Highly Permeable Mixed Matrix Hollow Fiber Membrane as a Latent Route for Hydrogen Purification from Hydrocarbons/Carbon Dioxide
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of MMHFMs
2.3. Characterizations
2.4. Permeation Experiment
3. Results and Discussion
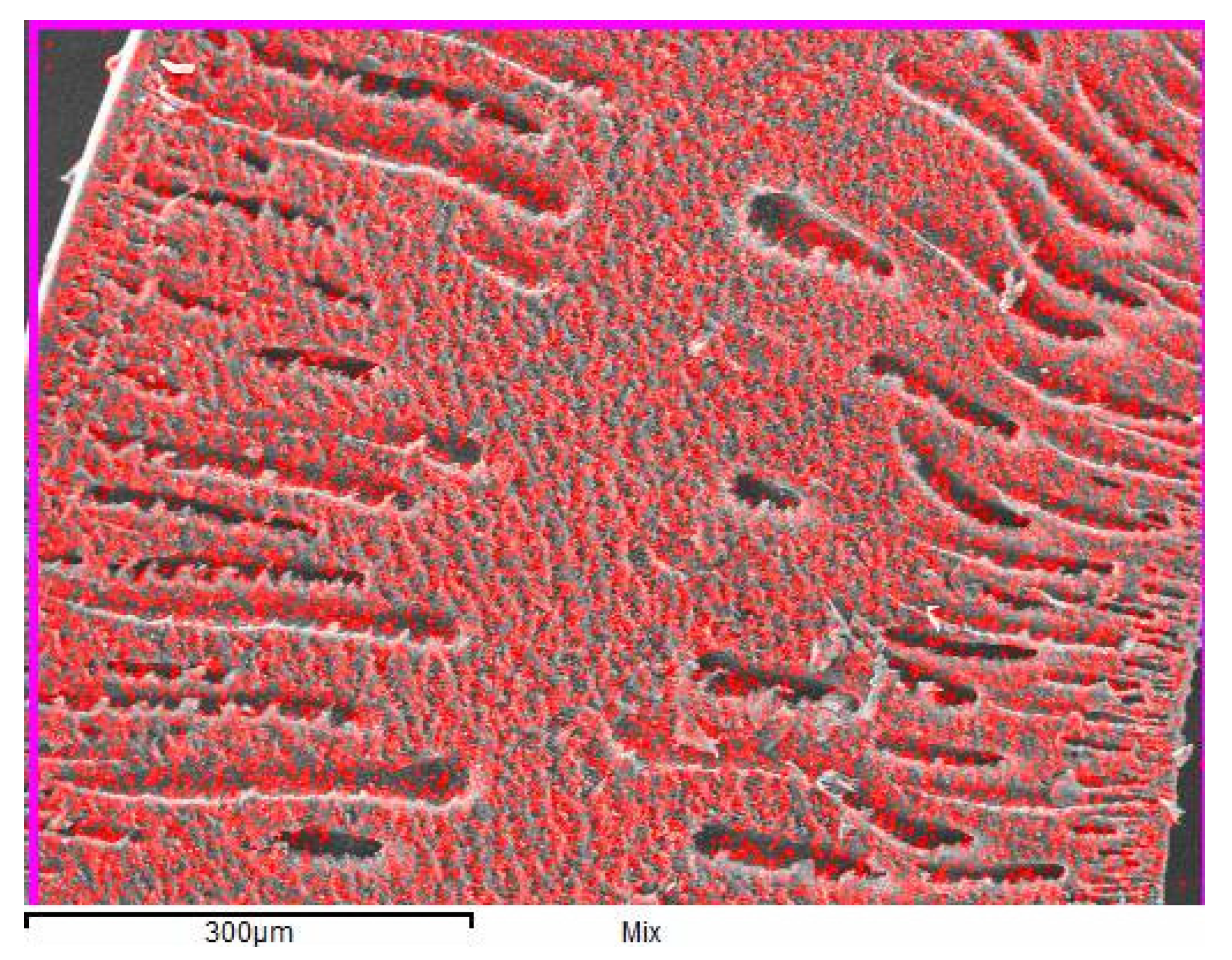
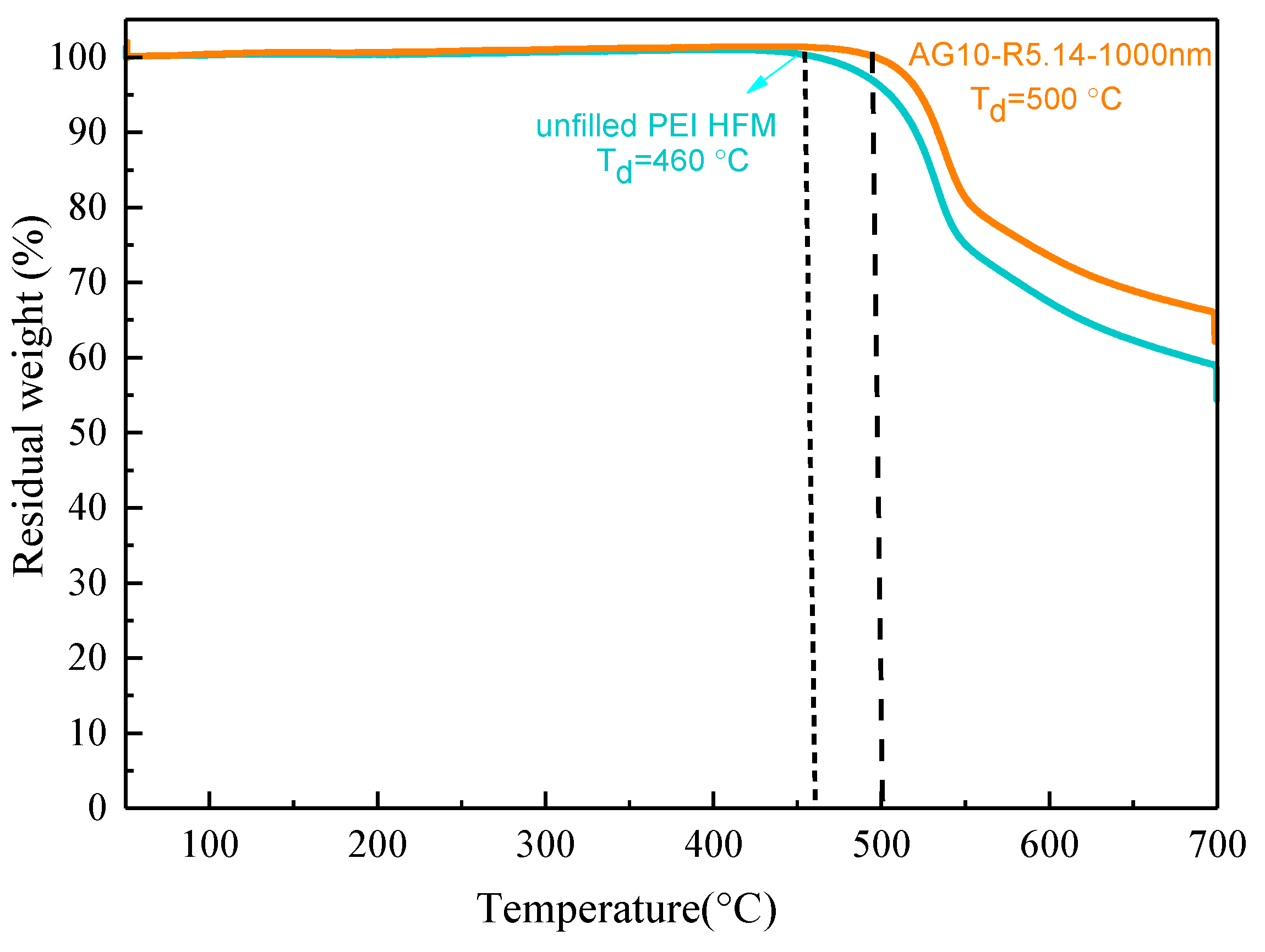
3.1. The Distribution of Alumina Particles in the Resultant Membrane
3.2. Morphological Structure Analysis
3.3. Single-Gas Permeance Test
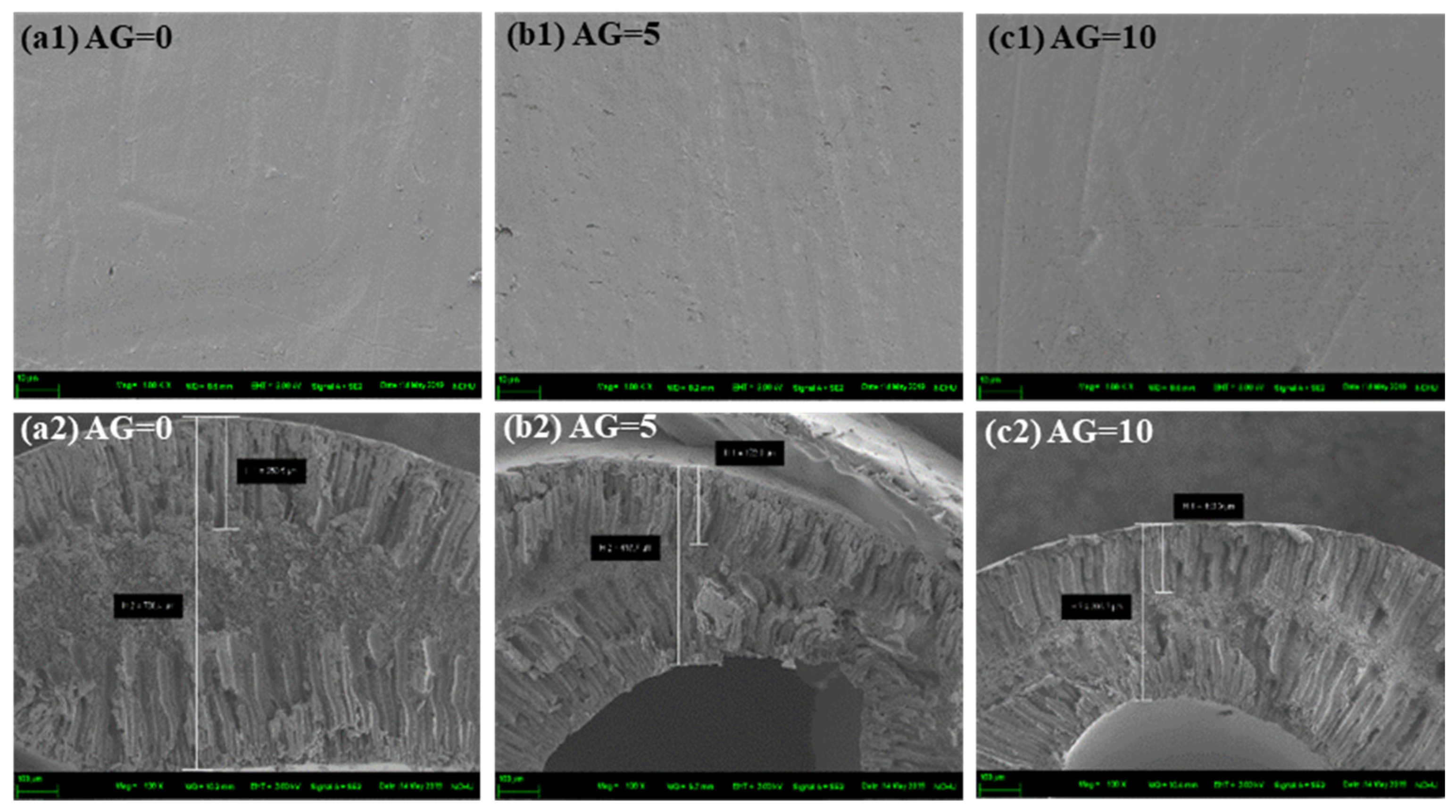
3.3.1. Effect of Air Gap Distance
3.3.2. Effect of the Flow Rate Ratio
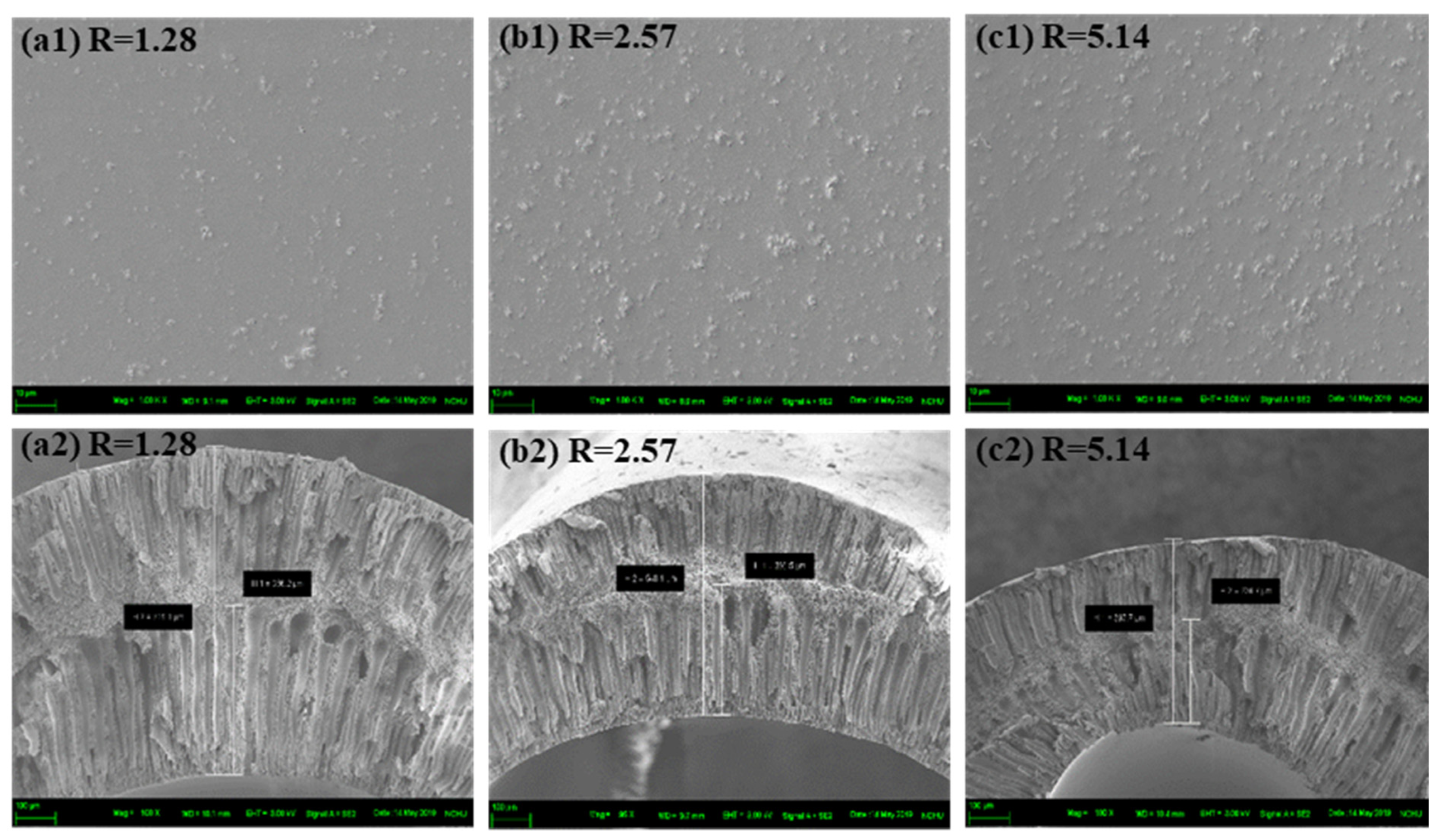
3.3.3. Effect of Filler Size
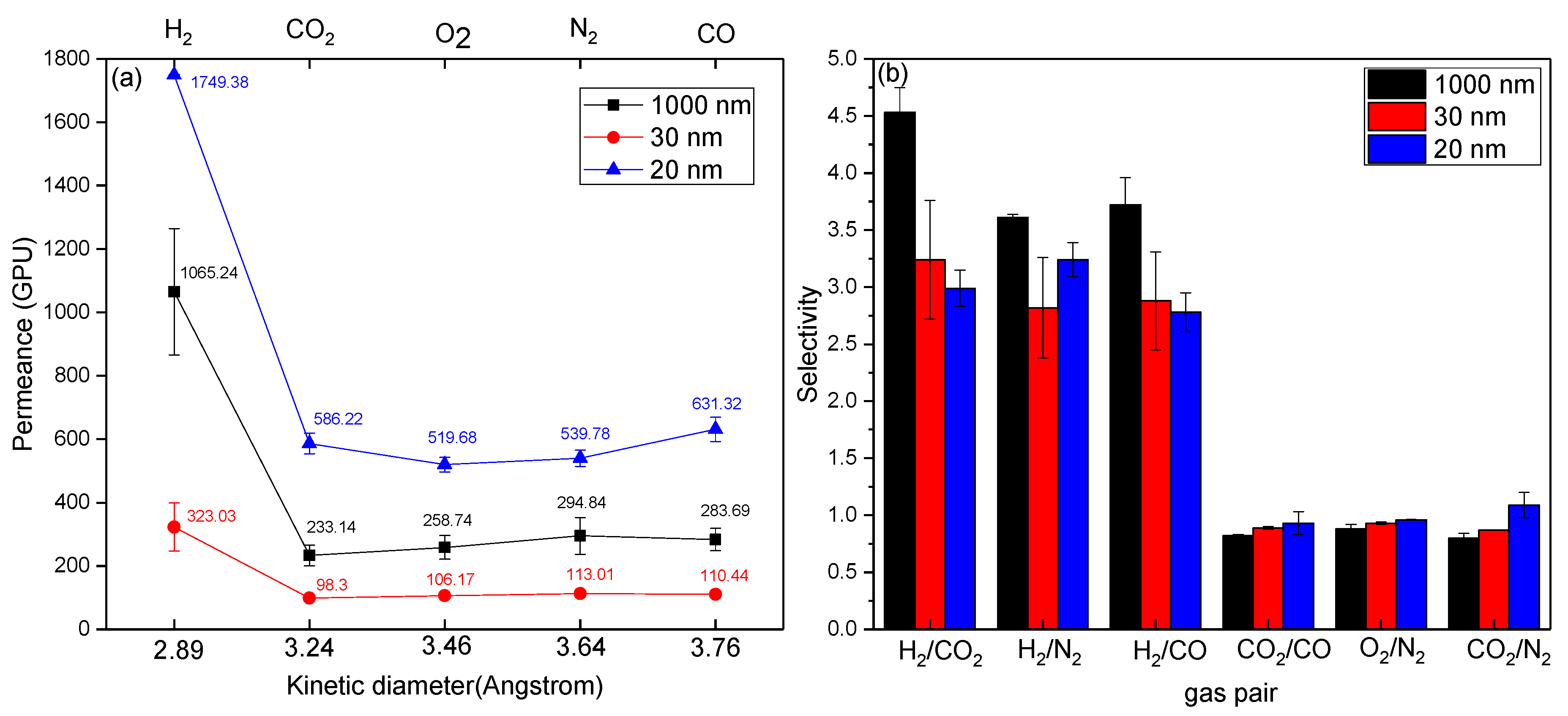
3.4. The Potential of Hydrogen Recovery from Either CO2 or Hydrocarbons
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lei, L.; Pan, F.; Lindbråthen, A.; Zhang, X.; Hillestad, M.; Nie, Y.; Bai, L.; He, X.; Guiver, M.D. Carbon hollow fiber membranes for a molecular sieve with precise-cutoff ultramicropores for superior hydrogen separation. Nat. Commun. 2021, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A review of hydrogen purification technologies for fuel cell vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Zhuang, G.-L.; Wey, M.-Y.; Tseng, H.-H. The viable fabrication of gas separation membrane used by reclaimed rubber from waste tires. Polymers 2020, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Lopez, D.R.; Smart, S.; Meulenberg, W.A.; Diniz da Costa, J.C. Mixed matrix carbon stainless steel (mmcss) hollow fibres for gas separation. Sep. Purif. Technol. 2017, 174, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Kumakiri, I.; Tamura, K.; Sasaki, Y.; Tanaka, K.; Kita, H. Influence of iron additive on the hydrogen separation properties of carbon molecular sieve membranes. Ind. Eng. Chem. Res. 2018, 57, 5370–5377. [Google Scholar] [CrossRef]
- Widiastuti, N.; Gunawan, T.; Fansuri, H.; Salleh, W.N.W.; Ismail, A.F.; Sazali, N. P84/zcc hollow fiber mixed matrix membrane with pdms coating to enhance air separation performance. Membranes 2020, 10, 267. [Google Scholar] [CrossRef]
- Roslan, R.A.; Lau, W.J.; Lai, G.S.; Zulhairun, A.K.; Yeong, Y.F.; Ismail, A.F.; Matsuura, T. Impacts of multilayer hybrid coating on psf hollow fiber membrane for enhanced gas separation. Membranes 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Janakiram, S.; Dai, Z.; Ansaloni, L.; Deng, L. Performance of mixed matrix membranes containing porous two-dimensional (2d) and three-dimensional (3d) fillers for CO2 separation: A review. Membranes 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laghaei, M.; Sadeghi, M.; Ghalei, B.; Shahrooz, M. The role of compatibility between polymeric matrix and silane coupling agents on the performance of mixed matrix membranes: Polyethersulfone/mcm-41. J. Membr. Sci. 2016, 513, 20–32. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.; Guiver, M.; Mikhailenko, S.; Wang, K.; Kaliaguine, S. Synthesis and characterization of sulfonated poly(ether ether ketone) for proton exchange membranes. J. Membr. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Ruan, X.; Yan, Z.; Yang, K.; Yu, M.; Li, H.; Zhao, W.; He, G. Imidazole functionalized graphene oxide/pebax mixed matrix membranes for efficient CO2 capture. Sep. Purif. Technol. 2016, 166, 171–180. [Google Scholar] [CrossRef]
- Wey, M.-Y.; Chen, H.-H.; Lin, Y.-T.; Tseng, H.-H. Thin carbon hollow fiber membrane with knudsen diffusion for hydrogen/alkane separation: Effects of hollow fiber module design and gas flow mode. Int. J. Hydrogen Energy 2020, 45, 7290–7302. [Google Scholar] [CrossRef]
- Awanis Hashim, N.; Liu, F.; Moghareh Abed, M.R.; Li, K. Chemistry in spinning solutions: Surface modification of pvdf membranes during phase inversion. J. Membr. Sci. 2012, 415-416, 399–411. [Google Scholar] [CrossRef]
- Raharjo, Y.; Wafiroh, S.; Nayla, M.; Yuliana, V.; Fahmi, M.Z. Primary study of cellulose acetate hollow fiber as a green membrane applied to hemodialysis. J. Chem. Technol. Metall. 2017, 52, 1021–1026. [Google Scholar]
- Hamid, N.; Ismail, A.; Matsuura, T.; Zularisam, A.; Lau, W.; Yuliwati, E.; Abdullah, M. Morphological and separation performance study of polysulfone/titanium dioxide (psf/tio2) ultrafiltration membranes for humic acid removal. Desalination 2011, 273, 85–92. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Ismail, A.; Matsuura, T.; Hashim, H.; Nordin, N.A.H.M.; Rahman, M.A.; Jaafar, J.; Jilani, A. Status and improvement of dual-layer hollow fiber membranes via co-extrusion process for gas separation: A review. J. Nat. Gas Sci. Eng. 2018, 52, 215–234. [Google Scholar] [CrossRef]
- Zhu, H.; Jie, X.; Wang, L.; Kang, G.; Liu, D.; Cao, Y. Enhanced gas separation performance of mixed matrix hollow fiber membranes containing post-functionalized s-mil-53. J. Energy Chem. 2018, 27, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Praneeth, K.; James, T.; Sridhar, S. Design of novel ultrafiltration systems based on robust polyphenylsulfone hollow fiber membranes for treatment of contaminated surface water. Chem. Eng. J. 2014, 248, 297–306. [Google Scholar]
- Li, G.; Kujawski, W.; Knozowska, K.; Kujawa, J. The effects of pei hollow fiber substrate characteristics on pdms/pei hollow fiber membranes for co2/n2 separation. Membranes 2021, 11, 56. [Google Scholar] [CrossRef]
- Nagy, E. Basic Equations of Mass Transport through a Membrane Layer; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hasbullah, H.; Kumbharkar, S.; Ismail, A.; Li, K. Preparation of polyaniline asymmetric hollow fiber membranes and investigation towards gas separation performance. J. Membr. Sci. 2011, 366, 116–124. [Google Scholar] [CrossRef]
- Zulhairun, A.; Ng, B.; Ismail, A.; Murali, R.S.; Abdullah, M. Production of mixed matrix hollow fiber membrane for co2/ch4 separation. Sep. Purif. Technol. 2014, 137, 1–12. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, T.; Xiong, R.; Wang, P.; Ma, J. Strong improvement of reverse osmosis polyamide membrane performance by addition of zif-8 nanoparticles: Effect of particle size and dispersion in selective layer. Chemosphere 2019, 233, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Muradov, N. 17—low-carbon production of hydrogen from fossil fuels. In Compendium of Hydrogen Energy; Subramani, V., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 489–522. [Google Scholar]
- Benali, M.; Aydin, B. Ethane/ethylene and propane/propylene separation in hybrid membrane distillation systems: Optimization and economic analysis. Sep. Purif. Technol. 2010, 73, 377–390. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Yun, G.-N.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Effects of pressure, contact time, permeance, and selectivity in membrane reactors: The case of the dehydrogenation of ethane. Sep. Purif. Technol. 2018, 194, 197–206. [Google Scholar] [CrossRef]
- Hamid, M.A.A.; Chung, Y.T.; Rohani, R.; Junaidi, M.U.M. Miscible-blend polysulfone/polyimide membrane for hydrogen purification from palm oil mill effluent fermentation. Sep. Purif. Technol. 2019, 209, 598–607. [Google Scholar] [CrossRef]
- Pinnau, I.; He, Z.; Morisato, A. Synthesis and gas permeation properties of poly(dialkylacetylenes) containing isopropyl-terminated side-chains. J. Membr. Sci. 2004, 241, 363–369. [Google Scholar] [CrossRef]
- Li, P.; Chung, T.; Paul, D. Gas sorption and permeation in pim-1. J. Membr. Sci. 2013, 432, 50–57. [Google Scholar] [CrossRef]
- Morisato, A.; Pinnau, I. Synthesis and gas permeation properties of poly(4-methyl-2-pentyne). J. Membr. Sci. 1996, 121, 243–250. [Google Scholar] [CrossRef]
- Bermeshev, M.V.; Syromolotov, A.V.; Starannikova, L.E.; Gringolts, M.L.; Lakhtin, V.G.; Yampolskii, Y.P.; Finkelshtein, E.S. Glassy polynorbornenes with si–o–si containing side groups. Novel materials for hydrocarbon membrane separation. Macromolecules 2013, 46, 8973–8979. [Google Scholar] [CrossRef]
- Pinnau, I.; Morisato, A.; He, Z. Influence of side-chain length on the gas permeation properties of poly (2-alkylacetylenes). Macromolecules 2004, 37, 2823–2828. [Google Scholar] [CrossRef]
- Mukaddam, M.; Litwiller, E.; Pinnau, I. Gas sorption, diffusion, and permeation in nafion. Macromolecules 2016, 49, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Fairuzov, D.; Gerzeliev, I.; Maximov, A.; Naranov, E. Catalytic dehydrogenation of ethane: A mini review of recent advances and perspective of chemical looping technology. Catalysts 2021, 11, 833. [Google Scholar] [CrossRef]
- Gärtner, C.A.; van Veen, A.C.; Lercher, J.A. Oxidative dehydrogenation of ethane: Common principles and mechanistic aspects. ChemCatChem 2013, 5, 3196–3217. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Iulianelli, A.; Basile, A. 10—membranes for hydrocarbon fuel processing and separation. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Basile, A., Nunes, S.P., Eds.; Woodhead Publishing: Shaston, UK, 2011; pp. 295–338. [Google Scholar]


| Condition | Value |
|---|---|
| Composition of spinning mixture (Al2O3:NMP:PEI) | 6:75:25 (weight ratio) |
| Bore liquid | D.I. water |
| External coagulant | tap water |
| Dope flow rate (mL/min) | 25.7 |
| Air gap (cm) | 0, 5, 10 |
| Bore liquid flow rate (mL/min) | 5, 10, 20 |
| Sample Code | Membrane Thickness (μm) | Length of Outer Finger-Like Structure (μm) |
|---|---|---|
| AG0-R5.14-1000 nm | 786.4 | 250.5 |
| AG5-R5.14-1000 nm | 443.7 | 173.0 |
| AG10-R5.14-1000 nm | 393.2 | 153.9 |
| Sample Code | Membrane Thickness (μm) | Length of Inner Finger-Like Structure (μm) |
|---|---|---|
| AG10-R1.28-1000 nm | 711.1 | 366.2 |
| AG10-R2.57-1000 nm | 548.2 | 294.3 |
| AG10-R5.14-1000 nm | 393.2 | 224.7 |
| Membrane | Hydrogen Permeance (GPU) | Thickness of Selective Layer (μm) | Hydrogen Permeability (Barrer) * | Selectivity | Ref. | ||
|---|---|---|---|---|---|---|---|
| H2/CO2 | H2/C2H6 | H2/C3H8 | |||||
| Unfilled PEI | 26.26 | - | - | 3.52 | - | - | This work |
| PEI/Al2O3 | 1065.24 | - | - | 4.53 | 5.77 | 5.39 | |
| PI/PSf | 348 | - | - | 4.4 | - | - | [28] |
| Poly(4-methyl-2-pentyne) | 94–141 | 40–60 | - | 1.51 | 1.35 | [29] | |
| Poly(6-methyl-2-heptyne) | 12.5–18.75 | 40–60 | - | 2.67 | - | [29] | |
| PIM-1 | 58.6 | 50 | - | 7.87 | - | [30] | |
| Poly(4-methyl-2-pentyne) | - | - | - | 1.56 | 1.23 | [31] | |
| Poly(trimethylsiloxy)silyl)-tricyclonone | - | - | - | - | 1.32 | [32] | |
| Poly(2-hexyne) | 2.66–4 | 40–60 | - | 10 | 4.57 | [33] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Wey, M.-Y.; Tseng, H.-H. Highly Permeable Mixed Matrix Hollow Fiber Membrane as a Latent Route for Hydrogen Purification from Hydrocarbons/Carbon Dioxide. Membranes 2021, 11, 865. https://doi.org/10.3390/membranes11110865
Lin Y-T, Wey M-Y, Tseng H-H. Highly Permeable Mixed Matrix Hollow Fiber Membrane as a Latent Route for Hydrogen Purification from Hydrocarbons/Carbon Dioxide. Membranes. 2021; 11(11):865. https://doi.org/10.3390/membranes11110865
Chicago/Turabian StyleLin, Yu-Ting, Ming-Yen Wey, and Hui-Hsin Tseng. 2021. "Highly Permeable Mixed Matrix Hollow Fiber Membrane as a Latent Route for Hydrogen Purification from Hydrocarbons/Carbon Dioxide" Membranes 11, no. 11: 865. https://doi.org/10.3390/membranes11110865
APA StyleLin, Y.-T., Wey, M.-Y., & Tseng, H.-H. (2021). Highly Permeable Mixed Matrix Hollow Fiber Membrane as a Latent Route for Hydrogen Purification from Hydrocarbons/Carbon Dioxide. Membranes, 11(11), 865. https://doi.org/10.3390/membranes11110865







