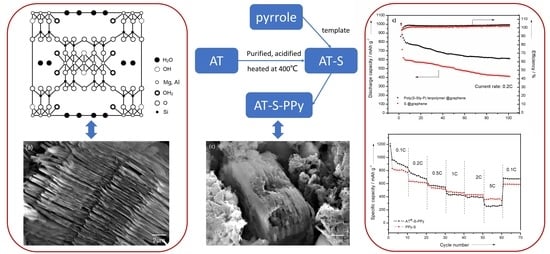
Lamellar Polypyrene Based on Attapulgite–Sulfur Composite for Lithium–Sulfur Battery
Abstract
1. Introduction
2. Experiment
2.1. Material Preparation
2.2. Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
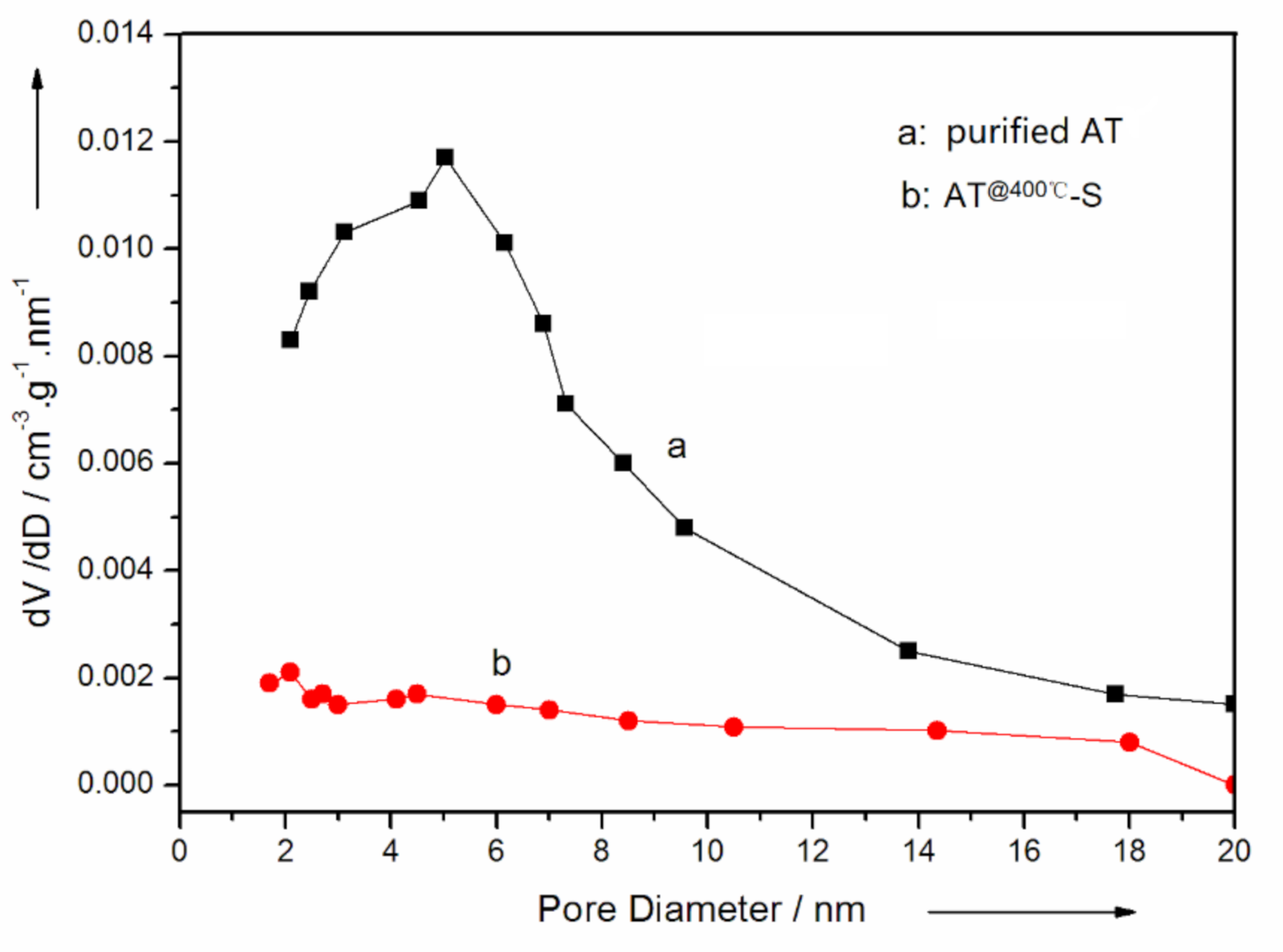
3.1. Optimum Conditions for Sulfur Adsorption of AT
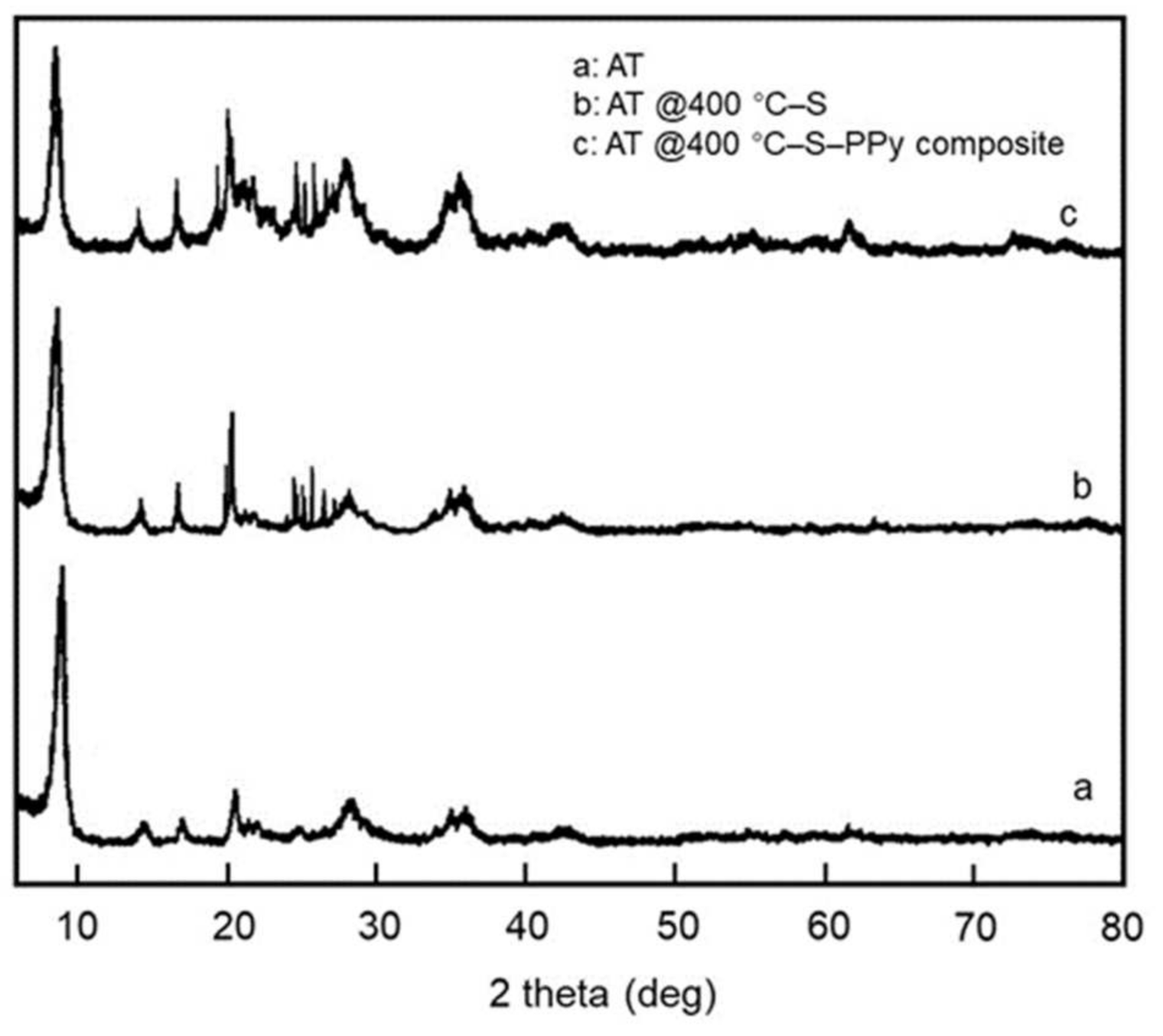
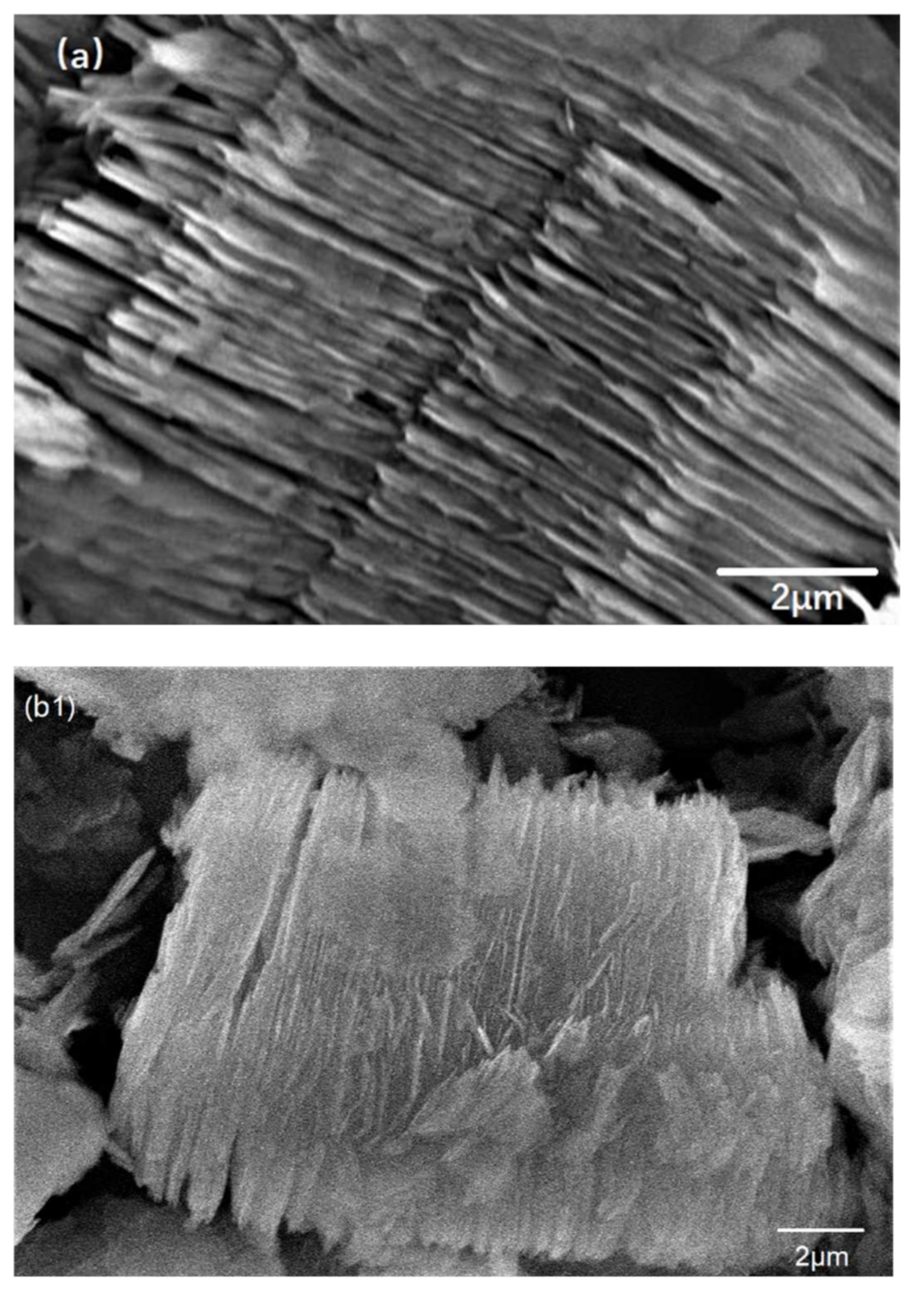
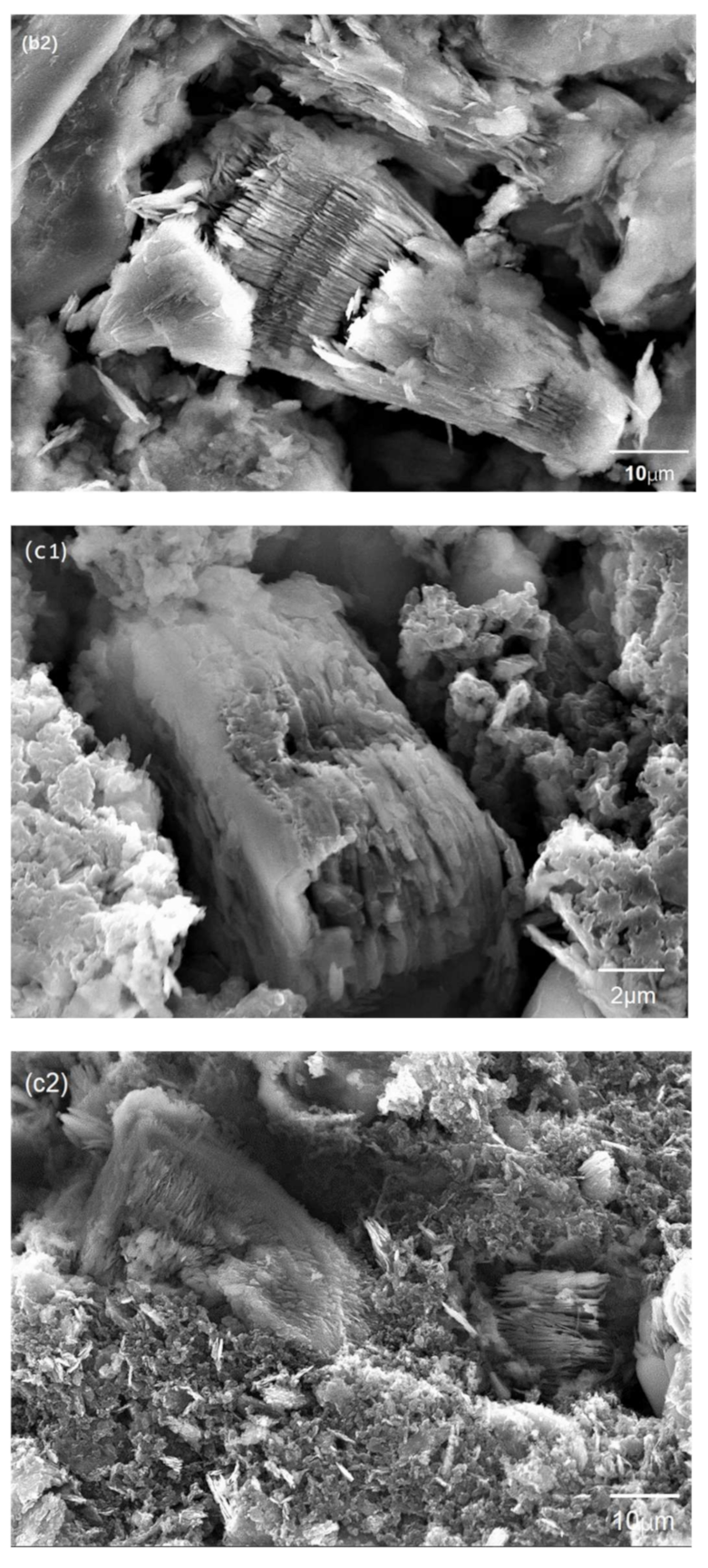
3.2. Characterization of AT @400 °C–S–PPy Composite
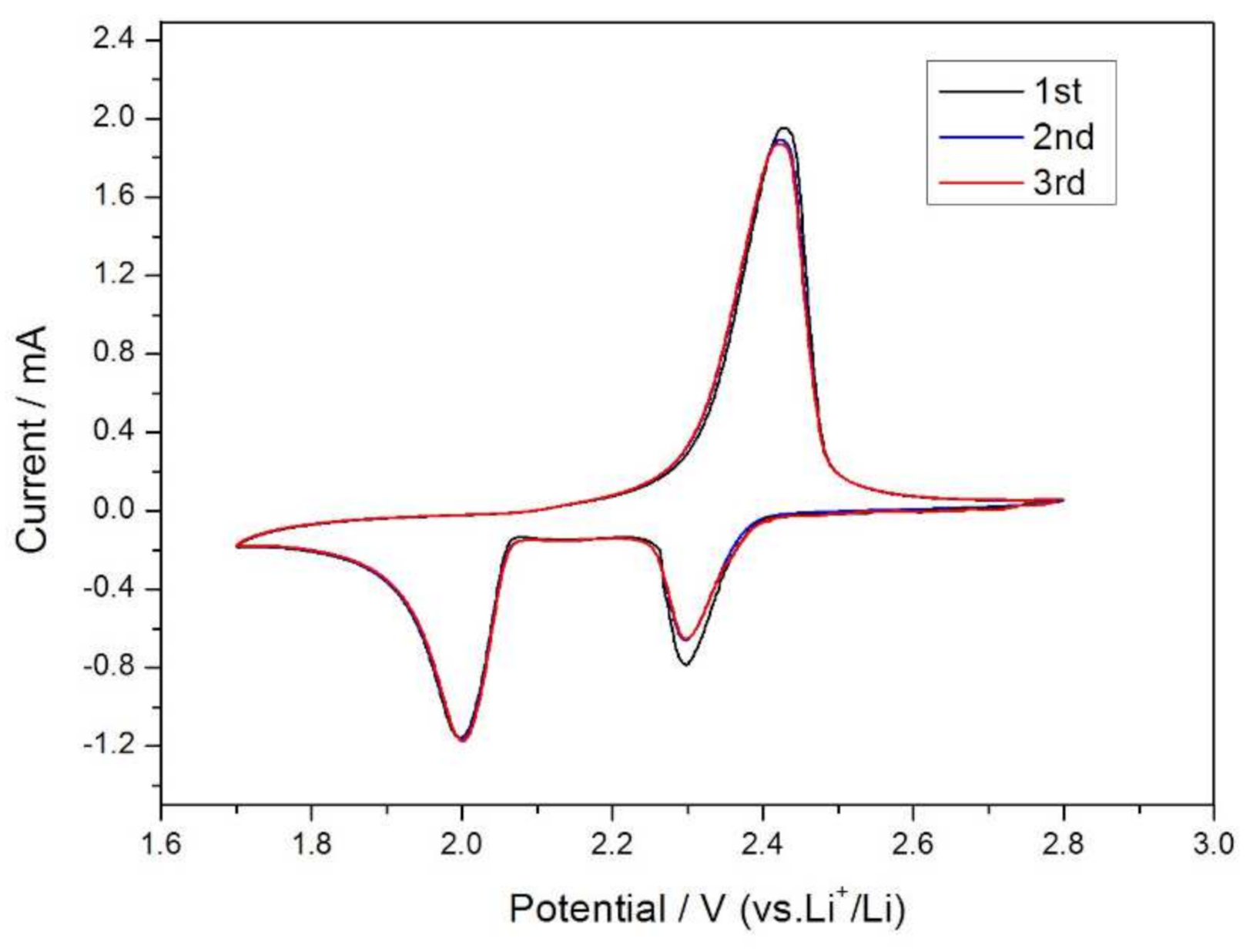
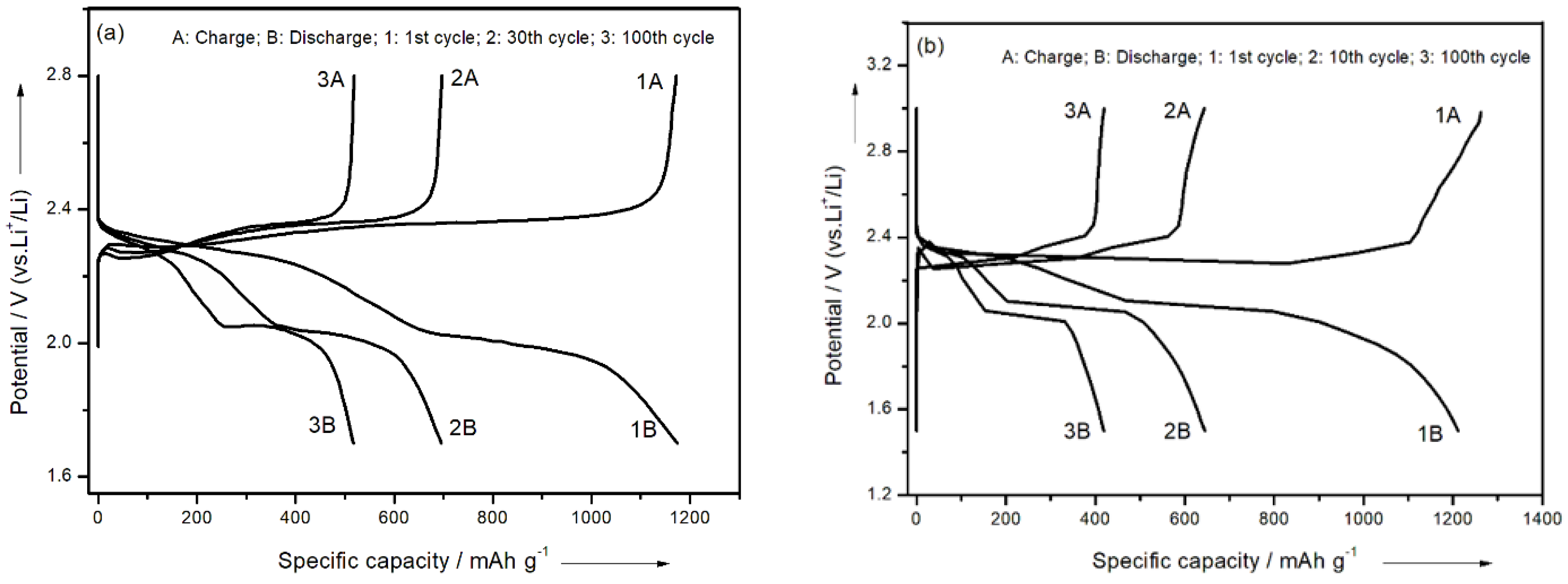
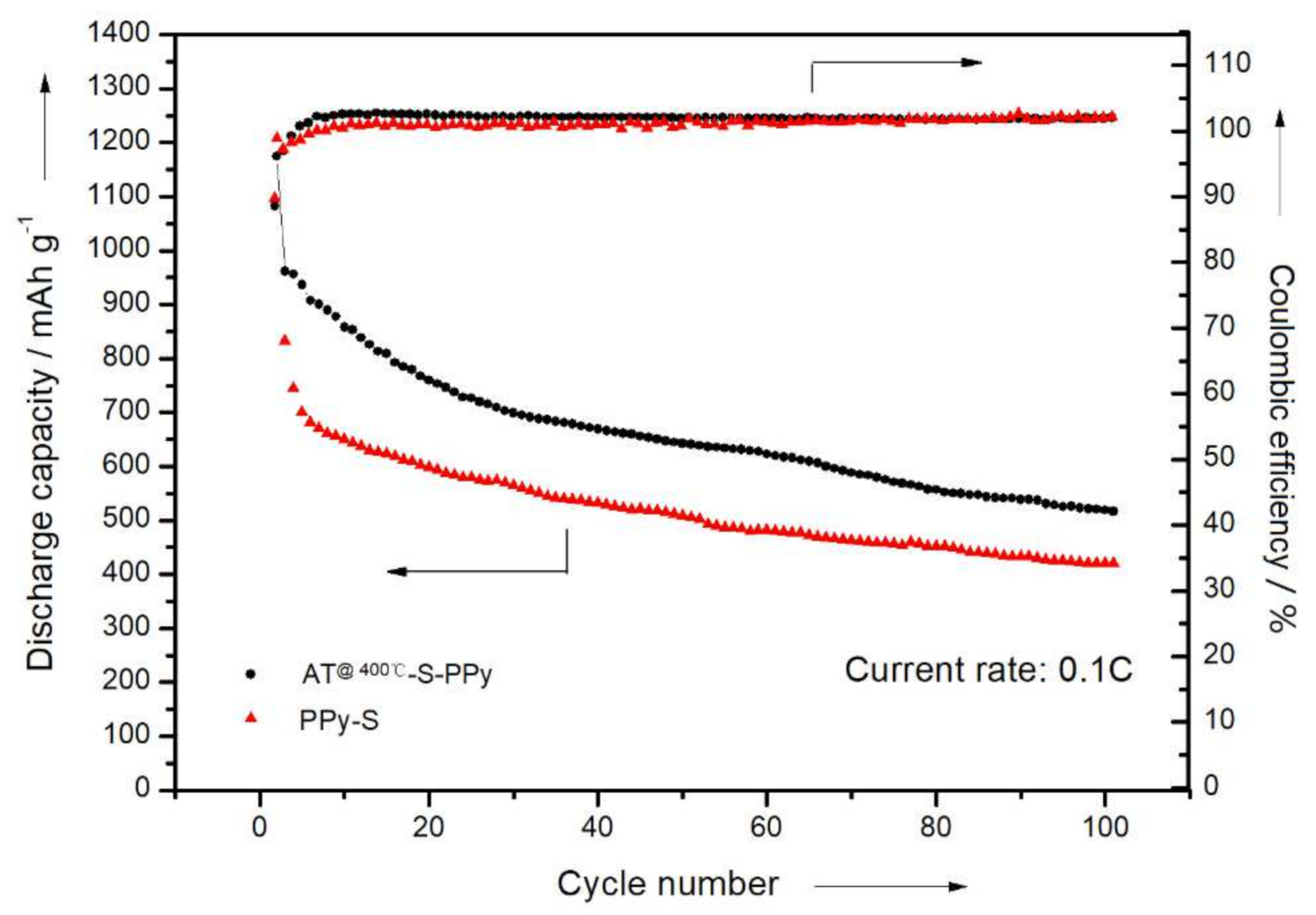
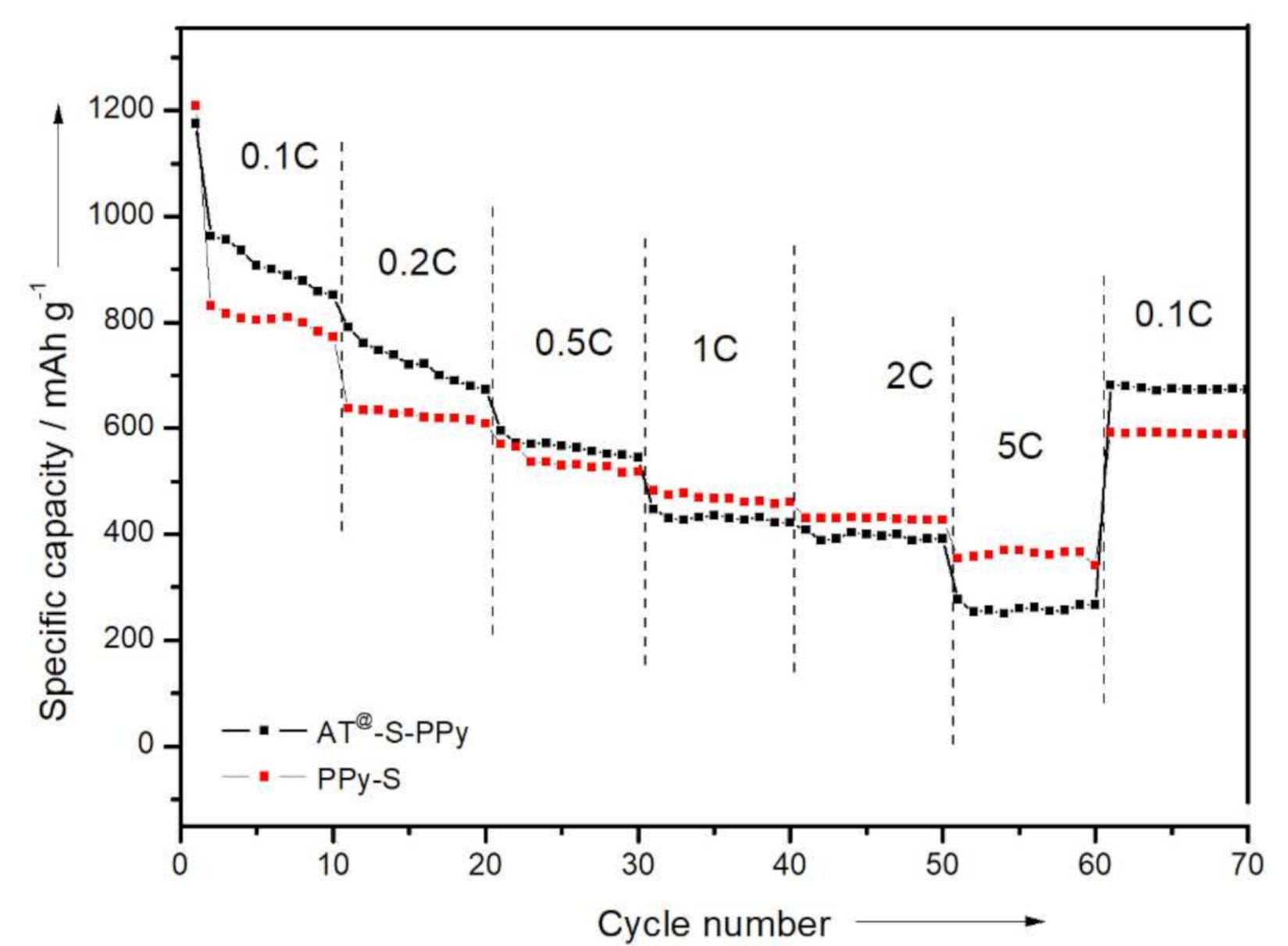
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dirlam, P.T.; Glass, R.S.; Char, K.; Pyun, J. The use of polymers in Li-S batteries: A review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1635–1668. [Google Scholar] [CrossRef]
- Rauh, R.D. A Lithium/Dissolved Sulfur Battery with an Organic Electrolyte. J. Electrochem. Soc. 1979, 126, 523. [Google Scholar] [CrossRef]
- Xiulei, J.; Kyu, T.L.; Linda, F.N. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. J. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; James, R.A. Polysulfide Shuttle Study in the li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Jiaqi, H.; Xiaofei, L.; Qiang, Z.; Chengmeng, C.; Mengqiang, Z.; Shumao, Z.; Wangcheng, Z.; Weizhong, Q.; Fei, W. Entrapment of sulfur in hierarchical porous graphene for lithium–sulfur batteries with high-rate performance from −40 to 60 °C. Nano Energy 2013, 2, 314–321. [Google Scholar] [CrossRef]
- Hailiang, W.; Yuan, Y.; Yongye, L.; Joshua, T.R.; Yanguang, L.; Ariel, J.; Yi, C.; Hongjie, D. Graphene-Wrapped Sulfur Particles as a Rechargeable Lithium-Sulfur Battery Cathode Material with High Capacity and Cycling Stability. J. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Barchasz, C.; Molton, F.; Duboc, C.; Leprêtre, J.-C.; Patoux, S.; Alloin, F. Lithium/Sulfur Cell Discharge Mechanism: An Original Approach for Intermediate Species Identification. Anal. Chem. 2012, 84, 3973–3980. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, G.; Xu, J.-L.; Zhang, M.; Kuo, C.-C.; Wang, S.-D. Conducting polymer-inorganic nanocomposite-based gas sensors: A review. Sci. Technol. Adv. Mater. 2020, 21, 768–786. [Google Scholar] [CrossRef]
- Saianand, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Saianand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Oh, S.-W.; Rhee, H.W.; Lee, C.; Kim, Y.C.; Kim, J.K.; Yu, J.W. The photovoltaic effect of the p–n heterojunction organic photovoltaic device using a nano template method. Curr. Appl. Phys. 2005, 5, 55–58. [Google Scholar] [CrossRef]
- Jérôme, C.; Labaye, D.; Jerome, R. Electrochemical formation of polypyrrole nanowires. Synth. Met. 2004, 142, 207–216. [Google Scholar] [CrossRef]
- Zhu, H.; Dang, Y.; Liu, Q.; Deng, X. Experimental and numerical simulation research of the physical and mechanical properties of Attapulgite clay. Sci. Sin. Phys. Mech. Astron. 2019, 49, 124601. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Ray, L.F.; Oliver, B.L.; Huada, R.J.; Theo, K. Near-infrared and mid-infrared spectroscopic study of sepiolites and palygorskites. Vib. Spectrosc. 2011, 27, 1–13. [Google Scholar] [CrossRef]
- Akyuz, S.; Akyüz, T.; Davies, J. FT-IR and FT-Raman spectral investigations of anatolian attapulgite and its interaction with 4,4′-bipyridyl. J. Mol. Struct. 1995, 349, 61–64. [Google Scholar] [CrossRef]
- Lu, Y.-P.; Zhang, M.-Q.; Feng, Q.; Long, T.; Ou, L.-M.; Zhang, G.-F. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Trans. Nonferrous Met. Soc. China 2011, 21, 208–213. [Google Scholar] [CrossRef]
- Zhang, T.; Li, T.; Liu, Y.; Li, Y.; Guo, G.; Cui, J.; Zhou, F. Preparation and Rheological Properties of Attapulgite Gel for Aqueous Suspensions. In Proceedings of the 2016 7th International Conference on Education, Management, Computer and Medicine (EMCM 2016), Shenyang, China, 29–31 December 2016; Atlantis Press: Zhengzhou, China, 2017; pp. 547–559. [Google Scholar] [CrossRef]
- Kawashima, S.; Chaouche, M.; Corr, D.J.; Shah, S.P. Influence of purified attapulgite clays on the adhesive properties of cement pastes as measured by the tack test. Cem. Concr. Compos. 2014, 48, 35–41. [Google Scholar] [CrossRef]
- Barrios, M.; González, L.; Rodríguez, M.; Pozas, J. Acid activation of a palygorskite with HCl: Development of physico-chemical, textural and surface properties. Appl. Clay Sci. 1995, 10, 247–258. [Google Scholar] [CrossRef]
- Tianhu, C.; Jian, W.; Chengsong, Q.; Shuchuan, P.; Genxian, S.; Yan, G. Effect of heat treatment on structure, morphology and surface properties of palygorskite. J. Chin. Ceram. Soc. 2006, 34, 1406–1410. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Chen, T.; Qing, C.; Zou, X.; Xie, J.; Zhang, X. Synthesis of palygorskite-supported Mn1−xCexO2 clusters and their performance in catalytic oxidation of formaldehyde. Appl. Clay Sci. 2018, 159, 50–59. [Google Scholar] [CrossRef]
- Park, J.H.; Ko, J.M.; Park, O.O.; Kim, D.-W. Capacitance properties of graphite/polypyrrole composite electrode prepared by chemical polymerization of pyrrole on graphite fiber. J. Power Source 2002, 105, 20–25. [Google Scholar] [CrossRef]
- Guo, B.; Liu, X.; Zhou, W.Y.; Lei, Y.; Jia, D. Adsorption of Ionic Liquid onto Halloysite Nanotubes: Mechanism and Reinforcement of the Modified Clay to Rubber. J. Macromol. Sci. Part B 2010, 49, 1029–1043. [Google Scholar] [CrossRef]
- Wu, L.; Dai, Y.; Zeng, W.; Huang, J.; Liao, B.; Pang, H. Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries. Nanotechnol. Rev. 2021, 10, 20–33. [Google Scholar] [CrossRef]
- Boki, K.; Sakamoto, N.; Minami, K. Inhibitory effect of 1,3-diglycerides during adsorption of β-carotene onto attapulgite and sepiolite. J. Am. Oil Chem. Soc. 2001, 78, 733–736. [Google Scholar] [CrossRef]
- Hannana, M.A.; Lipub, M.S.H.; Hussain, A.; Mohamed, A. A Rewiew of Lithum-Ion Battery State of Charge Estimation and Management System in Electric Vehicle Applications: Challenges and Recommendations. Renew. Sustain. Energ. Rev. 2017, 78, 834–854. [Google Scholar] [CrossRef]
- Sun, Z.; Xiao, M.; Wang, S.; Han, D.; Song, S.; Chen, G.; Meng, Y. Sulfur-rich polymeric materials with semi-interpenetrating network structure as a novel lithium–sulfur cathode. J. Mater. Chem. A 2014, 2, 9280–9286. [Google Scholar] [CrossRef]
- Shichao, Z.; Lan, Z.; Weikun, W. A Novel cathode material based on polyaniline used for lithium/sulfur secondary battery. Synth. Met. 2010, 160, 2041–2044. [Google Scholar] [CrossRef]
- Su, Y.-S.; Fu, Y.; Cochell, T.; Manthiram, A. A strategic approach to recharging lithium-sulphur batteries for long cycle life. Nat. Commun. 2013, 4, 2985. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ding, B.; Zhang, X. Effect of feeding ratios on the structure and electrochemical performance of graphite oxide/polypyrrole nanocomposites. Chin. Sci. Bull. 2011, 56, 2846–2852. [Google Scholar] [CrossRef][Green Version]
- Choi, Y.J.; Jung, B.S.; Lee, D.J.; Jeong, J.H.; Kim, K.W.; Ahn, H.J.; Cho, K.-K.; Gu, H.B. Electrochemical properties of sulfur electrode containing nano Al2O3 for lithium/sulfur cell. Phys. Scr. 2007, 62–65. [Google Scholar] [CrossRef]
- Kolosnitsyn, V.; Karaseva, E.V. Lithium-sulfur batteries: Problems and solutions. Russ. J. Electrochem. 2008, 44, 506–509. [Google Scholar] [CrossRef]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium-Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Barghamadi, M.; Best, A.S.; Bhatt, A.I.; Hollenkamp, A.; Musameh, M.; Rees, R.J.; Ruether, T. Lithium–sulfur batteries—the solution is in the electrolyte, but is the electrolyte a solution? Energy Environ. Sci. 2014, 7, 3902–3920. [Google Scholar] [CrossRef]
- Yamin, H.; Gorenshtein, A.; Penciner, J.; Sternberg, Y.; Peled, E. Lithium Sulfur Battery. Oxidation/Reduction Mechanisms of Polysulfides in THF Solutions. J. Electrochem. Soc. 1988, 19, 1045–1048. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.; Zheng, G.; Seh, Z.W.; Yao, H.; Cui, Y. Understanding the Role of Different Conductive Polymers in Improving the Nanostructured Sulfur Cathode Performance. Nano Lett. 2013, 13, 5534–5540. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, Y.; Chen, H.; Disalvo, F.J.; Abruña, H.D. Yolk–Shell Structure of Polyaniline-Coated Sulfur for Lithium–Sulfur Batteries. J. Am. Chem. Soc. 2013, 135, 16736–16743. [Google Scholar] [CrossRef]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse Vulcanization of Elemental Sulfur to Prepare Polymeric Electrode Materials for Li–S Batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef]
- Uzakbaiuly, B.; Mentbayeva, A.; Konarov, A.; Kurmanbayeva, I.; Zhang, Y.; Bakenov, Z. Evaluating Sulfur-Composite Cathode Material with Lithiated Graphite Anode in Coin Cell and Pouch Cell Configuration. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Chao, Y.; Wenjuan, W.; Zhigang, C.; Feng, L.; Tianhu, C. Sythesis of polypyrrole/attapulgite nanocomposite material and its conductivity. J. Chin. Ceram. Soc. 2009, 37, 2031–2036. [Google Scholar]
- Feng, H.X.; Liu, S.L.; Zhang, J.Q. Preparation and Characterization of Polypyrrole/Attapulgite with Organic Surface Modification Conducting Composite. Adv. Mater. Res. 2010, 160–162, 1126–1130. [Google Scholar] [CrossRef]


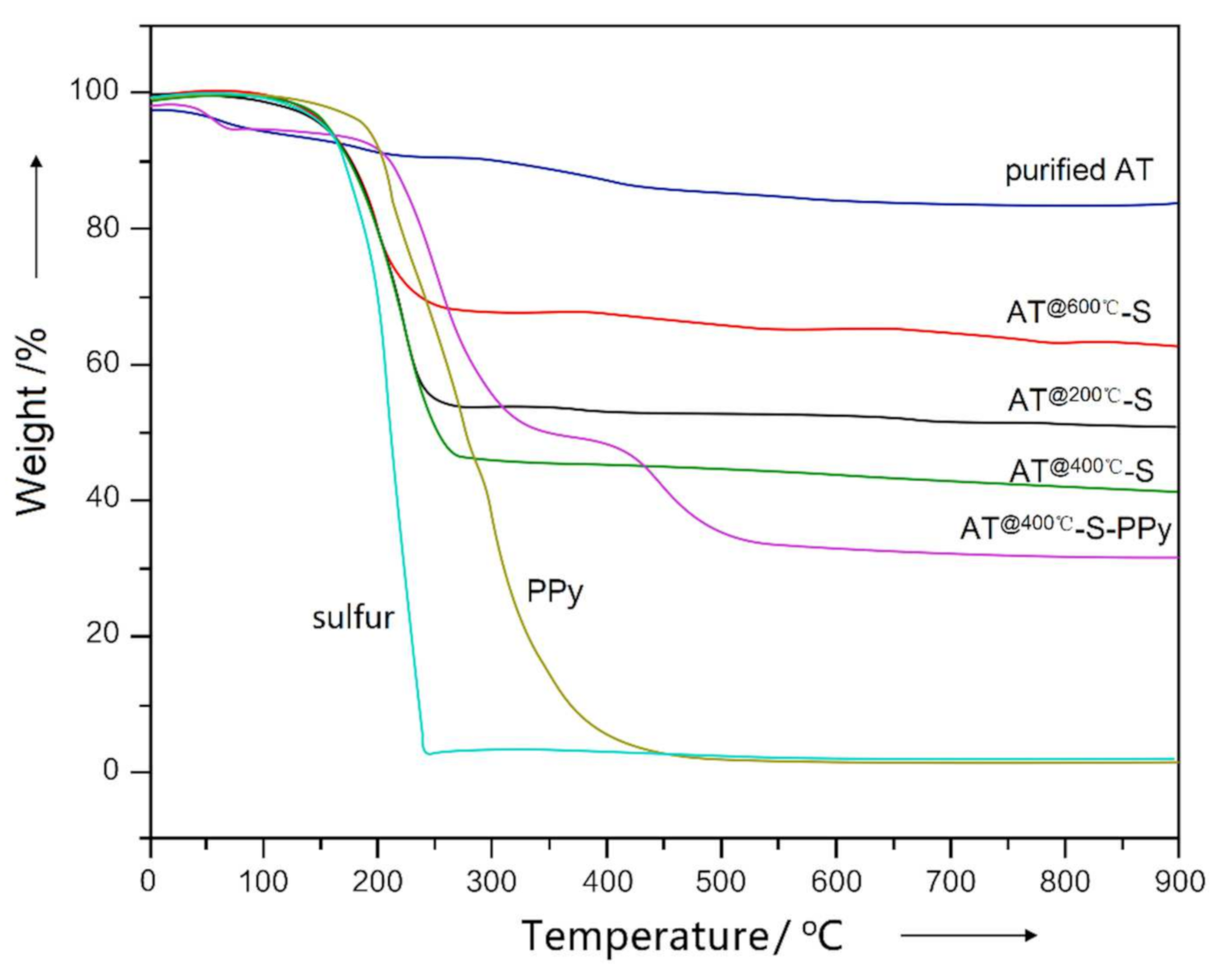
| AT System | Relative Sulfur Absorption Amount (wt.%) |
|---|---|
| AT1–S | 21 |
| AT2–S | 45 |
| AT @200 °C–S | 47 |
| AT @400 °C–S | 58 |
| AT @600 °C–S | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, R.; Wang, C.; Xiong, J. Lamellar Polypyrene Based on Attapulgite–Sulfur Composite for Lithium–Sulfur Battery. Membranes 2021, 11, 483. https://doi.org/10.3390/membranes11070483
Wang J, Xu R, Wang C, Xiong J. Lamellar Polypyrene Based on Attapulgite–Sulfur Composite for Lithium–Sulfur Battery. Membranes. 2021; 11(7):483. https://doi.org/10.3390/membranes11070483
Chicago/Turabian StyleWang, Jing, Riwei Xu, Chengzhong Wang, and Jinping Xiong. 2021. "Lamellar Polypyrene Based on Attapulgite–Sulfur Composite for Lithium–Sulfur Battery" Membranes 11, no. 7: 483. https://doi.org/10.3390/membranes11070483
APA StyleWang, J., Xu, R., Wang, C., & Xiong, J. (2021). Lamellar Polypyrene Based on Attapulgite–Sulfur Composite for Lithium–Sulfur Battery. Membranes, 11(7), 483. https://doi.org/10.3390/membranes11070483