Regulation of GABAA Receptors Induced by the Activation of L-Type Voltage-Gated Calcium Channels
Abstract
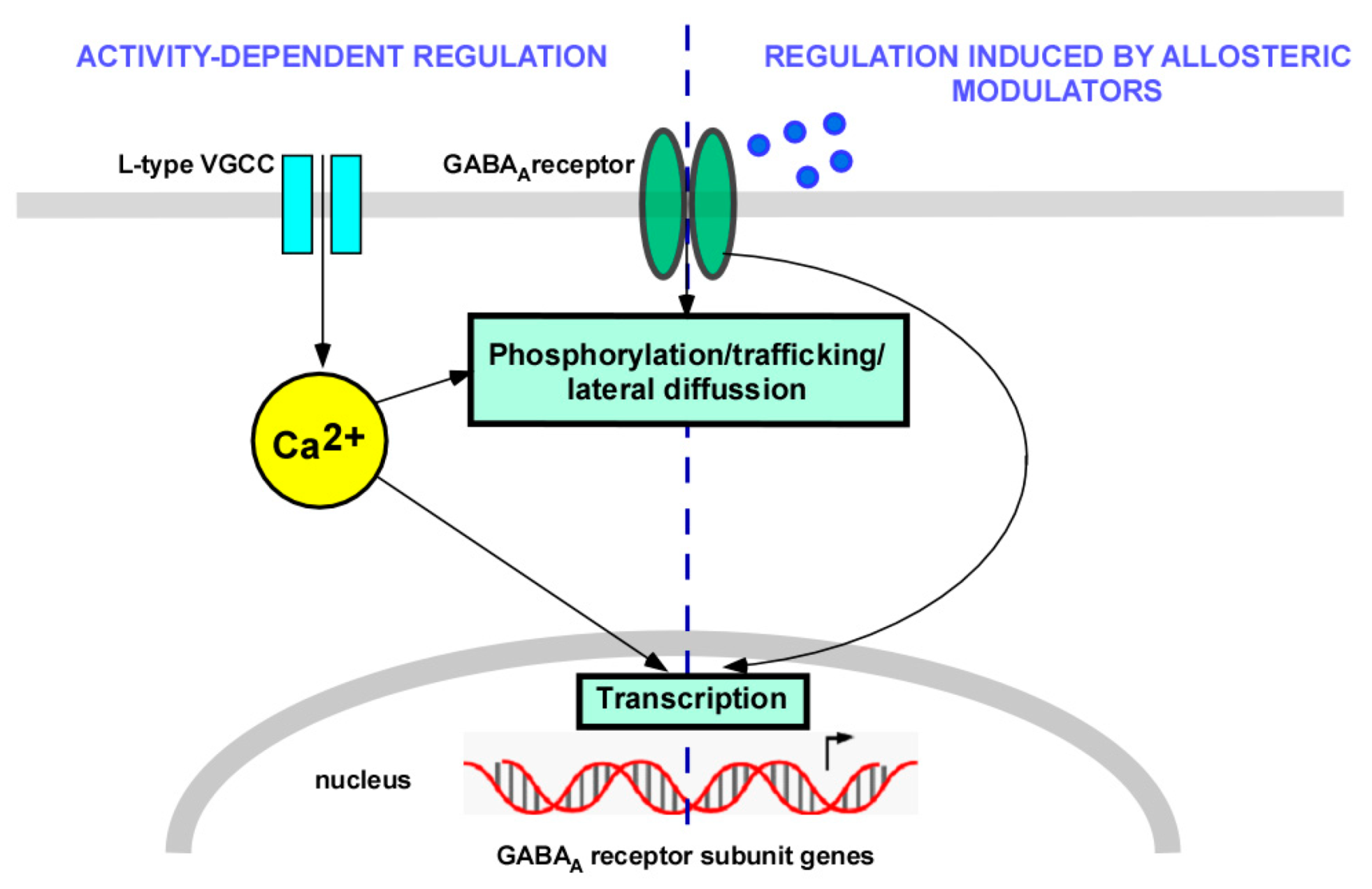
1. Introduction
2. Transcriptional Regulation
3. Trafficking Regulation
4. Phosphorylation Regulation
4.1. PKA
4.2. CaMKII
5. Regulation of Clustering/Lateral Diffusion
6. Regulation of GABAA Receptors by Other Sources of Calcium
7. Conclusions and Future Directions
- -
- To further investigate the physiological role of GABAA receptor plasticity, more in vivo studies are required.
- -
- Future experiments are needed to differentiate the specific mechanisms of GABAA receptor regulation that occur during normal development from the ones that persist into adulthood.
- -
- The plastic mechanisms of receptor regulation associated with neurodevelopmental disorders, such as epilepsy, autism, and schizophrenia, should be also investigated.
- -
- As activity-dependent plasticity can occur simultaneously at inhibitory and excitatory synapses, it is important to study how these two processes are coordinated.
Funding
Data Availability Statement
Conflicts of Interest
References
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABA-A receptor subtypes. Curr. Top. Med. Chem. 2005, 2, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.; Nicoll, R.A. The GABA A receptor b subunit is required for inhibitory transmission report the GABA A receptor b subunit is required for inhibitory transmission. Neuron 2018, 98, 718–725. [Google Scholar] [CrossRef]
- Martenson, J.S.; Yamasaki, T.; Chaudhury, N.H.; Albrecht, D.; Tomita, S. Assembly rules for GABAA receptor complexes in the brain. Elife 2017, 6, e27443. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.C.; Pendolino, V.; Kontou, G.; McGee, T.P.; Sheehan, D.F.; López-Doménech, G.; Farrant, M.; Kittler, J.T. An essential role for the tetraspanin LHFPL4 in the cell-type-specific targeting and clustering of synaptic GABAA receptors. Cell Rep. 2017, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Hoyos-Ramirez, E.; Martenson, J.S.; Morimoto-Tomita, M.; Tomita, S. GARLH family proteins stabilize GABAA receptors at synapses. Neuron 2017, 93, 1138–1152. [Google Scholar] [CrossRef]
- Ge, Y.; Kang, Y.; Cassidy, R.M.; Moon, K.M.; Lewis, R.; Wong, R.O.L.; Foster, L.J.; Craig, A.M. Clptm1 limits forward trafficking of GABAA receptors to scale inhibitory synaptic strength. Neuron 2018, 97, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, J.; Pelkey, K.A.; Pandey, S.; Chen, X.; Wang, Y.X.; Wu, K.; Ge, L.; Li, T.; Castellano, D.; et al. Shisa7 is a GABAAreceptor auxiliary subunit controlling benzodiazepine actions. Science 2019, 366, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Shepard, R.D.; Lu, W. Regulation of GABAARs by transmembrane accessory proteins. Trends Neurosci. 2021, 44, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, W.; Nelson, T.J.; Alkon, D.L. Theta rhythm of hippocampal CA1 neuron activity: Gating by GABAergic synaptic depolarization. J. Neurophysiol. 2001, 85, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Navarro, V.; Clemenceau, S.; Baulac, M.; Miles, R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 2002, 298, 1418–1421. [Google Scholar] [CrossRef]
- Timofeev, I.; Grenier, Ã.F.; Steriade, M. The role of cholride-dependent inhibition and the activity of fast-spiking neurons during cortical spike-wave electrographic seizures. Neuroscience 2002, 114, 1115–1132. [Google Scholar] [CrossRef]
- Fujiwara-Tsukamoto, Y.; Isomura, Y.; Kaneda, K.; Takada, M. Synaptic interactions between pyramidal cells and interneurone subtypes during seizure-like activity in the rat hippocampus. J. Physiol. 2004, 557, 961–979. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Macdonald, R.L. A structural look at GABA A receptor mutations linked to epilepsy syndromes. Brain Res. 2019, 1714, 234–247. [Google Scholar] [CrossRef]
- Brooks-Kayal, A.R.; Shumate, M.D.; Jin, H.; Rikhter, T.Y.; Coulter, D.A. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 1998, 4, 1166–1172. [Google Scholar] [CrossRef]
- Braat, S.; Kooy, R.F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 2015, 86, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Casanova, M.F.; Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Brown, G.L.; Edelson, S.M.; Slattery, J.C.; Adams, J.B. Neuropathological mechanisms of seizures in autism spectrum disorder. Front. Neurosci. 2016, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef]
- Maguire, J. Neuroactive steroids and GABaergic involvement in the neuroendocrine dysfunction associated with major depressive disorder and postpartum depression. Front. Cell. Neurosci. 2019, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Wong, A.H.C. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol. Sin. 2018, 39, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Rissman, R.A.; Mobley, W.C. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer’s disease. J. Neurochem. 2011, 117, 613–622. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Dolphin, A.C. Voltage-gated calcium channels and their auxiliary subunits: Physiology and pathophysiology and pharmacology. J. Physiol. 2016, 594, 5369–5390. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Benito, E.; Barco, A. CREB’s control of intrinsic and synaptic plasticity: Implications for CREB-dependent memory models. Trends Neurosci. 2010, 33, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Greer, P.L.; Greenberg, M.E. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron 2008, 59, 846–860. [Google Scholar] [CrossRef]
- Ghosh, A.; Carnahan, J.; Greenberg, M.E. Requirement for BDNF in activity-dependent survival of cortical neurons. Science 1994, 263, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Yap, E.L.; Greenberg, M.E. Activity-regulated transcription: Bridging the gap between neural activity and behavior. Neuron 2018, 100, 330–348. [Google Scholar] [CrossRef]
- Zieg, J.; Greer, P.L.; Greenberg, M.E. SnapShot: Ca2+-dependent transcription in neurons. Cell 2008, 134, 1080.e2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bengtson, C.P.; Bading, H. Nuclear calcium signaling. Adv. Exp. Med. Biol. 2012, 970, 377–405. [Google Scholar] [CrossRef]
- Steiger, J.L.; Russek, S.J. GABA A receptors: Building the bridge between subunit mRNAs, their promoters, and cognate transcription factors. Pharmacol. Ther. 2004, 101, 259–281. [Google Scholar] [CrossRef]
- Joyce, C.J. In silico comparative genomic analysis of GABAA receptor transcriptional regulation. BMC Genom. 2007, 8, 203. [Google Scholar] [CrossRef]
- Hu, Y.; Lund, I.V.; Gravielle, M.C.; Farb, D.H.; Brooks-kayal, A.R.; Russek, S.J. Surface expression of GABA A receptors is transcriptionally controlled by the interplay of cAMP-response element-binding protein and its binding partner inducible cAMP early repressor. J. Biol. Chem. 2008, 283, 9328–9340. [Google Scholar] [CrossRef]
- Li, Z.; Cogswell, M.; Hixson, K.; Brooks-Kayal, A.R.; Russek, S.J. Nuclear respiratory Factor 1 (NRF-1) controls the activity dependent transcription of the GABA-A receptor Beta 1 subunit gene in neurons. Front. Mol. Neurosci. 2018, 11, 285. [Google Scholar] [CrossRef]
- Nair, B.; Johar, K.; Priya, A.; Wong-Riley, M.T.T. Specificity protein 4 (Sp4) transcriptionally regulates inhibitory GABAergic receptors in neurons. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 1–9. [Google Scholar] [CrossRef]
- Quadrato, G.; Elnaggar, M.Y.; Duman, C.; Sabino, A.; Forsberg, K.; Di Giovanni, S. Modulation of GABAA receptor signaling increases neurogenesis and suppresses anxiety through NFATc4. J. Neurosci. 2014, 34, 8630–8645. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Kim, C.H.; Lee, D.; Sun, W.; Lee, H.W.; Kim, H. Early growth response 1 (Egr-1) directly regulates GABAA receptor α2, α4, and θ subunits in the hippocampus. J. Neurochem. 2015, 133, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.S.; Raol, Y.H.; Bandyopadhyay, S.; Lund, I.V.; Budreck, E.C.; Passini, M.A.; Wolfe, J.H.; Brooks-kayal, A.R.; Russek, S.J. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA A receptor α4 subunit expression. Proc. Nat. Acad. Sci. USA 2005, 102, 11894–11899. [Google Scholar] [CrossRef] [PubMed]
- Gangisetty, O.; Reddy, D.S. Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience 2010, 170, 865–880. [Google Scholar] [CrossRef]
- Grabenstatter, H.L.; Russek, S.J.; Brooks-Kayal, A.R. Molecular pathways controlling inhibitory receptor expression. Epilepsia 2012, 53 (Suppl. S9), 71–78. [Google Scholar] [CrossRef]
- Porcher, C.; Medina, I.; Gaiarsa, J.L. Mechanism of BDNF modulation in GABAergic synaptic transmission in healthy and disease brains. Front. Cell. Neurosci. 2018, 12, 273. [Google Scholar] [CrossRef]
- Riffault, B.; Medina, I.; Dumon, C.; Thalman, C.; Ferrand, N.; Friedel, P.; Gaiarsa, J.L.; Porcher, C. Pro-brain-derived neurotrophic factor inhibits gabaergic neurotransmission by activating endocytosis and repression of GABAA receptors. J. Neurosci. 2014, 34, 13516–13534. [Google Scholar] [CrossRef] [PubMed]
- Lund, I.V.; Hu, Y.; Raol, Y.S.H.; Benham, R.S.; Faris, R.; Russek, S.J.; Brooks-Kayal, A.R. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci. Signal. 2008, 1, ra9. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.S.; Hu, Y.; Lund, I.V.; Brooks-kayal, A.R.; Russek, S.J. Brain-derived neurotrophic factor ( BDNF ) -induced synthesis of early growth response Factor 3 ( Egr3 ) controls the levels of type A GABA receptor α4 subunits in hippocampal neurons. J. Biol. Chem. 2006, 281, 29431–29435. [Google Scholar] [CrossRef] [PubMed]
- Porcher, C.; Hatchett, C.; Longbottom, R.E.; McAinch, K.; Sihra, T.S.; Moss, S.J.; Thomson, A.M.; Jovanovic, J.N. Positive feedback regulation between γ-aminobutyric acid type A (GABAA) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J. Biol. Chem. 2011, 286, 21667–21677. [Google Scholar] [CrossRef]
- Bateson, A. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr. Pharm. Des. 2002, 8, 5–21. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Olivier, B. Mechanisms underlying tolerance after long-term benzodiazepine use: A future for subtype-selective GABA(A) receptor modulators? Adv. Pharmacol. Sci. 2012, 2012, 416864. [Google Scholar] [CrossRef]
- Gravielle, M.C. Neurochemistry international regulation of GABA A receptors by prolonged exposure to endogenous and exogenous ligands. Neurochem. Int. 2018, 118, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Gravielle, M.C. Activation-induced regulation of GABA A receptors: Is there a link with the molecular basis of benzodiazepine tolerance? Pharmacol. Res. 2016, 109, 92–100. [Google Scholar] [CrossRef]
- Mehta, A.K.; Ticku, M.K. Chronic GABA exposure down-regulates GABA-benzodiazepine receptor-ionophore complex in cultured cerebral cortical neurons. Mol. Brain Res. 1992, 16, 29–36. [Google Scholar] [CrossRef]
- Lyons, H.R.; Gibbs, T.T.; Farb, D.H. Turnover and down-regulation of GABA(A) receptor α1, β2S, and γ1 subunit mRNAs by neurons in culture. J. Neurochem. 2000, 74, 1041–1048. [Google Scholar] [CrossRef]
- Russek, S.J.; Bandyopadhyay, S.; Farb, D.H. An initiator element mediates autologous down-regulation of the human type A γ-aminobutyric acid receptor β1 subunit gene. Proc. Natl. Acad. Sci. USA 2000, 97, 8600–8605. [Google Scholar] [CrossRef]
- Lyons, H.R.; Land, M.B.; Gibbs, T.T.; Farb, D.H. Distinct signal transduction pathways for GABA-induced GABA(A) receptor down-regulation and uncoupling in neuronal culture: A role for voltage-gated calcium channels. J. Neurochem. 2001, 78, 1114–1126. [Google Scholar] [CrossRef]
- Xiang, K.; Tietz, E.I. Chronic benzodiazepine-induced reduction in GABAA receptor-mediated synaptic currents in hippocampal CA1 pyramidal neurons prevented by prior nimodipine injection. Neuroscience 2008, 157, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Foitzick, M.F.; Medina, N.B.; Iglesias García, L.C.; Gravielle, M.C. Benzodiazepine exposure induces transcriptional down-regulation of GABAA receptor α1 subunit gene via L-type voltage-gated calcium channel activation in rat cerebrocortical neurons. Neurosci. Lett. 2020, 721, 134801. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.J.; Messing, R.O. Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem. Int. 1999, 35, 95–101. [Google Scholar] [CrossRef]
- Xiang, K.; Earl, D.E.; Davis, K.M.; Giovannucci, D.R.; Greenfield, L.J., Jr.; Tietz, E.I. Chronic benzodiazepine administration potentiates high voltage-activated calcium currents in hippocampal CA1 neurons. J. Pharmacol. Exp. Ther. 2008, 327, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Shibasaki, M.; Kurokawa, K.; Tsujimura, A.; Ohkuma, S. Up-regulation of L-type high voltage-gated calcium channel subunits by sustained exposure to 1,4- and 1,5-benzodiazepines in cerebrocortical neurons. J. Neurochem. 2007, 103, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Mehmet, H.; Penrice, J.; Cooper, C.; Cady, E.; Wyatt, J.S.; Reynolds, E.O.R.; Edwards, A.D.; Squier, M.V. Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropathol. Appl. Neurobiol. 1997, 23, 16–25. [Google Scholar] [CrossRef]
- Wang, L.; Greenfield, L.J. Post-hypoxic changes in rat cortical neuron GABAA receptor function require L-type voltage-gated calcium channel activation. Neuropharmacology 2009, 56, 198–207. [Google Scholar] [CrossRef][Green Version]
- Gao, L.; Lyons, A.R.; Greenfield, L.J. Hypoxia alters GABAA receptor function and subunit expression in NT2-N neurons. Neuropharmacology 2004, 46, 318–330. [Google Scholar] [CrossRef]
- Xiang, K.; Earl, D.; Dwyer, T.; Behrle, B.L.; Tietz, E.I.; Greenfield, L.J. Hypoxia enhances high-voltage-activated calcium currents in rat primary cortical neurons via calcineurin. Epilepsy Res. 2012, 99, 293–305. [Google Scholar] [CrossRef][Green Version]
- Kurotani, T.; Yamada, K.; Yoshimura, Y.; Crair, M.C.; Komatsu, Y. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron 2008, 57, 905–916. [Google Scholar] [CrossRef]
- Saliba, R.S.; Gu, Z.; Yan, Z.; Moss, S.J. Blocking L-type voltage-gated Ca2+ channels with dihydropyridines reduces γ-aminobutyric acid type A receptor expression and synaptic inhibition. J. Biol. Chem. 2009, 284, 32544–32550. [Google Scholar] [CrossRef]
- Nakamura, Y.; Darnieder, L.M.; Deeb, T.Z.; Moss, S.J. Regulation of GABAARs by phosphorylation. In Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 72, pp. 97–146. [Google Scholar]
- Lorenz-Guertin, J.M.; Jacob, T.C. GABA type a receptor trafficking and the architecture of synaptic inhibition. Dev. Neurobiol. 2018, 78, 238–270. [Google Scholar] [CrossRef]
- McDonald, B.J.; Amato, A.; Connolly, C.N.; Benke, D.; Moss, S.J.; Smart, T.G. Adjacent phosphorylation sites on GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nat. Neurosci. 1998, 1, 23–28. [Google Scholar] [CrossRef]
- Moss, S.J.; Smart, T.G.; Blackstone, C.D.; Huganir, R.L. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science 1992, 257, 661–665. [Google Scholar] [CrossRef]
- Nakamura, Y.; Morrow, D.H.; Nathanson, A.J.; Henley, J.M.; Wilkinson, K.A.; Moss, S.J. Phosphorylation on Ser-359 of the α2 subunit in GABA type A receptors down-regulates their density at inhibitory synapses. J. Biol. Chem. 2020, 295, 12330–12342. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, J.P.; Carlson, S.L.; Morrow, A.L. Differential regulation of synaptic and extrasynaptic α4 GABA(A) receptor populations by protein kinase A and protein kinase C in cultured cortical neurons. Neuropharmacology 2016, 105, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Harney, S.C.; Frenguelli, B.G.; Lambert, J.J. Phosphorylation influences neurosteroid modulation of synaptic GABA A receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology 2003, 45, 873–883. [Google Scholar] [CrossRef]
- Carlson, S.L.; Kumar, S.; Werner, D.F.; Comerford, C.E.; Morrow, A.L. Ethanol activation of protein kinase a regulates GABAA α1 receptor function and trafficking in cultured cerebral Cortical Neurons. J. Pharmacol. Exp. Ther. 2013, 345, 317–325. [Google Scholar] [CrossRef]
- Carlson, S.L.; Bohnsack, J.P.; Morrow, A.L. Ethanol regulation of synaptic GABAA α4 receptors is prevented by protein kinase A activation. J. Pharmacol. Exp. Ther. 2016, 357, 10–16. [Google Scholar] [CrossRef]
- Carlson, S.L.; Bohnsack, J.P.; Patel, V.; Morrow, A.L. Regulation of extrasynaptic GABAA α4 receptors by ethanol-induced protein kinase A, but not protein kinase C activation in cultured rat cerebral cortical neurons. J. Pharmacol. Exp. Ther. 2016, 356, 148–156. [Google Scholar] [CrossRef]
- Kumar, S.; Ren, Q.; Beckley, J.H.; O’Buckley, T.K.; Gigante, E.D.; Santerre, J.L.; Werner, D.F.; Morrow, A.L. Ethanol activation of protein kinase A regulates GABA A receptor subunit expression in the cerebral cortex and contributes to ethanol-induced hypnosis. Front. Neurosci. 2012, 6, 44. [Google Scholar] [CrossRef]
- Lilly, S.M.; Zeng, X.J.; Tietz, E.I. Role of protein kinase A in GABAA receptor dysfunction in CA1 pyramidal cells following chronic benzodiazepine treatment. J. Neurochem. 2003, 85, 988–998. [Google Scholar] [CrossRef]
- Ali, N.J.; Olsen, R.W. Chronic benzodiazepine treatment of cells expressing recombinant GABAA receptors uncouples allosteric binding: Studies on possible mechanisms. J. Neurochem. 2001, 79, 1100–1108. [Google Scholar] [CrossRef]
- Houston, C.M.; Smart, T.G. CaMK-II modulation of GABAA receptors expressed in HEK293, NG108-15 and rat cerebellar granule neurons. Eur. J. Neurosci. 2006, 24, 2504–2514. [Google Scholar] [CrossRef]
- Houston, C.M.; Lee, H.H.C.; Hosie, A.M.; Moss, S.J.; Smart, T.G. Identification of the sites for CaMK-II-dependent phosphorylation of GABAA receptors. J. Biol. Chem. 2007, 282, 17855–17865. [Google Scholar] [CrossRef]
- Houston, C.M.; Hosie, A.M.; Smart, T.G. Distinct regulation of β2 and β3 subunit-containing cerebellar synaptic GABAa receptors by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 2008, 28, 7574–7584. [Google Scholar] [CrossRef]
- Sachidanandan, D.; Reddy, H.P.; Mani, A.; Hyde, G.J.; Bera, A.K. The neuropeptide orexin-A inhibits the GABAA receptor by PKC and Ca2+/CaMKII-dependent phosphorylation of its β1 subunit. J. Mol. Neurosci. 2017, 61, 459–467. [Google Scholar] [CrossRef]
- Kano, M.; Rexhausen, U.; Dreessen, J.; Konnerth, A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature 1992, 356, 601–604. [Google Scholar] [CrossRef]
- Kawaguchi, S.Y.; Hirano, T. Suppression of inhibitory synaptic potentiation by presynaptic activity through postsynaptic GABA(B) receptors in a Purkinje neuron. Neuron 2000, 27, 339–347. [Google Scholar] [CrossRef]
- Tanaka, S.; Kawaguchi, S.Y.; Shioi, G.; Hirano, T. Long-term potentiation of inhibitory synaptic transmission onto cerebellar Purkinje neurons contributes to adaptation of vestibulo-ocular reflex. J. Neurosci. 2013, 33, 17209–17220. [Google Scholar] [CrossRef][Green Version]
- Hirano, T.; Kawaguchi, S.Y. Regulation and functional roles of rebound potentiation at cerebellar stellate cell-Purkinje cell synapses. Front. Cell. Neurosci. 2014, 8, 42. [Google Scholar] [CrossRef]
- Hirano, T. Regulation and interaction of multiple types of synaptic plasticity in a purkinje neuron and their contribution to motor learning. Cerebellum 2018, 17, 756–765. [Google Scholar] [CrossRef]
- Kano, M.; Kano, M.; Fukunaga, K.; Konnerth, A. Ca2+-induced rebound potentiation of γ-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc. Natl. Acad. Sci. USA 1996, 93, 13351–13356. [Google Scholar] [CrossRef]
- Kawaguchi, S.Y.; Hirano, T. Sustained structural change of GABAA receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar purkinje neuron. J. Neurosci. 2007, 27, 6788–6799. [Google Scholar] [CrossRef]
- Kawaguchi, S.Y.; Hirano, T. Signaling cascade regulating long-term potentiation of GABAA receptor responsiveness in cerebellar Purkinje neurons. J. Neurosci. 2002, 22, 3969–3976. [Google Scholar] [CrossRef]
- Saliba, R.S.; Kretschmannova, K.; Moss, S.J. Activity-dependent phosphorylation of GABA A receptors regulates receptor insertion and tonic current. EMBO J. 2012, 31, 2937–2951. [Google Scholar] [CrossRef]
- Churn, S.B.; Rana, A.; Lee, K.; Travis Parsons, J.; De Blas, A.; Delorenzo, R.J. Calcium/calmodulin-dependent kinase II phosphorylation of the GABAA receptor α1 subunit modulates benzodiazepine binding. J. Neurochem. 2002, 82, 1065–1076. [Google Scholar] [CrossRef]
- Bogdanov, Y.; Michels, G.; Armstrong-Gold, C.; Haydon, P.G.; Lindstrom, J.; Pangalos, M.; Moss, S.J. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006, 25, 4381–4389. [Google Scholar] [CrossRef]
- Jacob, T.C.; Bogdanov, Y.D.; Magnus, C.; Saliba, R.S.; Kittler, J.T.; Haydon, P.G.; Moss, S.J. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J. Neurosci. 2005, 25, 10469–10478. [Google Scholar] [CrossRef]
- Oh, W.C.; Lutzu, S.; Castillo, P.E.; Kwon, H.B. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science 2016, 353, 1037–1040. [Google Scholar] [CrossRef]
- Bannai, H.; Lévi, S.; Schweizer, C.; Inoue, T.; Launey, T.; Racine, V.; Sibarita, J.B.; Mikoshiba, K.; Triller, A.; Levi, S.; et al. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron 2009, 62, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.; Kittler, J.T. Plasticity of GABAA receptor diffusion dynamics at the axon initial segment. Front. Cell. Neurosci. 2014, 8, 151. [Google Scholar] [CrossRef]
- Geisler, S.; Schöpf, C.L.; Stanika, R.; Kalb, M.; Campiglio, M.; Repetto, D.; Traxler, L.; Missler, M.; Obermair, G.J. Presynaptic α2δ-2 calcium channel subunits regulate postsynaptic GABAA receptor abundance and axonal wiring. J. Neurosci. 2019, 39, 2581–2605. [Google Scholar] [CrossRef] [PubMed]
- Marsden, K.C.; Beattie, J.B.; Friedenthal, J.; Carroll, R.C. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABAA receptors. J. Neurosci. 2007, 27, 14326–14337. [Google Scholar] [CrossRef]
- Marsden, K.C.; Shemesh, A.; Bayer, K.U.; Carroll, R.C. Selective translocation of Ca2+/calmodulin protein kinase IIα (CaMKIIα) to inhibitory synapses. Proc. Natl. Acad. Sci. USA 2010, 107, 20559–20564. [Google Scholar] [CrossRef]
- Petrini, E.M.; Barberis, A. Diffusion dynamics of synaptic molecules during inhibitory postsynaptic plasticity. Front. Cell. Neurosci. 2014, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Luscher, B.; Fuchs, T.; Kilpatrick, C.L. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 2011, 70, 385–409. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.H.; Haditsch, U.; Tu, W.H.; Cochrane, K.; Ahmadian, G.; Tran, L.; Paw, J.; Wang, Y.T.; Mansuy, I.; et al. Interaction of calcineurin and type-A GABA receptor γ2 subunits produces long-term depression at CA1 inhibitory synapses. J. Neurosci. 2003, 23, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.; Arancibia-Carcamo, I.L.; MacAskill, A.F.; Smith, K.R.; Griffin, L.D.; Kittler, J.T. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the γ2 subunit. Proc. Natl. Acad. Sci. USA 2010, 107, 16679–16684. [Google Scholar] [CrossRef] [PubMed]
- Bannai, H.; Niwa, F.; Sherwood, M.W.; Shrivastava, A.N.; Arizono, M.; Miyamoto, A.; Sugiura, K.; Lévi, S.; Triller, A.; Mikoshiba, K. Bidirectional control of synaptic GABAAR clustering by glutamate and calcium. Cell Rep. 2015, 13, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.L.; Pilli, J.; Lorenz-Guertin, J.M.; Das, S.; Moon, C.E.; Graff, N.; Jacob, T.C. Depolarizing, inhibitory GABA type A receptor activity regulates GABAergic synapse plasticity via ERK and BDNF signaling. Neuropharmacology 2018, 128, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.; Szulc, B.; Walker, M.C.; Kittler, J.T. Activation of calcineurin underlies altered trafficking of α2 subunit containing GABAA receptors during prolonged epileptiform activity. Neuropharmacology 2015, 88, 82–90. [Google Scholar] [CrossRef]
- Han, D.Y.; Guan, B.J.; Wang, Y.J.; Hatzoglou, M.; Mu, T.W. L-type calcium channel blockers enhance trafficking and function of epilepsy-associated α1(D219N) subunits of GABAA receptors. ACS Chem. Biol. 2015, 10, 2135–2148. [Google Scholar] [CrossRef]
- Nicholson, M.W.; Sweeney, A.; Pekle, E.; Alam, S.; Ali, A.B.; Duchen, M.; Jovanovic, J.N. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/ Ca2+/calcineurin signalling downstream of GABAA receptors. Mol. Psychiatry 2018, 23, 1851–1867. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravielle, M.C. Regulation of GABAA Receptors Induced by the Activation of L-Type Voltage-Gated Calcium Channels. Membranes 2021, 11, 486. https://doi.org/10.3390/membranes11070486
Gravielle MC. Regulation of GABAA Receptors Induced by the Activation of L-Type Voltage-Gated Calcium Channels. Membranes. 2021; 11(7):486. https://doi.org/10.3390/membranes11070486
Chicago/Turabian StyleGravielle, María Clara. 2021. "Regulation of GABAA Receptors Induced by the Activation of L-Type Voltage-Gated Calcium Channels" Membranes 11, no. 7: 486. https://doi.org/10.3390/membranes11070486
APA StyleGravielle, M. C. (2021). Regulation of GABAA Receptors Induced by the Activation of L-Type Voltage-Gated Calcium Channels. Membranes, 11(7), 486. https://doi.org/10.3390/membranes11070486





