Graphene/Fe3O4 Nanocomposite as a Promising Material for Chemical Current Sources: A Theoretical Study
Abstract
:1. Introduction
2. Methods
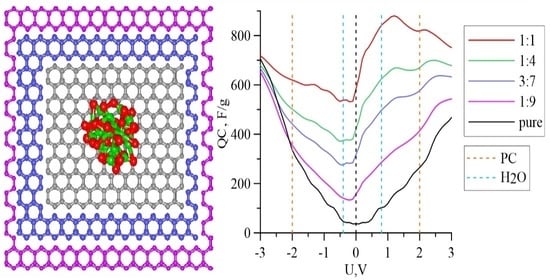
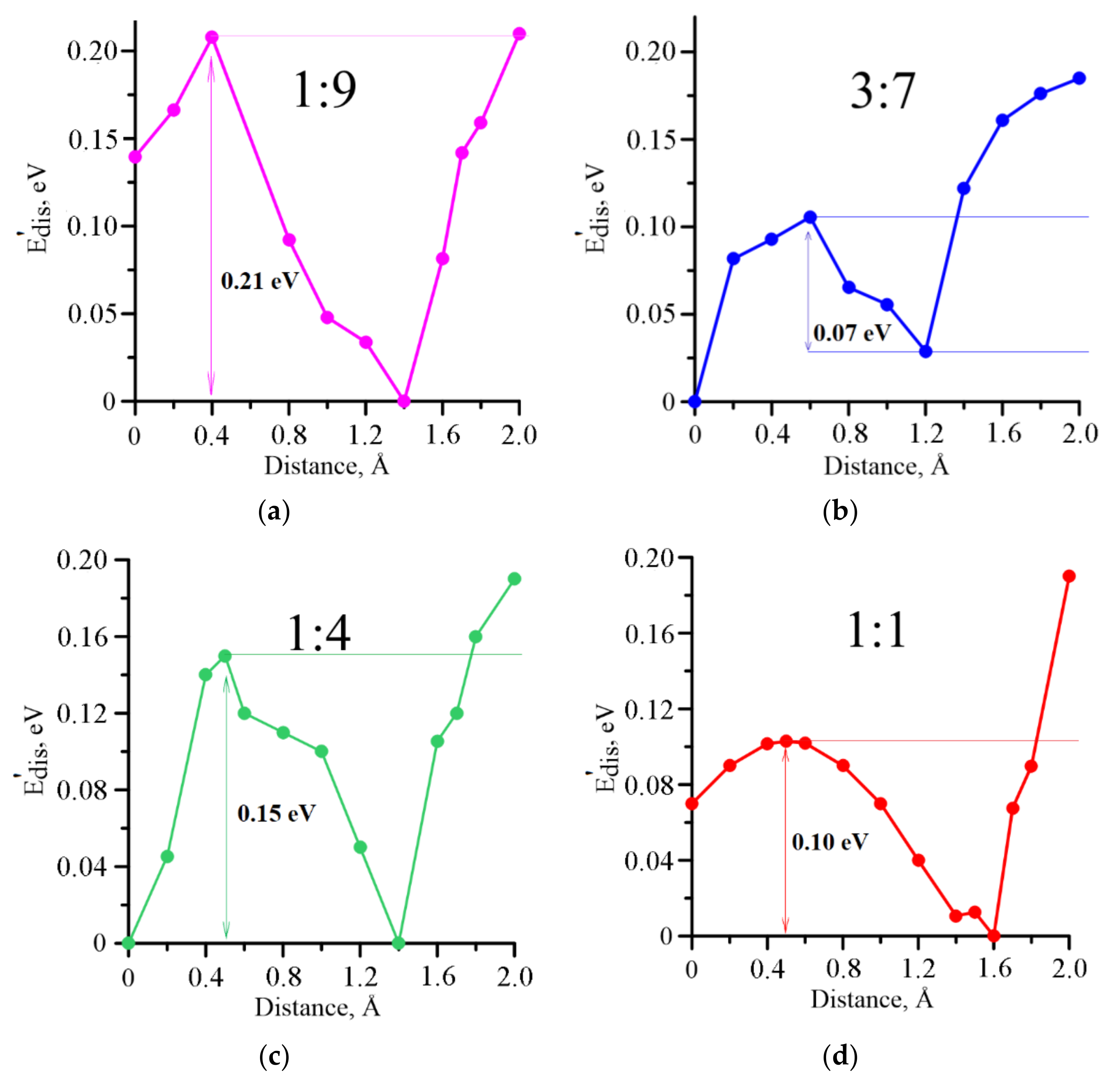
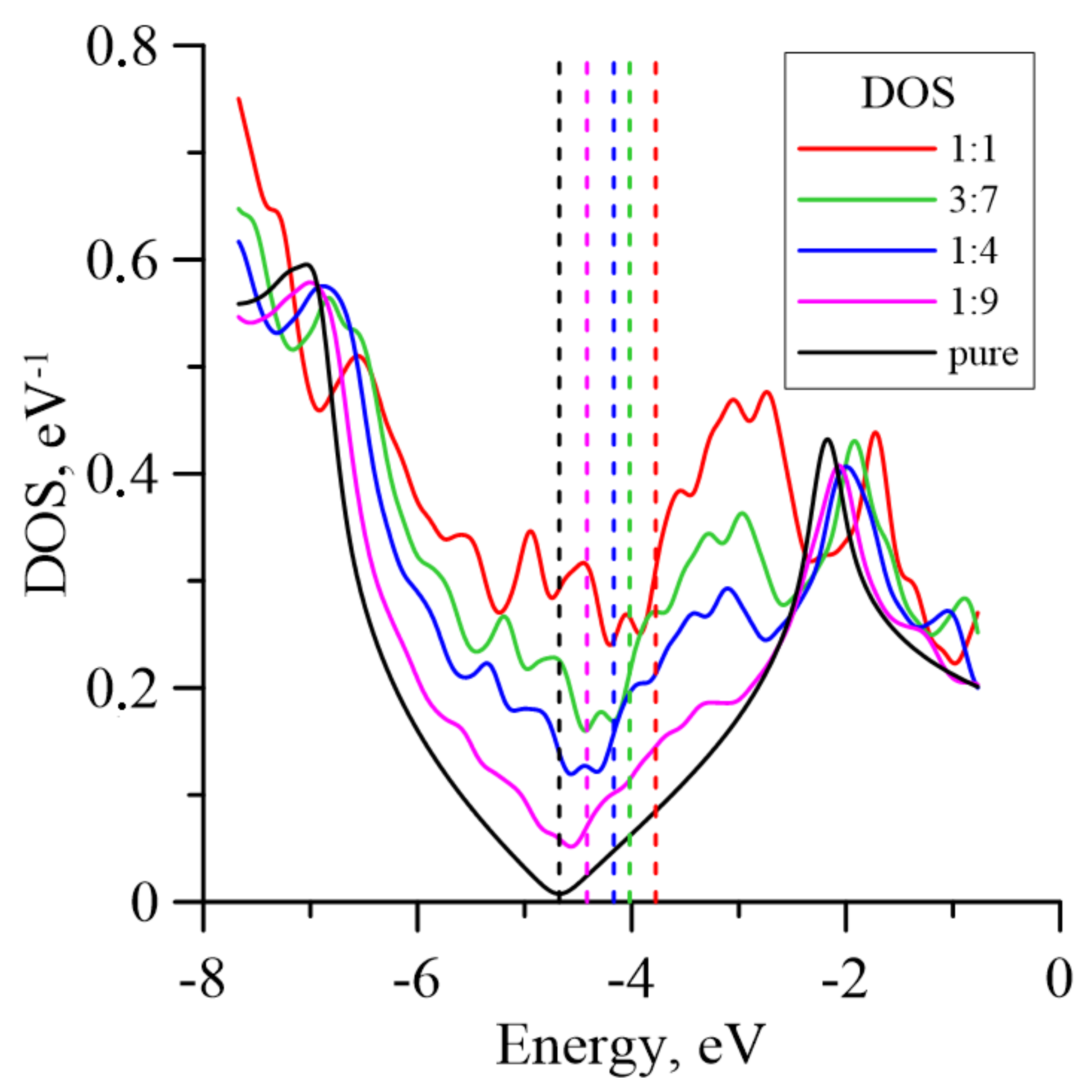
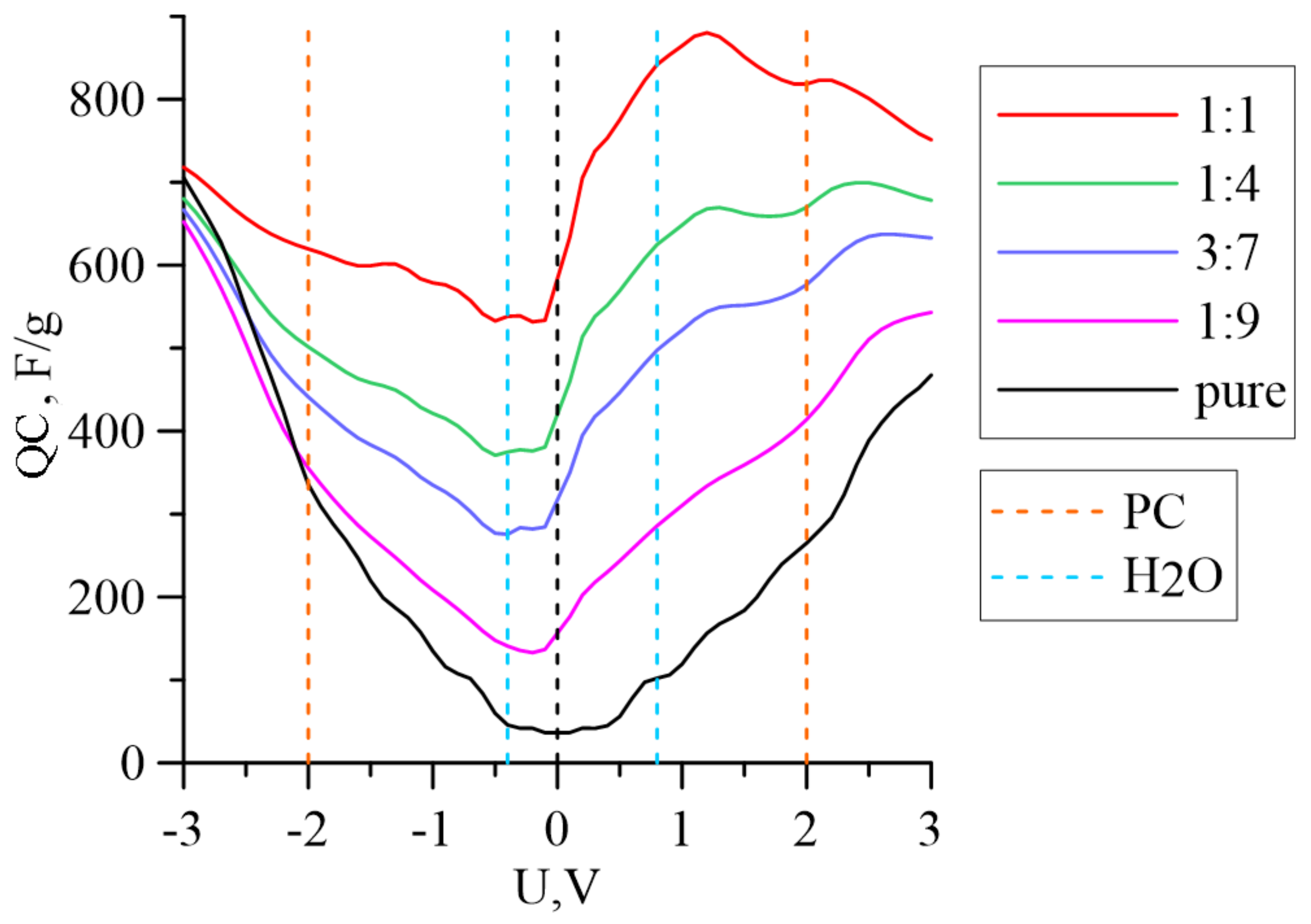
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azhar, A.; Yamauchi, Y.; Allah, A.E.; Alothman, Z.A.; Badjah, A.Y.; Naushad, M.; Habila, M.; Wabaidur, S.; Wang, J.; Zakaria, M.B. Nanoporous Iron Oxide/Carbon Composites through In-Situ Deposition of Prussian Blue Nanoparticles on Graphene Oxide Nanosheets and Subsequent Thermal Treatment for Supercapacitor Applications. Nanomaterials 2019, 9, 776. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Nan, H.; Hu, X.; Zhang, W.; Qiao, L.; Zeng, Y.; Tian, H. Graphene decorated iron oxide negative electrodes with high capacity, excellent rate performance, and wide working voltage for aqueous battery-supercapacitor hybrid devices. J. Alloys Compd. 2021, 864, 158147. [Google Scholar] [CrossRef]
- Samuel, J.; Shah, A.; Kumar, D.; Singh, L.R.; Mahato, M. Preparation, characterization and some electrochemical study of waste derived iron Oxide-Carbon nanocomposite. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Zhu, W.; Kierzek, K.; Wang, S.; Li, S.; Holze, R.; Chen, X. Improved performance in lithium ion battery of CNT-Fe3O4@graphene induced by three-dimensional structured construction. Colloids Surf. A Physicochem. Eng. 2021, 621, 126014. [Google Scholar] [CrossRef]
- Fan, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2019, 10, 1902485. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, R.; Mu, L.; Xu, S. Fe3O4 anodes for lithium batteries: Production techniques and general applications. Comptes R. Chim. 2019, 22, 96–102. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J. In situ growth of monodisperse Fe3O4 nanoparticles on graphene as flexible paper for supercapacitor. J. Mater. Chem. A 2014, 2, 12068–12074. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, M.; Yue, W.; Jiang, Y.; Wang, Y.; Ren, Y.; Hu, F. Sandwich-Structured Graphene-Fe3O4@Carbon Nanocomposites for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 9709–9715. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, J.; Sim, D.H.; Tay, Y.Y.; Lu, Z.; Zhang, X.; Sharma, Y.; Srinivasan, M.; Zhang, H.; Hnga, H.H.; et al. Achieving high specific charge capacitances in Fe3O4/reduced graphene oxide nanocomposites. J. Mater. Chem. 2011, 21, 3422–3427. [Google Scholar] [CrossRef]
- Huang, J.-L.; Fan, L.-Q.; Gu, Y.; Geng, C.-L.; Luo, H.; Huang, Y.-F.; Lin, J.-M.; Wu, J.-H. One-step solvothermal synthesis of high-capacity Fe3O4/reduced graphene oxide composite for use in Li-ion capacitor. J. Alloys Compd. 2019, 788, 1119–1126. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Zhang, X.; Sun, X.; Wang, K.; Ma, Y. High Performance Lithium-Ion Hybrid Capacitors Employing Fe3O4−Graphene Composite Anode and Activated Carbon Cathode. ACS Appl. Mater. Interfaces 2017, 9, 17136–17144. [Google Scholar] [CrossRef]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterisation of reduced graphene oxide: Effects of reduction variables on electrical conductivity. MSEB 2015, 193, 49–60. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Ahmad, I.; Israr, M. Graphene/Fe3O4 nanocomposite: Interplay between photo-Fenton type reaction, and carbon purity for the removal of methyl orange. Ceram. Int. 2018, 44, 2643–2648. [Google Scholar] [CrossRef]
- Taufik, A.; Saleh, R. The Role of graphene in Fe3O4/graphene Composites on the Adsorption of Methylene Blue and Their Kinetic Study. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Sanur-Bali, Indonesia, 19–20 October 2016; Volume 196, p. 012003. [Google Scholar] [CrossRef] [Green Version]
- Nene, A.; Takahashi, M.; Somani, P.R.; Aryal, H.R.; Wakita, K.; Umeno, M. Synthesis and characterization of graphene-Fe3O4 nanocomposite. Carbon-Sci. Tech. 2016, 8, 13–24. [Google Scholar]
- Eskusson, J.; Rauwel, P.; Nerut, J.; Janes, A. A Hybrid Capacitor Based on Fe3O4-Graphene Nanocomposite/Few-Layer Graphene in Different Aqueous Electrolytes. J. Electrochem. Soc. 2016, 163, A2768–A2775. [Google Scholar] [CrossRef]
- Gu, S.; Zhu, A. Graphene nanosheets loaded Fe3O4 nanoparticles as a promising anode material for lithium ion batteries. J. Alloy Compd. 2020, 813, 152160. [Google Scholar] [CrossRef]
- Mi, W.; Yang, H.; Cheng, Y.; Chen, G.; Bai, H. Magnetic and electronic properties of Fe3O4/graphene heterostructures: First principles perspective. J. Appl. Phys. 2013, 113, 083711. [Google Scholar] [CrossRef] [Green Version]
- Al-Bagawi, A.H.; Bayoumy, A.M.; Ibrahim, M.A. Molecular modeling analyses for graphene functionalized with Fe3O4 and NiO. Heliyon 2020, 6, e04456. [Google Scholar] [CrossRef]
- Shunaev, V.V.; Slepchenkov, M.M.; Glukhova, O.E. Single-Shell Carbon Nanotubes Covered with Iron Nanoparticles for Ion-Lithium Batteries: Thermodynamic Stability and Charge Transfer. Top. Catal. 2018, 61, 1716–1720. [Google Scholar] [CrossRef]
- Shunaev, V.V.; Ushakov, A.V.; Glukhova, O.E. Increase of γ-Fe2O3/CNT composite quantum capacitance by structural design for performance optimization of electrode materials. Int. J. Quantum Chem. 2020, 120, e26165. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Lin, J.-Y.; Mo, C.-Y. Improved storage capacity and rate capability of Fe3O4–graphene anodes for lithium-ion batteries. Electrochim. Acta 2011, 58, 119–124. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Zheng, G.; Witek, H.A.; Bobadova-Parvanova, P.; Irle, S.; Musaev, D.G.; Prabhakar, R.; Morokuma, K.; Lundberg, M.; Elstner, M.; Kohler, C.; et al. Parameter Calibration of Transition-Metal Elements for the Spin-Polarized Self-Consistent-Charge Density-Functional Tight-Binding (DFTB) Method: Sc, Ti, Fe, Co, and Ni. J. Chem. Theory Comput. 2007, 3, 1349–1367. [Google Scholar] [CrossRef] [Green Version]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. J. Chem. Phys. 1995, 23, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Wood, B.C.; Ogitsu, T.; Otani, M.; Biener, J. First-Principles-Inspired Design Strategies for Graphene-Based Supercapacitor Electrodes. J. Phys. Chem. C 2014, 118, 4–15. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, G.; Fan, X.; Zheng, W. Improving the Quantum Capacitance of Graphene-Based Supercapacitors by the Doping and Co-Doping: First-Principles Calculations. ACS Omega 2019, 4, 13209–13217. [Google Scholar] [CrossRef] [Green Version]
- Kolosov, D.A.; Glukhova, O.E. Boron-Decorated Pillared Graphene as the Basic Element for Supercapacitors: An Ab Initio Study. Appl. Sci. 2021, 11, 3496. [Google Scholar] [CrossRef]
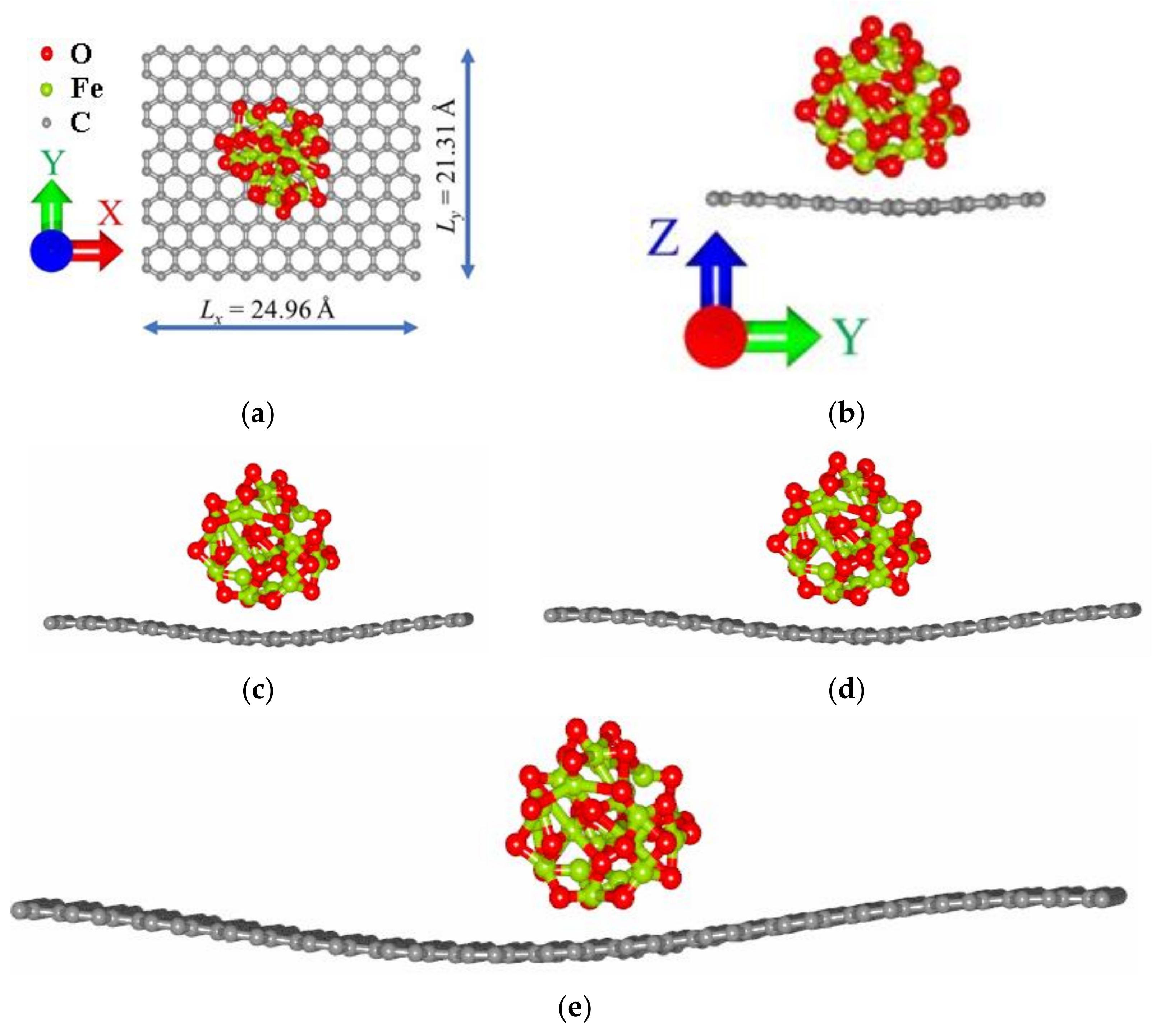
| m(Fe3O4):m(G) | Lx, Å | Ly, Å | Eb, eV | Ef, eV | Δq(Fe3O4)/n(C), me |
|---|---|---|---|---|---|
| 1:9 | 62.41 | 59.63 | −2.36 | −4.42 | 1.15 |
| 1:4 | 37.44 | 38.34 | −2.65 | −4.27 | 3.23 |
| 3:7 | 32.45 | 29.80 | −1.53 | −4.02 | 4.29 |
| 1:1 | 24.96 | 21.31 | −1.99 | −3.78 | 7.10 |
| m(Fe3O4):m(G) | −0.4 V (red. H2O) | 0.8 V (ox. H2O) | −2.0 V (red. PC) | 2.0 V (ox. PC) |
|---|---|---|---|---|
| Pure graphene | 45.8 | 102.2 | 335.8 | 265.0 |
| 1:9 | −15.8 | 129.2 | 198.6 | 257.3 |
| 1:4 | −41.5 | 180.7 | 123.9 | 259.3 |
| 3:7 | −45.6 | 204.6 | 81.1 | 249.4 |
| 1:1 | −36.4 | 267.2 | 44.8 | 243.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shunaev, V.V.; Glukhova, O.E. Graphene/Fe3O4 Nanocomposite as a Promising Material for Chemical Current Sources: A Theoretical Study. Membranes 2021, 11, 642. https://doi.org/10.3390/membranes11080642
Shunaev VV, Glukhova OE. Graphene/Fe3O4 Nanocomposite as a Promising Material for Chemical Current Sources: A Theoretical Study. Membranes. 2021; 11(8):642. https://doi.org/10.3390/membranes11080642
Chicago/Turabian StyleShunaev, Vladislav V., and Olga E. Glukhova. 2021. "Graphene/Fe3O4 Nanocomposite as a Promising Material for Chemical Current Sources: A Theoretical Study" Membranes 11, no. 8: 642. https://doi.org/10.3390/membranes11080642
APA StyleShunaev, V. V., & Glukhova, O. E. (2021). Graphene/Fe3O4 Nanocomposite as a Promising Material for Chemical Current Sources: A Theoretical Study. Membranes, 11(8), 642. https://doi.org/10.3390/membranes11080642