Enhanced Performance of Membrane Distillation Using Surface Heating Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
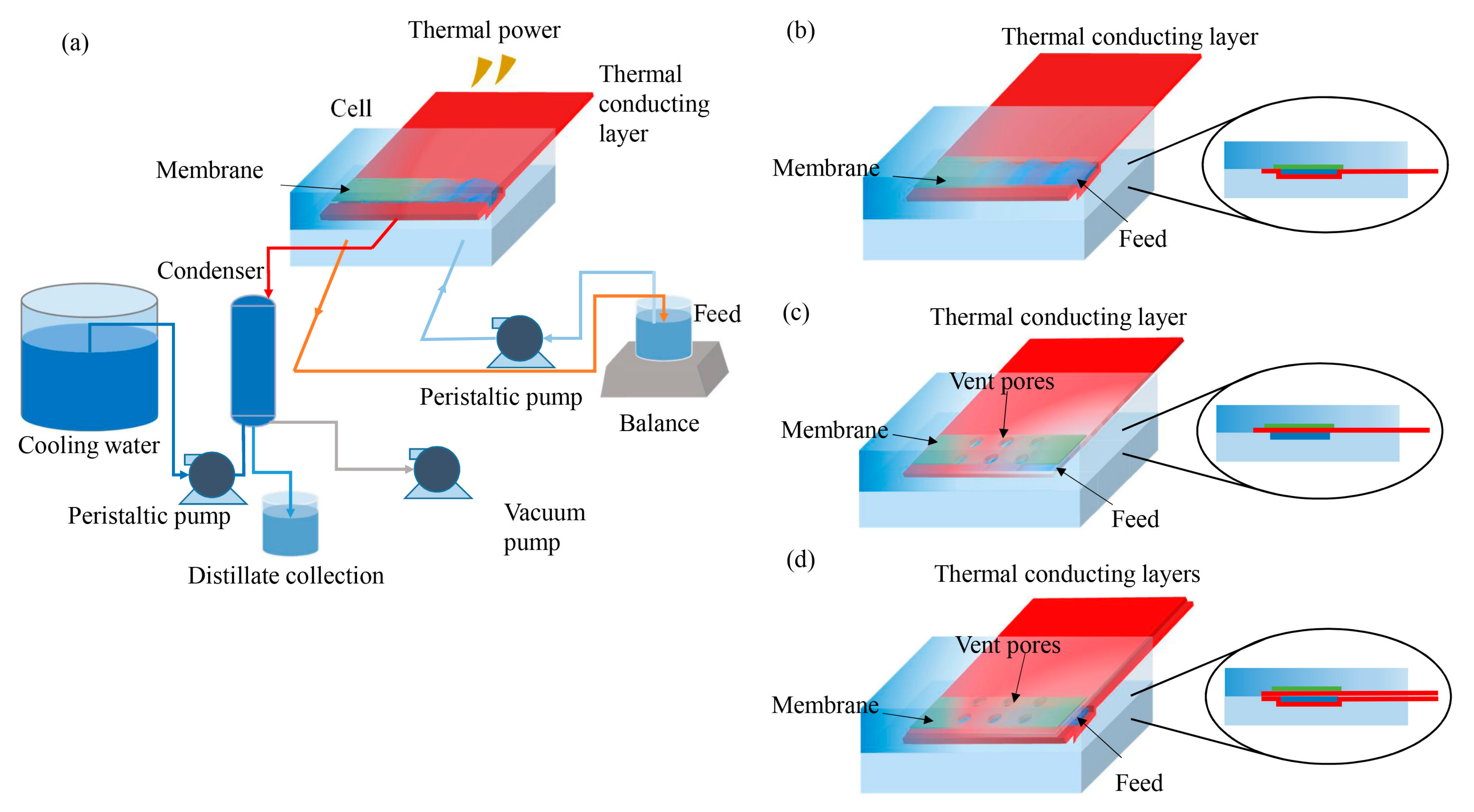
2.2. Surface Heating System Design
2.3. Surface-Heating VMD Experiments
2.4. System Performance
2.4.1. Distillate Flux
2.4.2. Rejection
2.4.3. Temperature Polarization Coefficient
2.4.4. Heat Transfer Performance
3. Results and Discussion
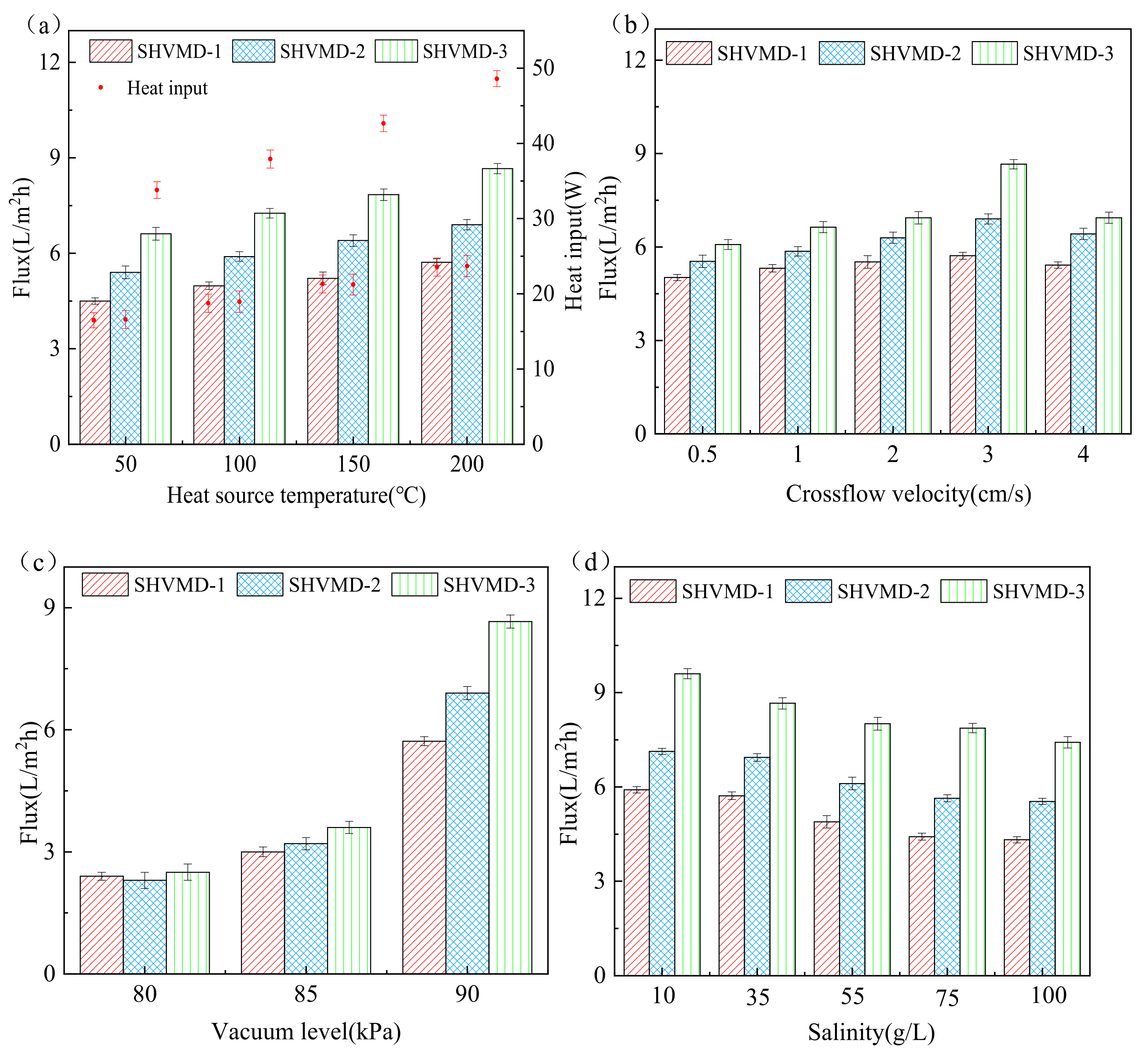
3.1. Effect of Operating Conditions on Distillate Flux
3.2. TPC
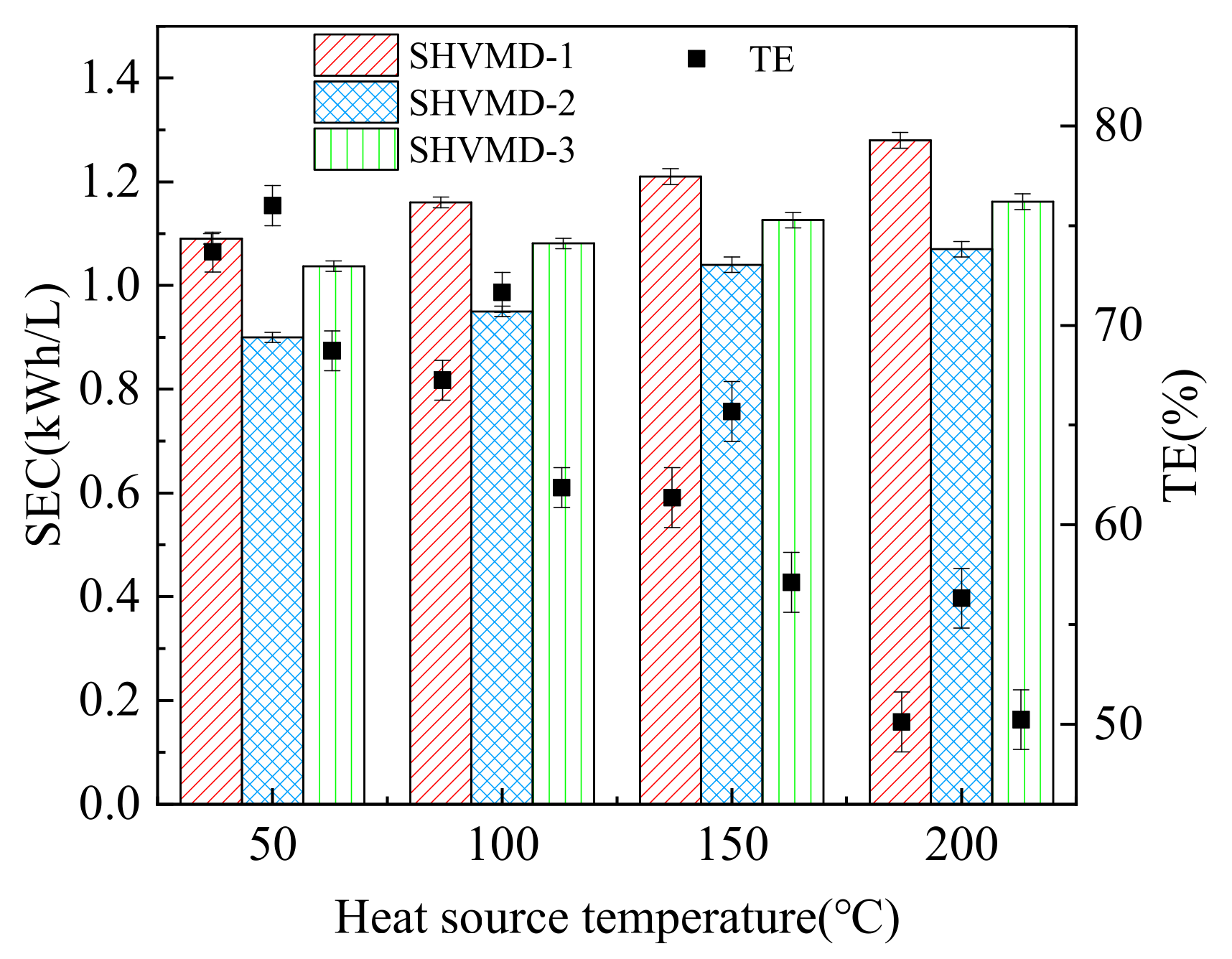
3.3. Energy Performance
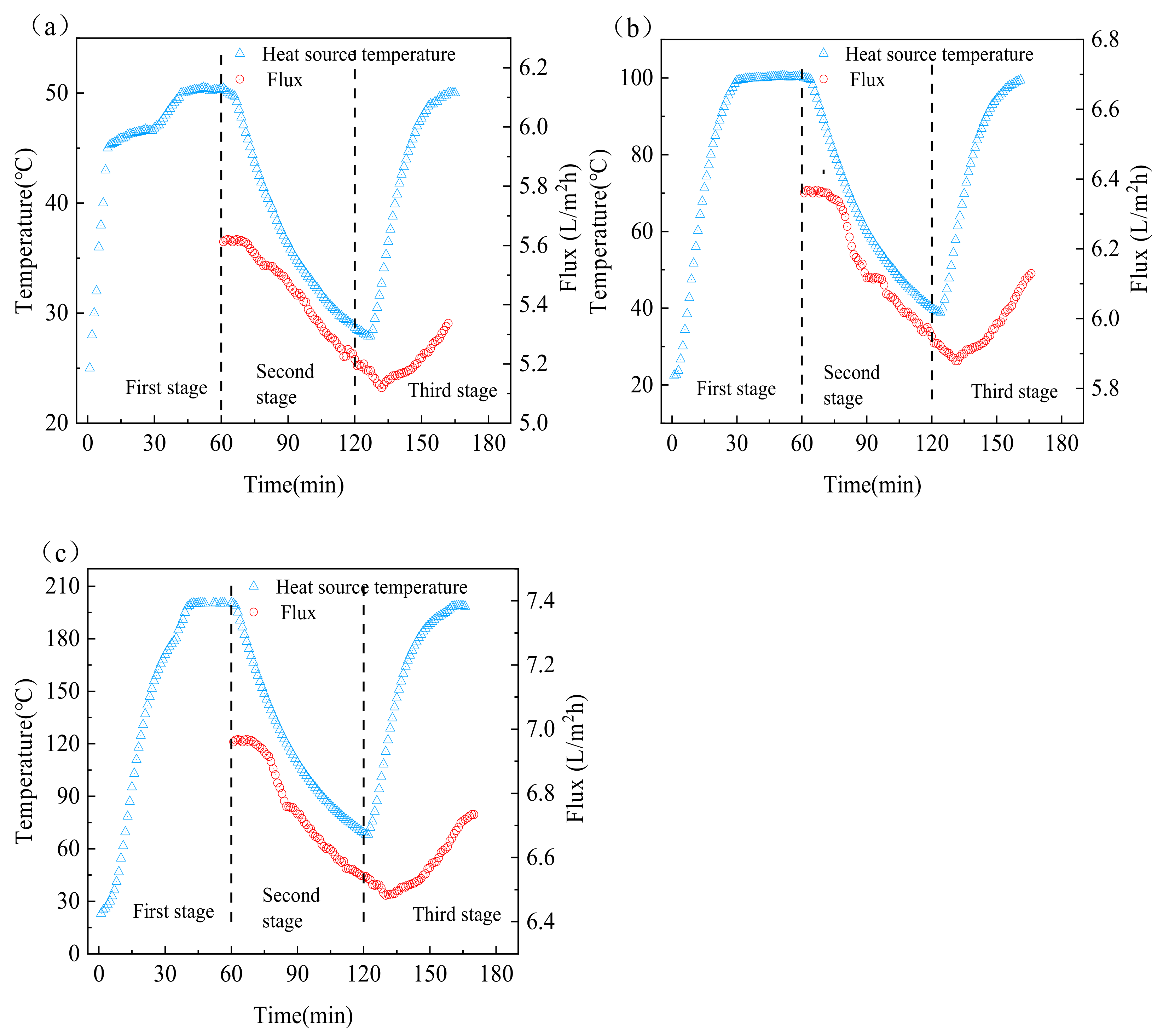
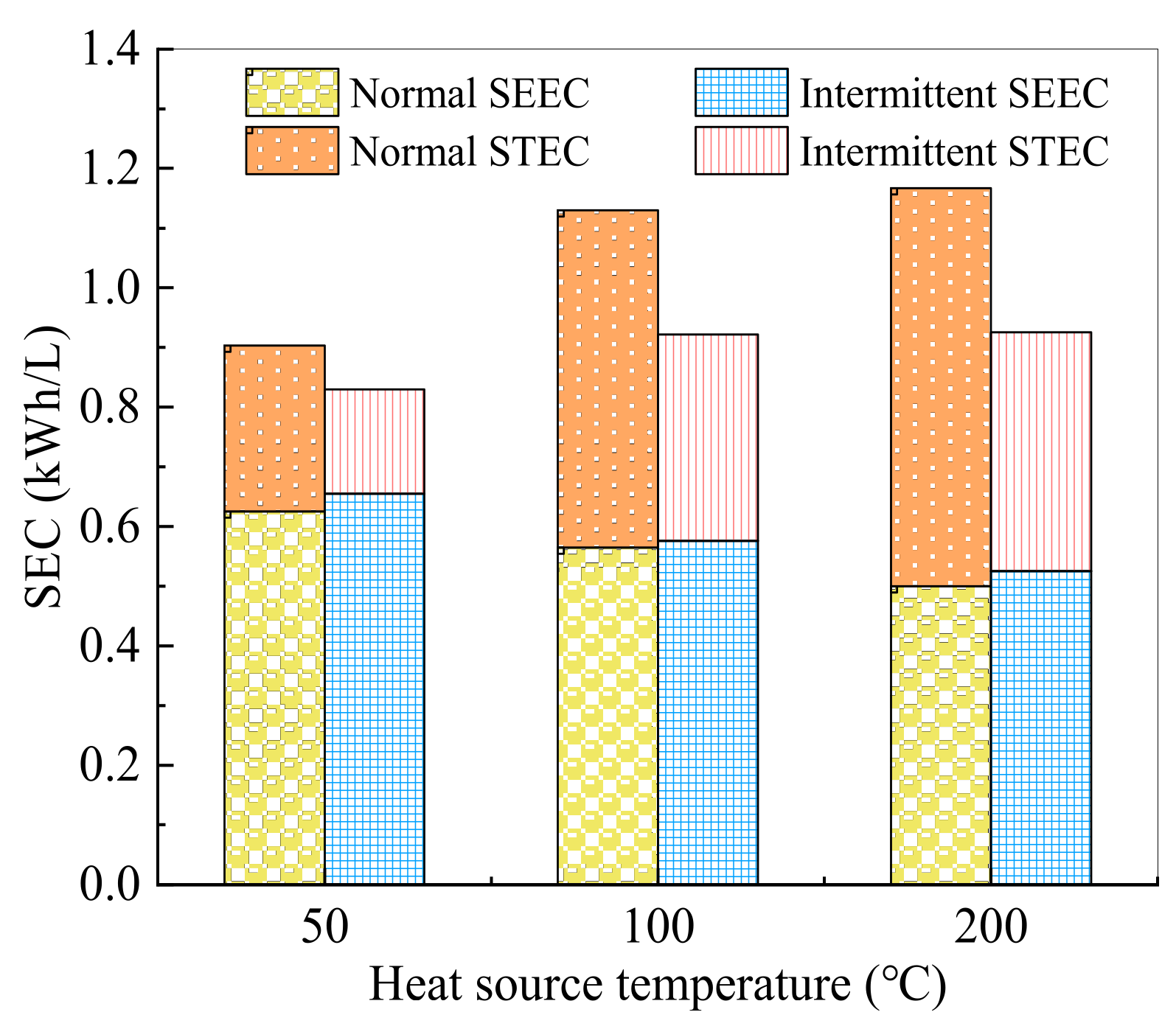
3.4. Intermittent Surface Heating Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | effective area of the membrane, m2 |
| Am | cross-section area of the shim, m2 |
| C | specific heat capacity, J/kg·°C |
| d | heat conduction distance on the shim, m |
| E | rate of electrical energy input, kJ/s |
| ∆Hv | latent heat of evaporation, kJ/kg |
| J | permeate flux, L/m2·h |
| kf | conductivity of the feed, mS/cm |
| kp | conductivity of the permeate, mS/cm |
| Km | coefficient of thermal conductivity, W/m·K |
| ∆m | mass reduction in feed, kg |
| Qin | total heat transfer to the system, W |
| Qv | heat transfer across the VMD, W |
| R | rejection rate, % |
| SEC | specific energy consumption of the system, kWh/L |
| SEEC | specific electrical energy consumption, kWh/L |
| STEC | specific thermal energy consumption, kWh/L |
| t | operation time, h |
| ΔT | temperature difference of the distance, K |
| Tbf | bulk temperature of the feed, °C |
| Tmf | membrane surface temperature on feed side, °C |
| TE | thermal efficiency, % |
| TPC | temperature polarization coefficient |
| ρ | density of feed solution, kg/L |
References
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Hoekstra, A.Y. Water scarcity challenges to business. Nat. Clim. Chang. 2014, 4, 318–320. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S.-M. The state of desalination and brine production: A global outlook. Sci. Total. Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Salih, H.H.; Dastgheib, S.A. Treatment of a hypersaline brine, extracted from a potential CO2 sequestration site, and an industrial wastewater by membrane distillation and forward osmosis. Chem. Eng. J. 2017, 325, 415–423. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; Garcia-Payo, M.C.; El-Balcouri, H.; Riaza, A. Treatment of reverse osmosis brine by direct contact membrane distillation: Chemical pretreatment approach. Desalination 2017, 420, 79–90. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Bio-Technol. 2017, 16, 591–609. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Ali, E.; Orfi, J.; AlAnsary, H.; Soukane, S.; Elcik, H.; Alpatova, A.; Ghaffour, N. Cost analysis of multiple effect evaporation and membrane distillation hybrid desalination system. Desalination 2021, 517, 115258. [Google Scholar] [CrossRef]
- Mezher, T.; Fath, H.; Abbas, Z.; Khaled, A. Techno-economic assessment and environmental impacts of desalination technologies. Desalination 2011, 266, 263–273. [Google Scholar] [CrossRef]
- Suarez, A.; Fernandez, P.; Ramon Iglesias, J.; Iglesias, E.; Riera, F.A. Cost assessment of membrane processes: A practical example in the dairy wastewater reclamation by reverse osmosis. J. Membr. Sci. 2015, 493, 389–402. [Google Scholar] [CrossRef]
- Chen, Q.; Muhammad, B.; Akhtar, F.H.; Ybyraiymkul, D.; Muhammad, W.S.; Li, Y.; Ng, K.C. Thermo-economic analysis and optimization of a vacuum multi-effect membrane distillation system. Desalination 2020, 483, 114413. [Google Scholar] [CrossRef]
- Burhan, M.; Shahzad, M.W.; Ybyraiymkul, D.; Oh, S.J.; Ghaffour, N.; Ng, K.C. Performance investigation of MEMSYS vacuum membrane distillation system in single effect and multi-effect mode. Sustain. Energy Technol. Assess. 2019, 34, 9–15. [Google Scholar] [CrossRef]
- Chen, Q.; Burhan, M.; Akhtar, F.H.; Ybyraiymkul, D.; Shahzad, M.W.; Li, Y.; Ng, K.C. A decentralized water/electricity cogeneration system integrating concentrated photovoltaic/thermal collectors and vacuum multi-effect membrane distillation. Energy 2021, 230, 120852. [Google Scholar] [CrossRef]
- Mericq, J.-P.; Laborie, S.; Cabassud, C. Vacuum membrane distillation of seawater reverse osmosis brines. Water Res. 2010, 44, 5260–5273. [Google Scholar] [CrossRef] [PubMed]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, S.-H.; Lee, S. Comparison of performance and economics of reverse osmosis, membrane distillation, and pressure retarded osmosis hybrid systems. Desalin. Water Treat. 2017, 77, 19–29. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. Air gap membrane distillation: A detailed study of high saline solution. Desalination 2017, 403, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane distillation and related operations—A review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Suleman, M.; Asif, M.; Jamal, S.A. Temperature and concentration polarization in membrane distillation: A technical review. Desalin. Water Treat. 2021, 229, 52–68. [Google Scholar] [CrossRef]
- Yang, L.; Ding, Z.; Ma, R. Study on temperature polarization in membrane distillation. Membr. Sci. Technol. 2004, 24, 4–9. [Google Scholar]
- Anvari, A.; Kekre, K.M.; Yancheshme, A.A.; Yao, Y.; Ronen, A. Membrane distillation of high salinity water by induction heated thermally conducting membranes. J. Membr. Sci. 2019, 589, 117253. [Google Scholar] [CrossRef]
- Dudchenko, A.V.; Chen, C.; Cardenas, A.; Rolf, J.; Jassby, D. Frequency-dependent stability of CNT Joule heaters in ionizable media and desalination processes. Nat. Nanotechnol. 2017, 12, 557–563. [Google Scholar] [CrossRef]
- Buenaventura Pouyfaucon, A.; Garcia-Rodriguez, L. Solar thermal-powered desalination: A viable solution for a potential market. Desalination 2018, 435, 60–69. [Google Scholar] [CrossRef]
- Wu, J.; Zodrow, K.R.; Szemraj, P.B.; Li, Q. Photothermal nanocomposite membranes for direct solar membrane distillation. J. Mater. Chem. A 2017, 5, 23712–23719. [Google Scholar] [CrossRef]
- Soomro, M.I.; Kim, W.-S. Performance and economic investigations of solar power tower plant integrated with direct contact membrane distillation system. Energy Convers. Manag. 2018, 174, 626–638. [Google Scholar] [CrossRef]
- Kabeel, A.E.; Abdelgaied, M.; El-Said, E.M.S. Study of a solar-driven membrane distillation system: Evaporative cooling effect on performance enhancement. Renew. Energy 2017, 106, 192–200. [Google Scholar] [CrossRef]
- Dongare, P.D.; Alabastri, A.; Pedersen, S.; Zodrow, K.R.; Hogan, N.J.; Neumann, O.; Wu, J.; Wang, T.; Deshmukh, A.; Elimelech, M.; et al. Nanophotonics-enabled solar membrane distillation for off-grid water purification. Proc. Natl. Acad. Sci. USA 2017, 114, 6936–6941. [Google Scholar] [CrossRef] [Green Version]
- Anvari, A.; Yancheshme, A.A.; Kekre, K.M.; Ronen, A. State-of-the-art methods for overcoming temperature polarization in membrane distillation process: A review. J. Membr. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N.; Rommel, M.; Koschikowski, J.; Wieghaus, M. Performance evaluation of the “large SMADES” autonomous desalination solar-driven membrane distillation plant in Aqaba, Jordan. Desalination 2007, 217, 17–28. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Li, Y.-W.; Chang, H. Optimal design and control of solar driven air gap membrane distillation desalination systems. Appl. Energy 2012, 100, 193–204. [Google Scholar] [CrossRef]
- Alanezi, A.A.; Abdallah, H.; El-Zanati, E.; Ahmad, A.; Sharif, A.O. Performance investigation of O-ring vacuum membrane distillation module for water desalination. J. Chem. 2016, 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Pei, J.; Jiang, H.; Hu, X. Water desalination under one sun using graphene-based material modified PTFE membrane. Desalination 2018, 442, 1–7. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, P.; Xiao, Y.; Liang, Y.; Liang, H.; Cheng, Z.; Qu, L. High rate production of clean water based on the combined photo-electro-thermal effect of graphene architecture. Adv. Mater. 2018, 30, 1706805. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Rao, U.; Dudley, M.; Ebrahimi, N.D.; Lou, J.; Han, F.; Hoek, E.M.V.; Tilton, N.; Cath, T.Y.; et al. Conducting thermal energy to the membrane/water interface for the enhanced desalination of hypersaline brines using membrane distillation. J. Membr. Sci. 2021, 626, 119188. [Google Scholar] [CrossRef]
- Lokare, O.R.; Tavakkoli, S.; Khanna, V.; Vidic, R.D. Importance of feed recirculation for the overall energy consumption in membrane distillation systems. Desalination 2018, 428, 250–254. [Google Scholar] [CrossRef]
- Wang, D.C.; Wu, J.Y. Influence of intermittent heat source on adsorption ice maker using waste heat. Energy Convers. Manag. 2005, 46, 985–998. [Google Scholar] [CrossRef]
- Wang, D.C.; Wu, J.Y.; Shan, H.G.; Wang, R.Z. Experimental study on the dynamic characteristics of adsorption heat pumps driven by intermittent heat source at heating mode. Appl. Therm. Eng. 2005, 25, 927–940. [Google Scholar]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, B.; Wu, J.; Li, R.; Wang, P. Hydrophobic light-to-heat conversion membranes with self-healing ability for interfacial solar heating. Adv. Mater. 2015, 27, 4889–4894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olatunji, S.O.; Camacho, L.M. Heat and mass transport in modeling membrane distillation configurations: A review. Front. Energy Res. 2018, 6, 130. [Google Scholar] [CrossRef]
- Qtaishat, M.; Matsuura, T.; Kruczek, B.; Khayet, M. Heat and mass transfer analysis in direct contact membrane distillation. Desalination 2008, 219, 272–292. [Google Scholar] [CrossRef]
- Miladi, R.; Frikha, N.; Kheiri, A.; Gabsi, S. Energetic performance analysis of seawater desalination with a solar membrane distillation. Energy Convers. Manag. 2019, 185, 143–154. [Google Scholar] [CrossRef]
- Luo, A.; Lior, N. Critical review of membrane distillation performance criteria. Desalin. Water Treat. 2016, 57, 20093–20140. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; Antonio Sanmartino, J.; Khayet, M.; Garcia-Payo, C.; Chaouch, M. Fouling in membrane distillation, osmotic distillation and osmotic membrane distillation. Appl. Sci. 2017, 7, 334. [Google Scholar] [CrossRef] [Green Version]
- Koutsoyiannis, D. Clausius-Clapeyron equation and saturation vapour pressure: Simple theory reconciled with practice. Eur. J. Phys. 2012, 33, 1021–1022. [Google Scholar] [CrossRef] [Green Version]
- Alsaadi, A.S.; Francis, L.; Amy, G.L.; Ghaffour, N. Experimental and theoretical analyses of temperature polarization effect in vacuum membrane distillation. J. Membr. Sci. 2014, 471, 138–148. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, F.; Liu, S.; Wang, K.; Zhang, X. Enhanced Performance of Membrane Distillation Using Surface Heating Process. Membranes 2021, 11, 866. https://doi.org/10.3390/membranes11110866
Han F, Liu S, Wang K, Zhang X. Enhanced Performance of Membrane Distillation Using Surface Heating Process. Membranes. 2021; 11(11):866. https://doi.org/10.3390/membranes11110866
Chicago/Turabian StyleHan, Fei, Shuxun Liu, Kang Wang, and Xiaoyuan Zhang. 2021. "Enhanced Performance of Membrane Distillation Using Surface Heating Process" Membranes 11, no. 11: 866. https://doi.org/10.3390/membranes11110866
APA StyleHan, F., Liu, S., Wang, K., & Zhang, X. (2021). Enhanced Performance of Membrane Distillation Using Surface Heating Process. Membranes, 11(11), 866. https://doi.org/10.3390/membranes11110866






