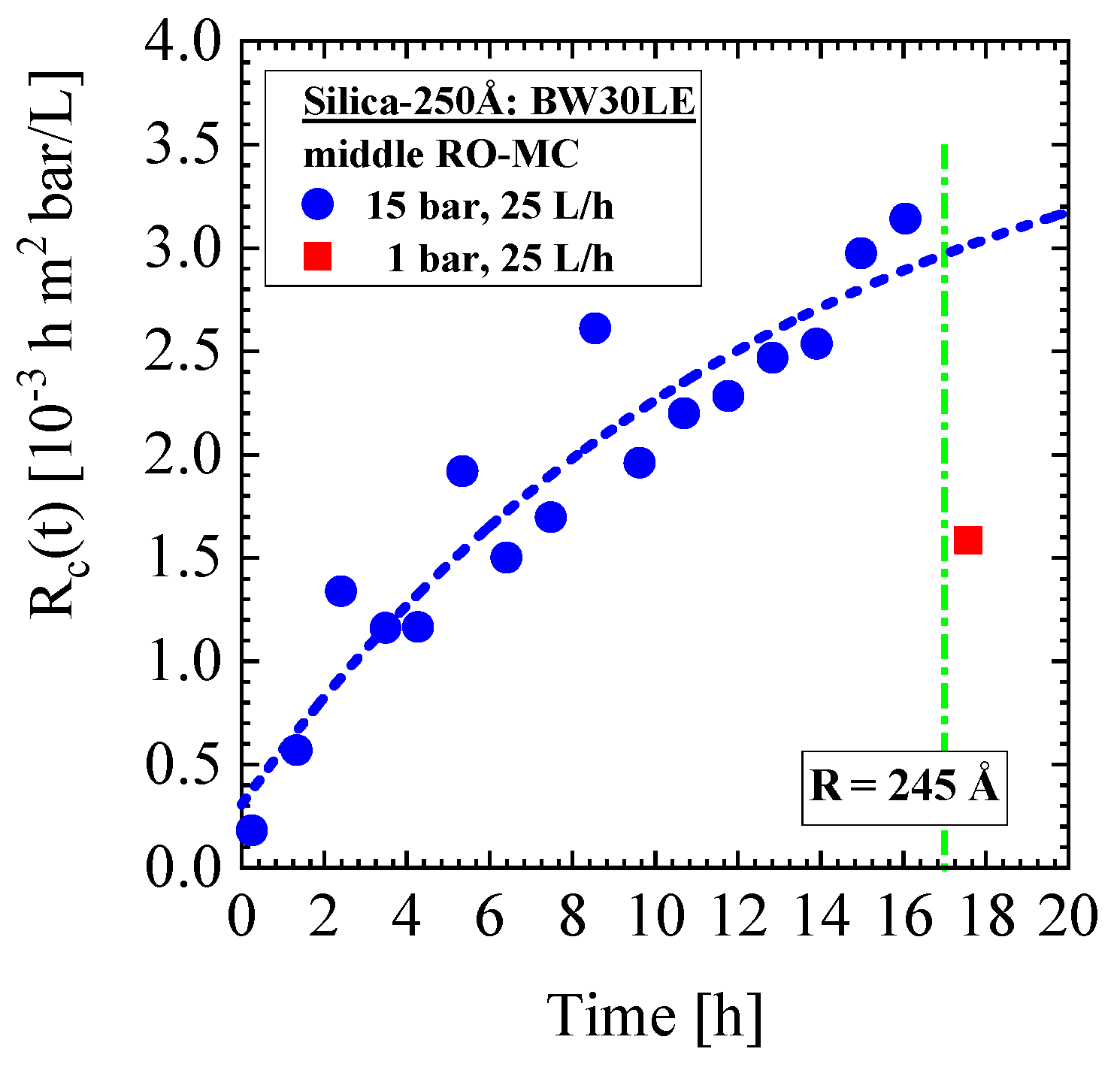
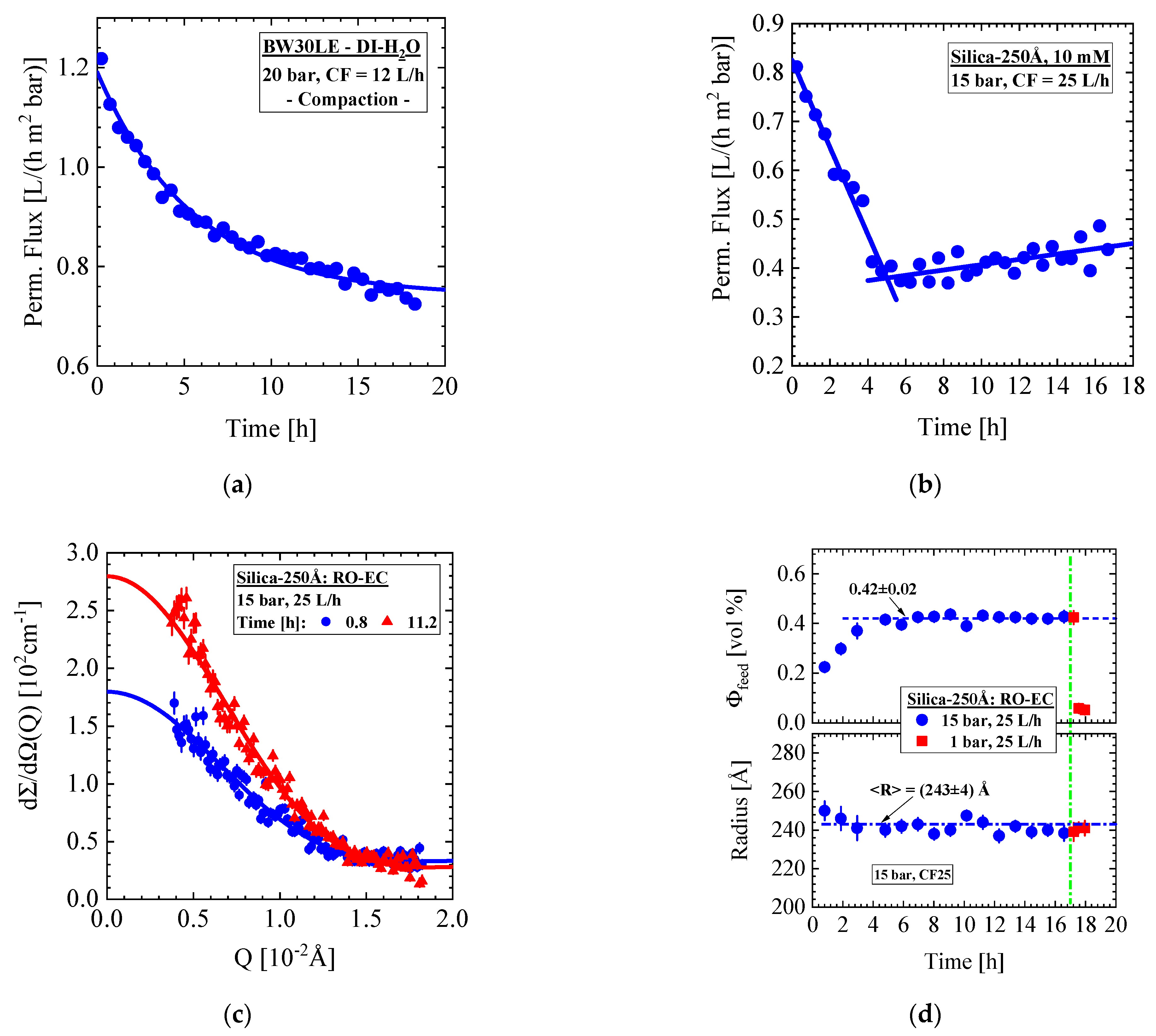
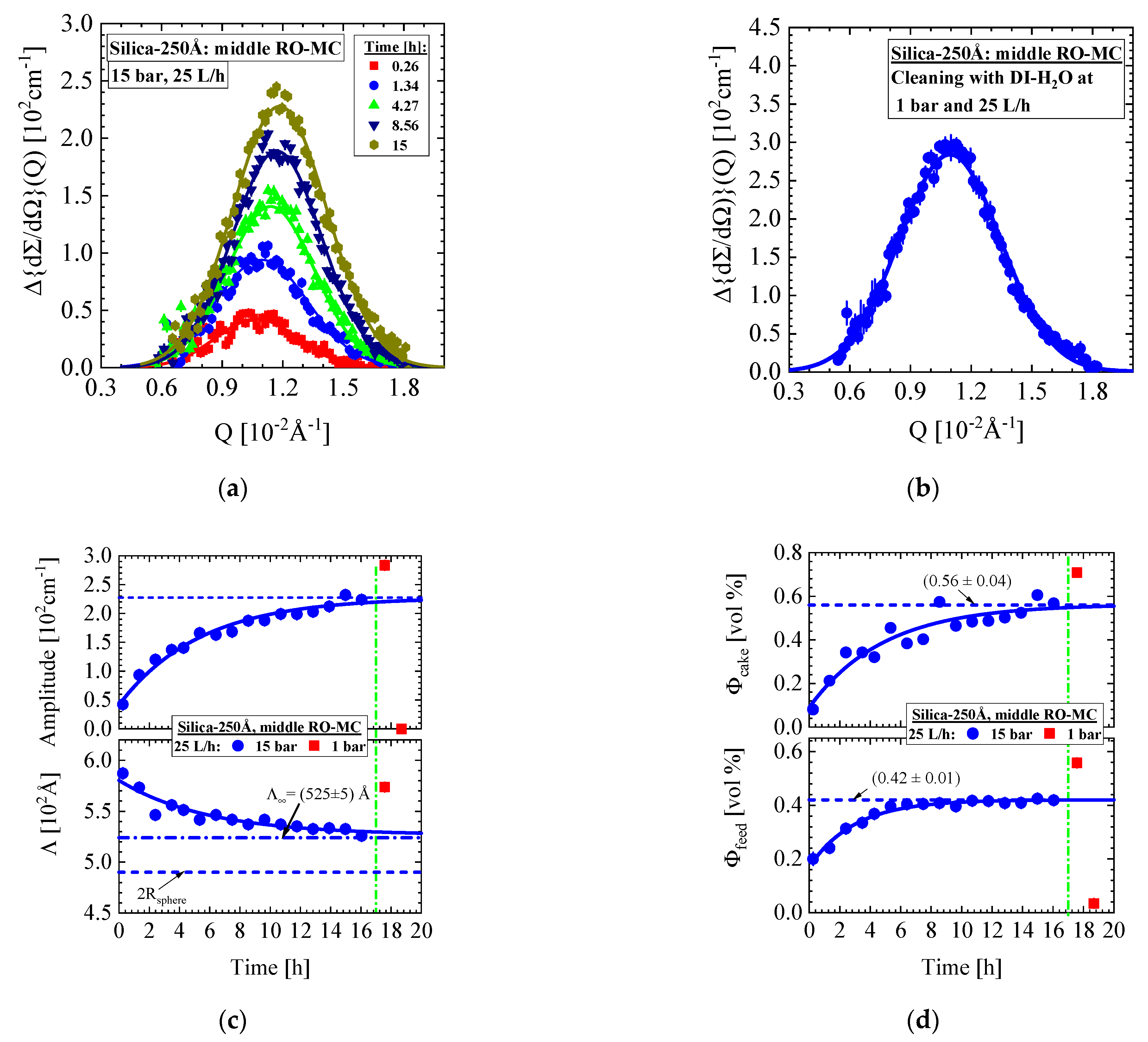
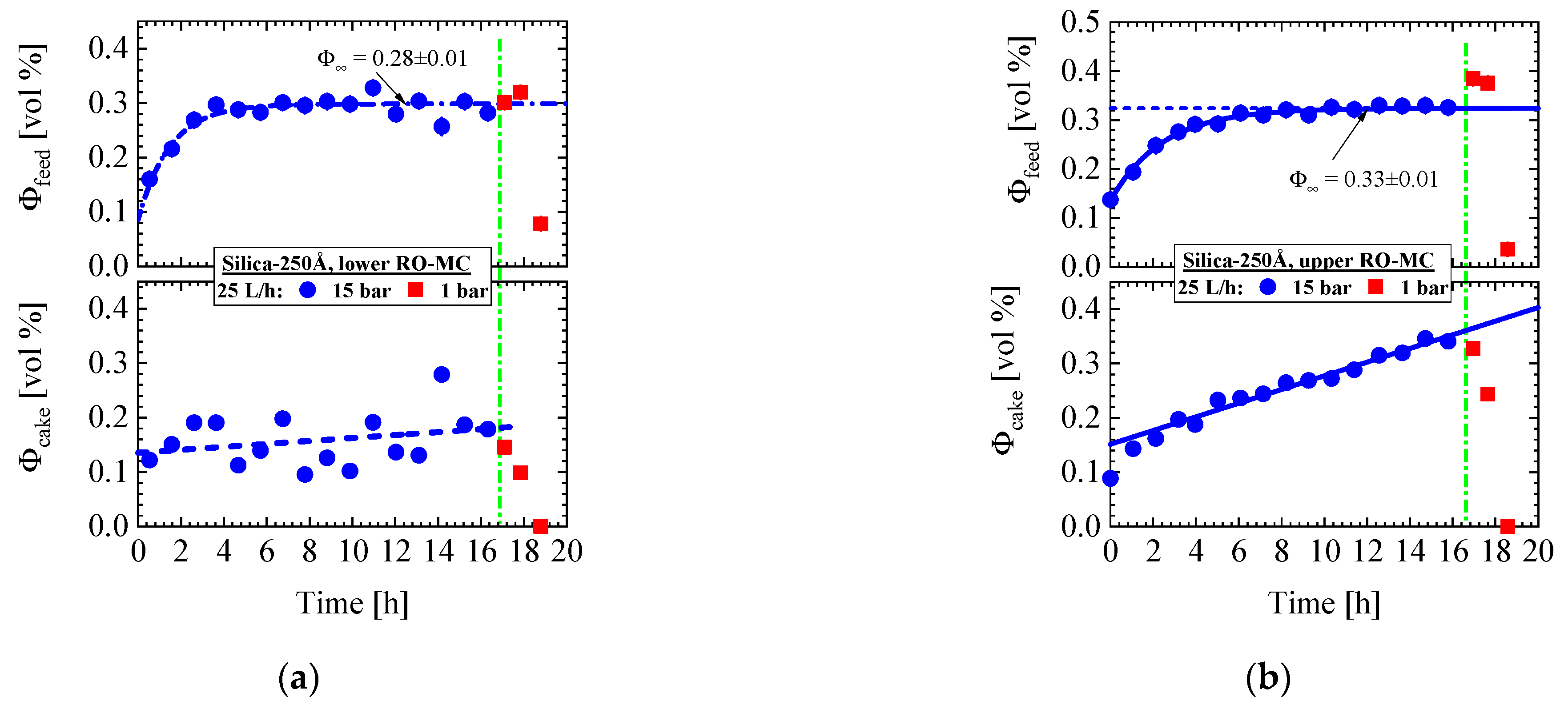
- Silica-250Å dispersion in Figure 9 and Figure 10 (0.4 vol%, 10 mM, BW30LE membrane): Gas-like silica dispersion, no concentration polarization at 15 bar and 25 L/h cross-flow, but temporal cake layer formation of increasing concentration and thickness and declining porosity. The nominal silica volume fraction of the feed in the RO-EC cell was achieved after about 4 h, whereas in the middle part of the membrane after 10 h. In parallel, the cake layer achieved a volume fraction of (0.56 ± 0.04) vol% (averaged over the channel height) after 14 h (Figure 10b). The upper and lower windows (Figure 11) show slightly smaller volume fractions of individual colloids and cake layer. Cleaning with DI-H2O at 1 bar and 25 L/h led the cake layer to re-dissolve after some delay. The cake porosity could be determined in the same feed at 25 bar and 1 bar, which allowed us to determine the cake compressibility. The fouling process appeared rather homogeneous at all three positions of the membrane.
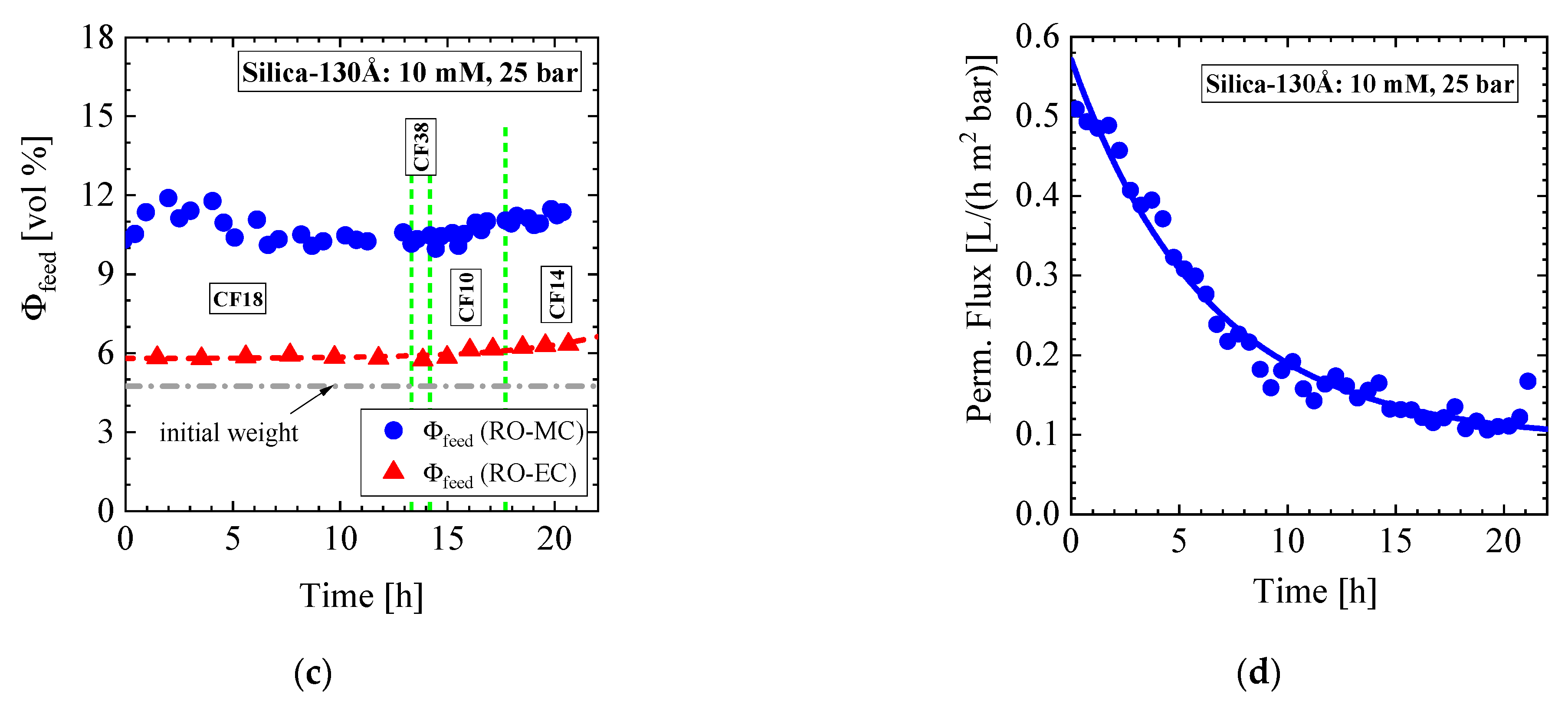
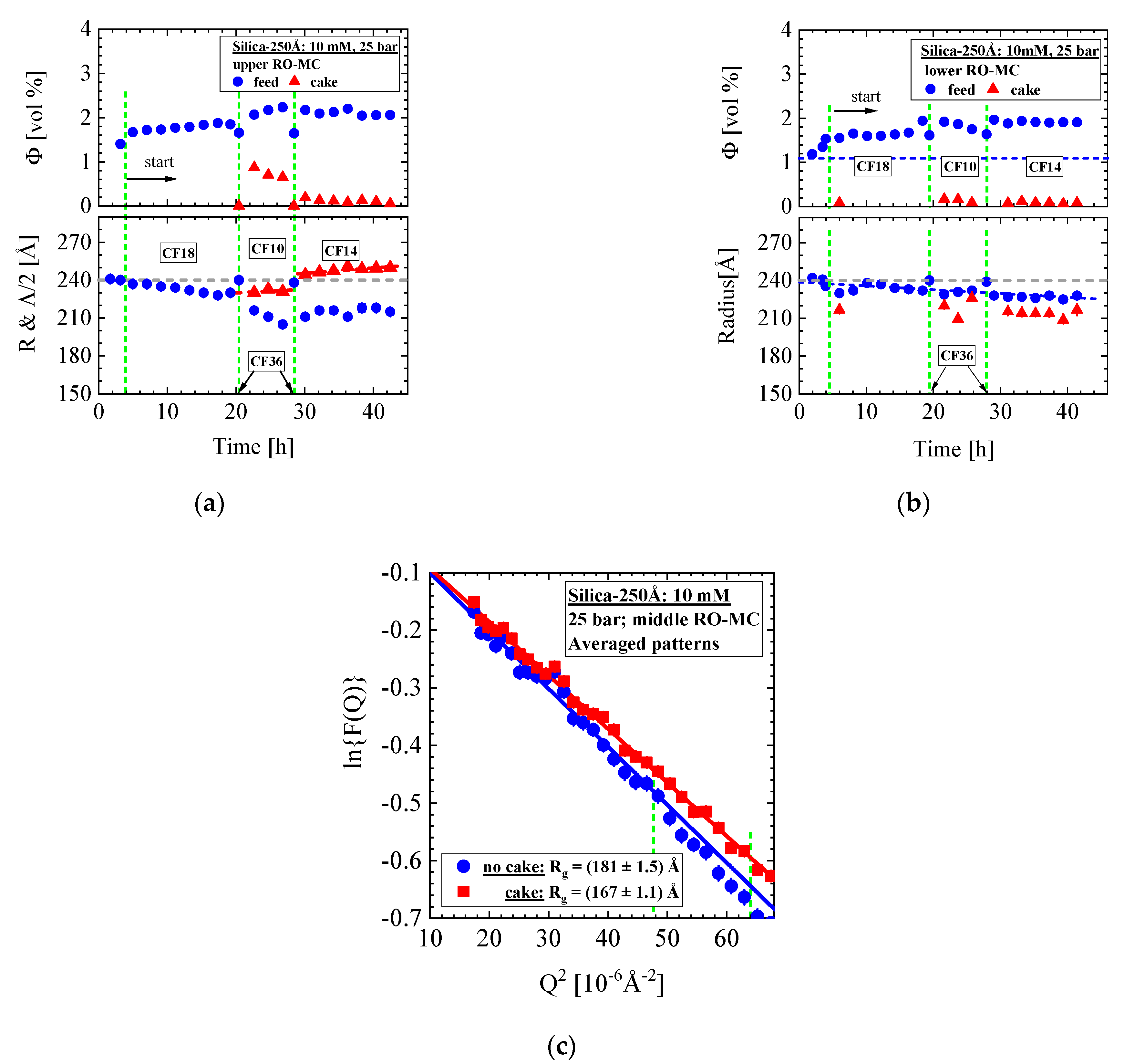
- Silica-250Å dispersion in Figure 12 and Figure 13 (1.09 vol%, 10 mM, RO98 pHt membrane): The liquid-like dispersion shows concentration polarization as concluded from comparison of the volume fraction of (0.92 ± 0.01) vol% in the RO-EC cell with the over the process averaged feed concentration of (2.1 ± 0.2) vol% in the middle part of the RO-MC cell. Stable cake layer formation is observed for CF = 18 L/h but becomes unstable for the lower cross-flow of 10 and 14 L/h. During cake formation, we observed smaller silica sizes in the feed. Before changing cross-flow, the cake layer was completely removed after applying a cross-flow of 36 L/h. At CF = 14 L/h, the porosity became smaller than for simple cubic crystals, suggesting partial sintering of the silica colloids and also explaining the remaining cake thickness of about 1.6 μm. These observations indicate partially irreversible cake formation (Figure 13e).
5.1. Cake Layer Formation and Cleaning
5.2. Change of Colloid Radius during Cake Formation
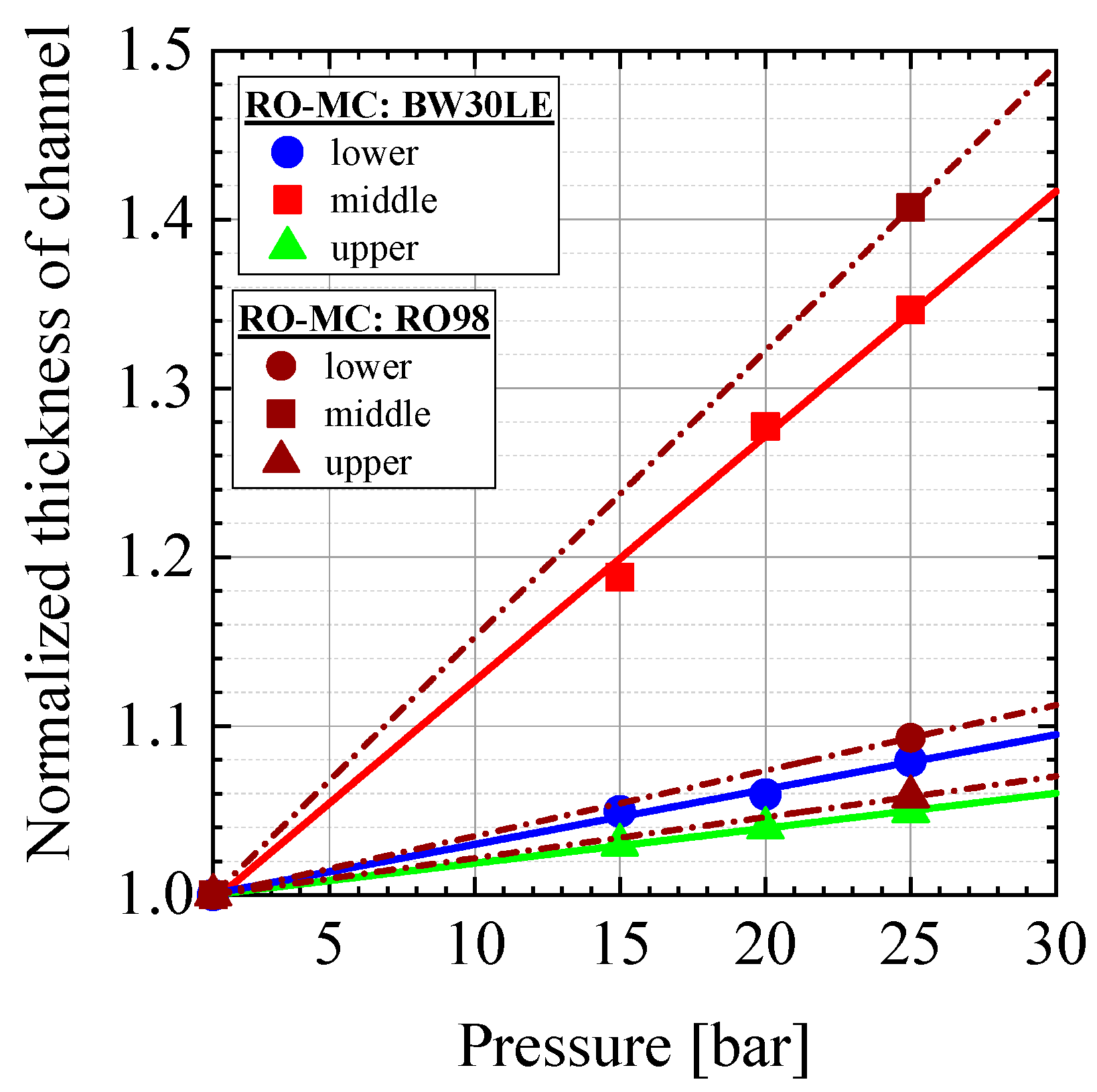
5.3. Permeate Flux
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Operando SANS Device for Desalination
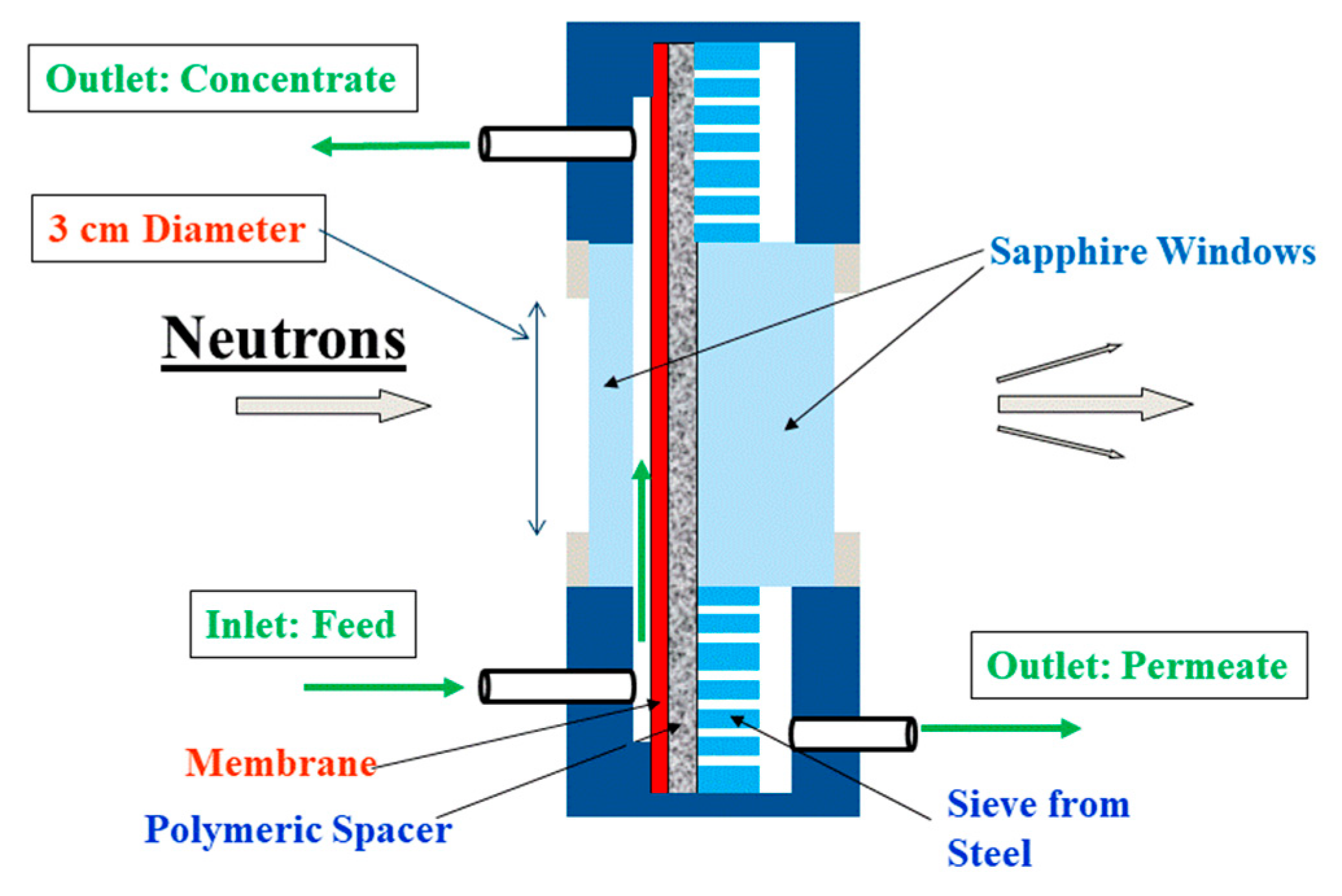
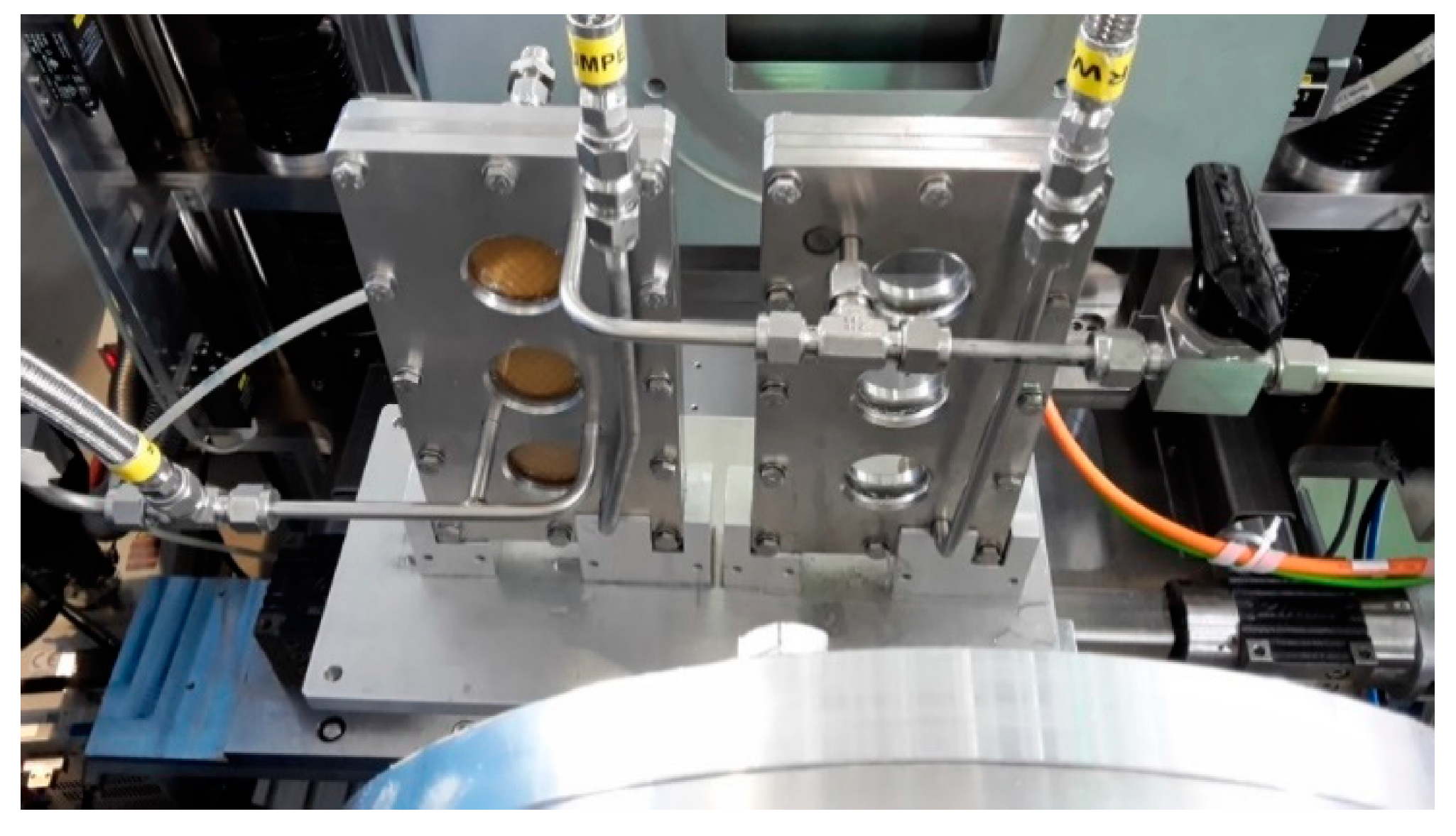
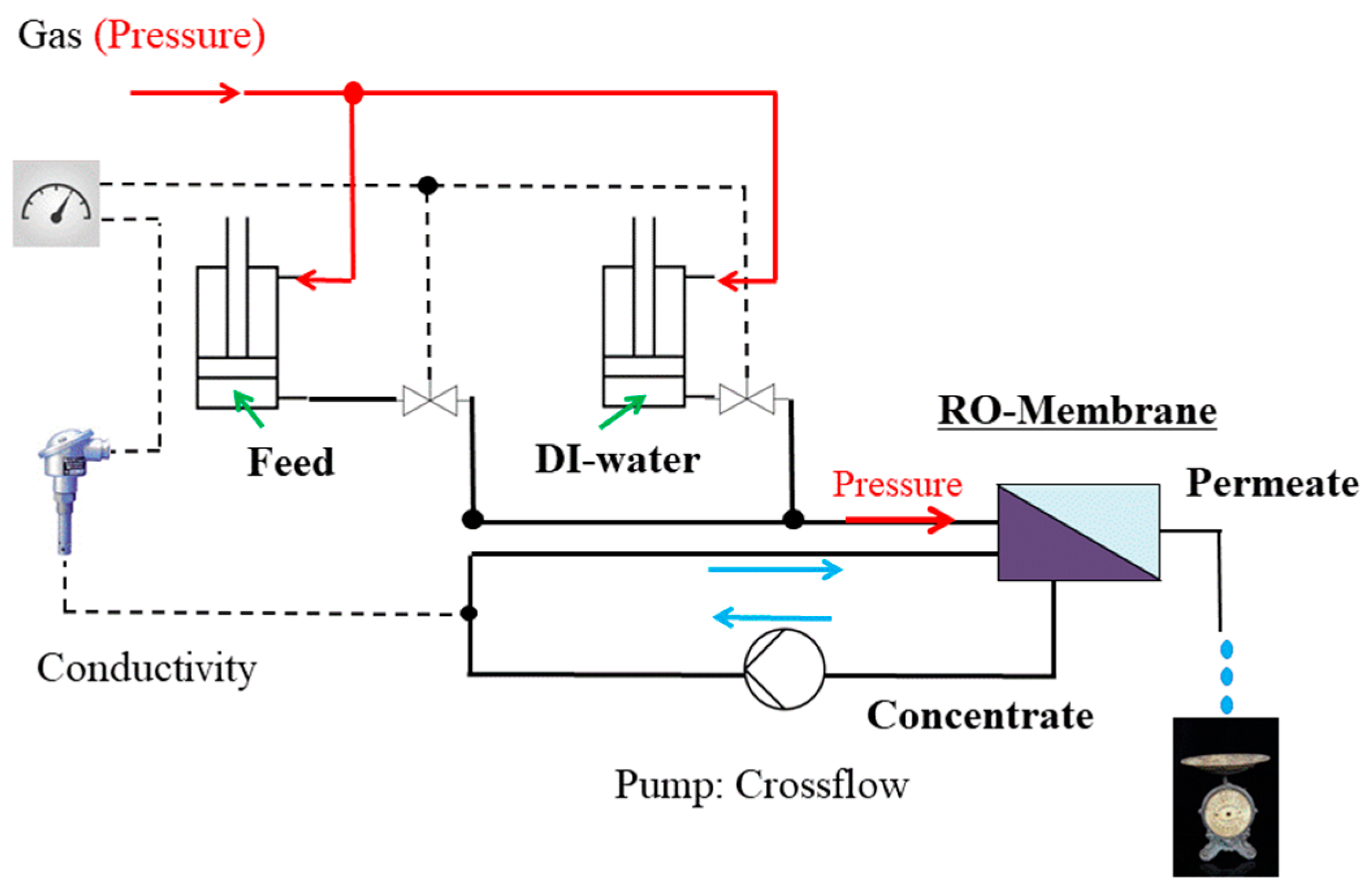
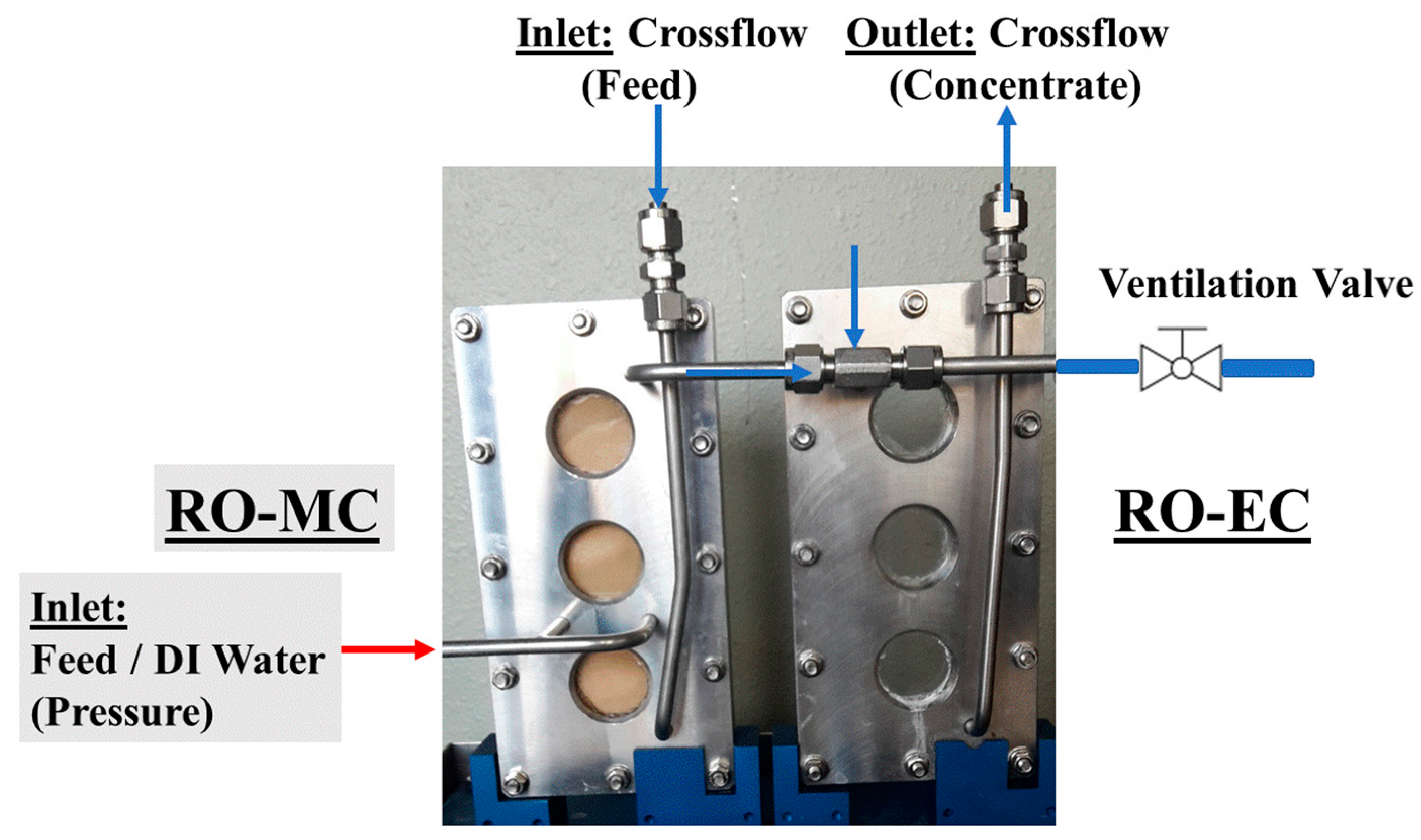
| Position/Pressure | Total Channel Height (cm) | BW30LE (RO98) (μm) | Spacer (μm) | Channel Height of Feed (cm) | Cross-Section of Channel (cm2) | |
|---|---|---|---|---|---|---|
| 25 bar | ||||||
| RO-EC | 0.172 | ----------- | ----------- | 0.172 | 0.77 | |
| RO-MC | upper | 0.142 | 150 (300) | 215 | 0.106 (0.091) | 0.48 (0.41) |
| middle | 0.172 | 0.136 (0.121) | 0.61 (0.54) | |||
| lower | 0.145 | 0.109 (0.094) | 0.49 (0.42) | |||
| 20 bar | ||||||
| RO-EC | 0.157 | ----------- | ----------- | 0.157 | 0.71 | |
| RO-MC | upper | 0.141 | 150 | 215 | 0.105 | 0.47 |
| middle | 0.165 | 0.129 | 0.58 | |||
| lower | 0.143 | 0.107 | 0.48 | |||
| 15 bar | ||||||
| RO-EC | 0.150 | ----------- | ----------- | 0.150 | 0.68 | |
| RO-MC | upper | 0.140 | 150 | 215 | 0.104 | 0.47 |
| middle | 0.156 | 0.120 | 0.54 | |||
| lower | 0.142 | 0.106 | 0.48 | |||
| 1 bar | ||||||
| RO-EC | 0.137 | ----------- | ----------- | 0.137 | 0.62 | |
| RO-MC | 0.137 | 150 (300) | 215 | 0.101 (0.086) | 0.45 | |
Appendix B. SANS Scattering Laws

Appendix C. Crystal Structure, Cake Compressibility, and Hydraulic Resistance
| Properties of Cubic Lattice-Volume of Elementary Cell VEC = a3 | |||
|---|---|---|---|
| Crystal structure | Simple cubic | Body-centered cubic (bcc) | Body-centered cubic (fcc) |
| Radius of colloid (R) | 2R = a (a = 2R) | 2R = √3 a/2 (a = 4R/√3) | 2R = a/√2 (a = 4R/√2) |
| Volume fraction of colloids (QP) | (4π/3) (a/2)3/a3 = π/6 = 0.52 | 2 × (4π/3) R3/a3 = 0.68 | 4 × (4π/3) R3/a3 = 0.74 |
| Porosity of the crystal (ε) | 0.48 | 0.32 | 0.26 |
| Thickness of cake | ΦCL/0.52 × 820 μm | ||
References
- Koo, T.; Lee, Y.J.; Sheikholeslami, R. Silica fouling and cleaning of reverse osmosis membranes. Desalination 2001, 139, 43–56. [Google Scholar] [CrossRef]
- Den, W.; Wang, C.-J. Removal of silica from brackish water by electrocoagulation pretreatment to prevent fouling of reverse osmosis membranes. Sep. Purif. Technol. 2008, 59, 318–325. [Google Scholar] [CrossRef]
- Oren, Y.; Korngold, E.; Daltrophe, N.; Messalem, R.; Volkman, Y.; Aronov, L.; Weismann, M.; Bouriakov, N.; Glueckstern, P.; Gilron, J. Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination 2010, 261, 321–330. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Braun, G.; Hater, W.; Kolk, C.Z.; Dupoiron, C.; Harrer, T.; Götz, T. Investigations of silica scaling on reverse osmosis membranes. Desalination 2010, 250, 982–984. [Google Scholar] [CrossRef]
- Milne, N.A.; O’Reilly, T.; Sanciolo, P.; Ostarcevic, E.; Beighton, M.; Taylor, K.; Mullett, M.; Tarquin, A.J.; Gray, S.R. Chemistry of silica scale mitigation for RO desalination with particular reference to remote operations. Water Res. 2014, 65, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Latour, I.; Miranda, R.; Blanco, A. Silica removal in industrial effluents with high silica content and low hardness. Environ. Sci. Pollut. Res. 2014, 21, 9832–9842. [Google Scholar] [CrossRef] [PubMed]
- Neofotistou, E.; Demadis, K.D. Use of antiscalants for mitigation of silica (SiO2) fouling and deposition: Fundamentals and applications in desalination systems. Desalination 2004, 167, 257–272. [Google Scholar] [CrossRef]
- Salvador Cob, S.; Hofs, B.; Maffezzoni, C.; Adamus, J.; Siegers, W.G.; Cornelissen, E.R.; Genceli Güner, F.E.; Witkamp, G.J. Silica removal to prevent silica scaling in reverse osmosis membranes. Desalination 2014, 344, 137–143. [Google Scholar] [CrossRef]
- Sanciolo, P.; Milne, N.; Taylor, K.; Mullet, M.; Gray, S. Silica scale mitigation for high recovery reverse osmosis of groundwater for a mining process. Desalination 2014, 340, 49–58. [Google Scholar] [CrossRef]
- Sheikholeslami, R.; Bright, J. Silica and metals removal by pretreatment to prevent fouling of reverse osmosis membranes. Desalination 2002, 143, 255–267. [Google Scholar] [CrossRef]
- Higgin, R.; Howe, K.J.; Mayer, T.M. Synergistic behavior between silica and alginate: Novel approach for removing silica scale from RO membranes. Desalination 2010, 250, 76–81. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Bhattacharjee, S.; Elimelech, M. Effect of Membrane Surface Roughness on Colloid-Membrane DLVO Interactions. Langmuir 2003, 19, 4836–4847. [Google Scholar] [CrossRef]
- Zhu, X.; Elimelech, M. Colloidal Fouling of Reverse Osmosis Membranes: Measurements and Fouling Mechanisms. Environ. Sci. Technol. 1997, 31, 3654–3662. [Google Scholar] [CrossRef]
- Wang, F.; Shi, H.; Tarabara, V.V. Combined precipitative and colloidal fouling of reverse osmosis membranes. J. Environ. Eng. 2019, 145, 04019040. [Google Scholar] [CrossRef]
- Rathinam, K.; Abraham, S.; Oren, Y.; Schwahn, D.; Petry, W.; Kaufman, Y.; Kasher, R. Surface-Induced Silica Scaling during Brackish Water Desalination: The Role of Surface Charge and Specific Chemical Groups. Environ. Sci. Technol. 2019, 53, 5202–5211. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Elimelech, M.; Shon, H.K.; Hong, S. Combined organic and colloidal fouling in forward osmosis: Fouling reversibility and the role of applied pressure. J. Membr. Sci. 2014, 460, 206–212. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: London, UK, 1992. [Google Scholar]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: New York, NY, USA, 1991. [Google Scholar]
- Elimelech, M.; Gregory, J.; Jia, X.; Williams, R.A. Particle Deposition and Aggregation Measurement. Modelling and Simulation; Butterworth-Heinemann: Boston, UK, 1995. [Google Scholar]
- Vigil, G.; Xu, Z.H.; Steinberg, S.; Israelachvili, J.N. Interactions of Silica Surfaces. J. Colloid Interface Sci. 1994, 166, 367–385. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Giesche, H. Synthesis of monodispersed silica powders II. Controlled growth reaction and continuous production process. J. Eur. Ceram. Soc. 1994, 14, 205–214. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Kim, A.S.; Elimelech, M. Influence of crossflow membrane filter geometry and shear rate on colloidal fouling in reverse osmosis and nanofiltration separations. Environ. Eng. Sci. 2002, 19, 357–372. [Google Scholar] [CrossRef]
- Schwahn, D.; Feilbach, H.; Starc, T.; Pipich, V.; Kasher, R.; Oren, Y. Design and Test of Reverse Osmosis Pressure Cell for In-Situ Small-Angle Neutron Scattering Studies. Desalination 2017, 405, 40–50. [Google Scholar] [CrossRef][Green Version]
- Pipich, V.; Dickmann, M.; Frielinghaus, H.; Kasher, R.; Hugenschmidt, C.; Petry, W.; Oren, Y.; Schwahn, D. Morphology of thin film composite membranes explored by small-angle neutron scattering and positron-annihilation lifetime spectroscopy. Membranes 2020, 10, 48. [Google Scholar] [CrossRef]
- Pipich, V.; Schlenstedt, K.; Dickmann, M.; Kasher, R.; Meier-Haack, J.; Hugenschmidt, C.; Petry, W.; Oren, Y.; Schwahn, D. Morphology and porous structure of standalone aromatic polyamide films as used in RO membranes—An exploration with SANS, PALS, and SEM. J. Membr. Sci. 2019, 573, 167–176. [Google Scholar] [CrossRef]
- Heinz Maier-Leibnitz Zentrum. Available online: http://www.mlz-garching.de/instrumente-und-labore/nanostrukturen/kws-3.html (accessed on 5 May 2021).
- Kim, K.M.; Kim, H.M.; Lee, W.J.; Lee, C.W.; Kim, T.; Lee, J.K.; Jeong, J.; Paek, S.M.; Oh, J.M. Surface treatment of silica nanoparticles for stable and charge-controlled colloidal silica. Int. J. Nanomed. 2014, 9, 29–40. [Google Scholar]
- O’Brien, R.W.; White, L.R. Electrophoretic mobility of a spherical colloidal particle. J. Chem. Soc. Faraday Trans. II 1978, 74, 1607–1626. [Google Scholar] [CrossRef]
- Yotsumoto, H.; Yoon, R.H. Application of extended DLVO theory II. Stability of silica suspensions. J. Colloid Interface Sci. 1993, 157, 434–441. [Google Scholar] [CrossRef]
- Laven, J.; Stein, H.N. The electroviscous behavior of aqueous dispersions of amorphous silica (Ludox). J. Colloid Interface Sci. 2001, 238, 8–15. [Google Scholar] [CrossRef]
- Goegelein, C.; Naegele, G.; Buitenhuis, J.; Tuinier, R.; Dhont, J.K.G. Polymer depletion-driven cluster aggregation and initial phase separation in charged nanosized colloids. J. Chem. Phys. 2009, 130, 204905. [Google Scholar] [CrossRef]
- Kim, A.S.; Hoek, E.M.V. Cake structure in dead-end membrane filtration: Monte Carlo simulations. Environ. Eng. Sci. 2002, 19, 373–385. [Google Scholar] [CrossRef]
- Tarabara, V.V.; Koyuncu, I.; Wiesner, M.R. Effect of hydrodynamic and solution ionic strength on permeate flux in crossflow filtration: Direct experiment observation of filter cake cross-sections. J. Membr. Sci. 2004, 241, 65–78. [Google Scholar] [CrossRef]
- ElSherbiny, I.M.A.; Khalil, A.S.G.; Ulbricht, M. Influence of Surface Micro-Patterning and Hydrogel Coating on Colloidal Silica Fouling of Polyamide Thin-Film Composite Membranes. Membranes 2019, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Roe, R.J. Methods of X-ray and Neutron Scattering in Polymer Science; University Press: Oxford, UK, 2000. [Google Scholar]
- Singh, D. A Quantitative Study of Colloidal Fouling in Membrane Process. Ph.D. Thesis, National University of Singapore, Singapore, 2007. [Google Scholar]
- Song, L.; Elimelech, M. Theory of concentration polarization in crossflow filtration. J. Chem. Soc. Faraday Trans. 1995, 91, 3389–3398. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Nakao, S.; Smolders, C.A. Flux limitation in Ultrafiltration. Osmotic pressure model and gel layer model. J. Membr. Sci. 1984, 20, 115–124. [Google Scholar] [CrossRef]
- Petsev, D.N.; Starov, V.M.; Ivanov, I.B. Concentrated dispersions of charged colloidal particles: Sedimentation, ultrafiltration and diffusion. Colloids Surf. A Phys. Eng. Asp. 1993, 81, 65–81. [Google Scholar] [CrossRef]
- Alefeld, B.; Schwahn, D.; Springer, T. New Developments of Small-Angle Neutron Scattering Instruments with Focussing. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detectors Assoc. Equip. 1989, 274, 210–216. [Google Scholar] [CrossRef]
- Goerigk, G.; Varga, Z. Comprehensive Upgrade of the High-Resolution Small-Angle Neutron Scattering Instrument KWS-3 at FRM II. J. Appl. Cryst. 2011, 44, 337–342. [Google Scholar] [CrossRef]
- Sears, V.F. Neutron scattering lengths and cross sections. Neutron News 1992, 3, 26–37. [Google Scholar] [CrossRef]
- Guinier, A. X-ray Diffraction in Crystals, Imperfect Crystals, and Amorphous Bodies; Freeman: San Francisco, CA, USA, 1963. [Google Scholar]
- Singh, G.; Song, L. Cake Compressibility of Silica Colloids in Membrane Filtration Processes. Ind. Eng. Chem. Res. 2006, 45, 7633–7638. [Google Scholar] [CrossRef]
- Brezonik, P.L.; Arnold, W.A. Water Chemistry, an Introduction of the Chemistry of Natural and Engineering Aquatic Systems; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Schäfer, B.; Hecht, M.; Harting, J.; Nirschl, H. Agglomeration and filtration of colloidal suspensions with DLVO interactions in simulation and experiment. J. Colloid Interface Sci. 2010, 349, 186–195. [Google Scholar] [CrossRef]
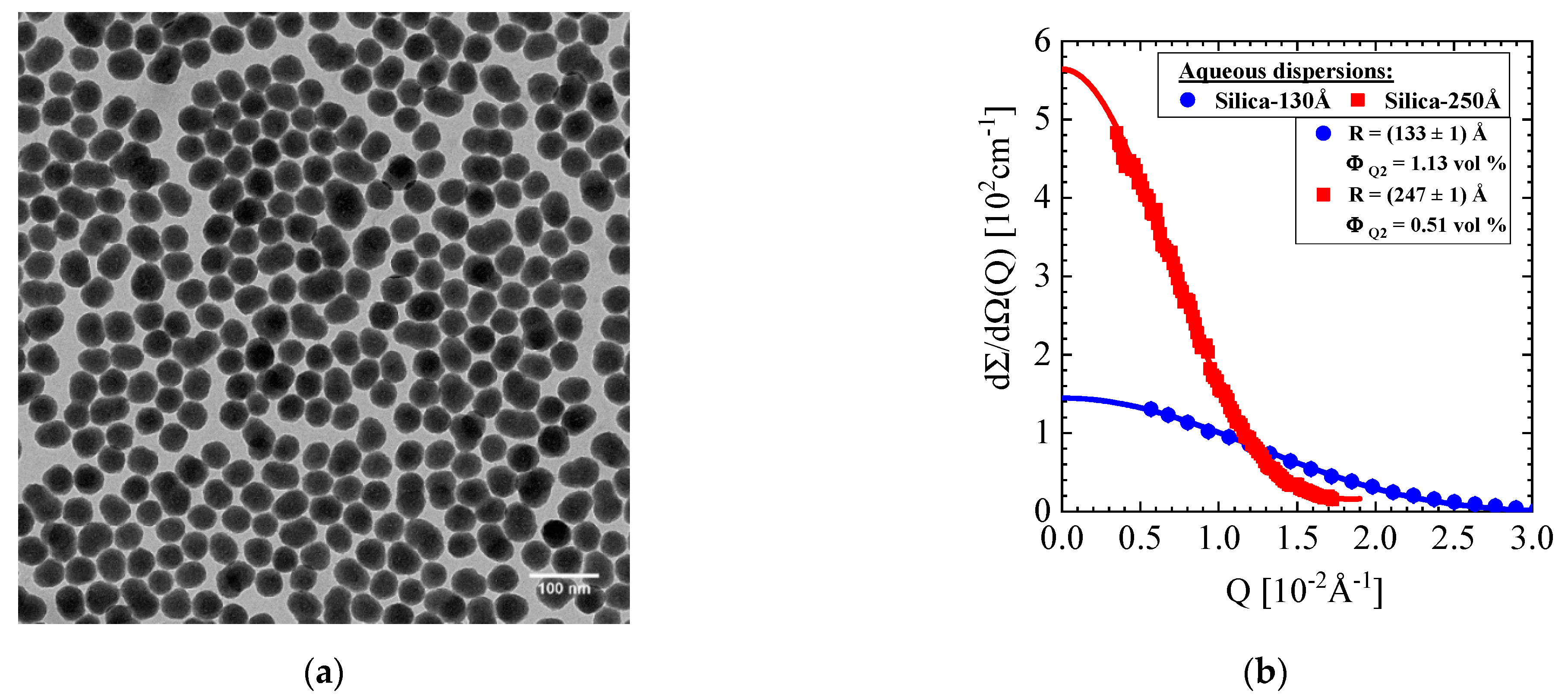






| Silica Colloid | R (Å) (Silica-130Å) | R (Å) (Silica-250Å) | dS (Amorphous Silica) (g/mL) | ρ (SiO2) (1010cm−2) | Δρ (SiO2) (1010 cm−2) | (1012 cm) |
|---|---|---|---|---|---|---|
| TEM | 116 (± 19) | 215 (± 24) | 2.196 (2.11; [24]) | --------- | --------- | --------- |
| DLS | 160 | 255 | --------- | --------- | --------- | |
| SANS | 133 ± 1 | 247 ± 1 | 3.47 | 4.03 | −0.569; 0.958 |
| Silica Colloid | Silica-130Å | Silica-250Å | |||
|---|---|---|---|---|---|
| Membrane | RO-BW30LE | RO98 pHt | RO-BW30LE | RO98 pHt | |
| Nominal concentration (vol%) | 1 | 1 | 4.74 | 0.4 | 1.09 |
| Ionic strength (mM) | 1.2 | 10 | |||
| pH | 9.5 | 9.6 | 9.6 | ||
| Zeta potential (mV) | −92 | −77 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipich, V.; Starc, T.; Buitenhuis, J.; Kasher, R.; Petry, W.; Oren, Y.; Schwahn, D. Silica Fouling in Reverse Osmosis Systems–Operando Small-Angle Neutron Scattering Studies. Membranes 2021, 11, 413. https://doi.org/10.3390/membranes11060413
Pipich V, Starc T, Buitenhuis J, Kasher R, Petry W, Oren Y, Schwahn D. Silica Fouling in Reverse Osmosis Systems–Operando Small-Angle Neutron Scattering Studies. Membranes. 2021; 11(6):413. https://doi.org/10.3390/membranes11060413
Chicago/Turabian StylePipich, Vitaliy, Thomas Starc, Johan Buitenhuis, Roni Kasher, Winfried Petry, Yoram Oren, and Dietmar Schwahn. 2021. "Silica Fouling in Reverse Osmosis Systems–Operando Small-Angle Neutron Scattering Studies" Membranes 11, no. 6: 413. https://doi.org/10.3390/membranes11060413
APA StylePipich, V., Starc, T., Buitenhuis, J., Kasher, R., Petry, W., Oren, Y., & Schwahn, D. (2021). Silica Fouling in Reverse Osmosis Systems–Operando Small-Angle Neutron Scattering Studies. Membranes, 11(6), 413. https://doi.org/10.3390/membranes11060413









