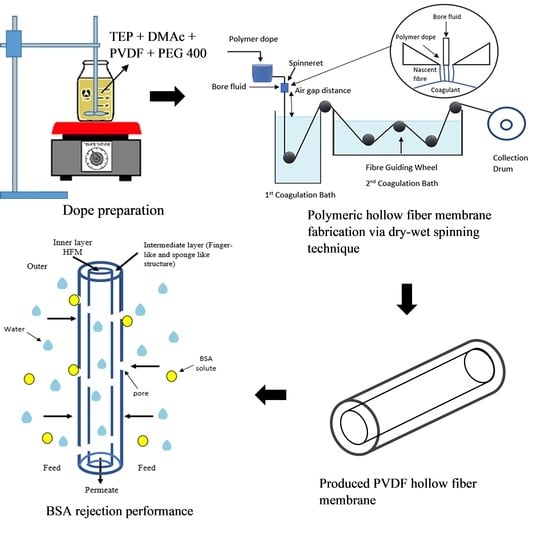
Fabrication of High Performance PVDF Hollow Fiber Membrane Using Less Toxic Solvent at Different Additive Loading and Air Gap
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Single Layer PVDF Hollow Fiber Membrane
2.2.1. Preparation of Polymer Dope Solution
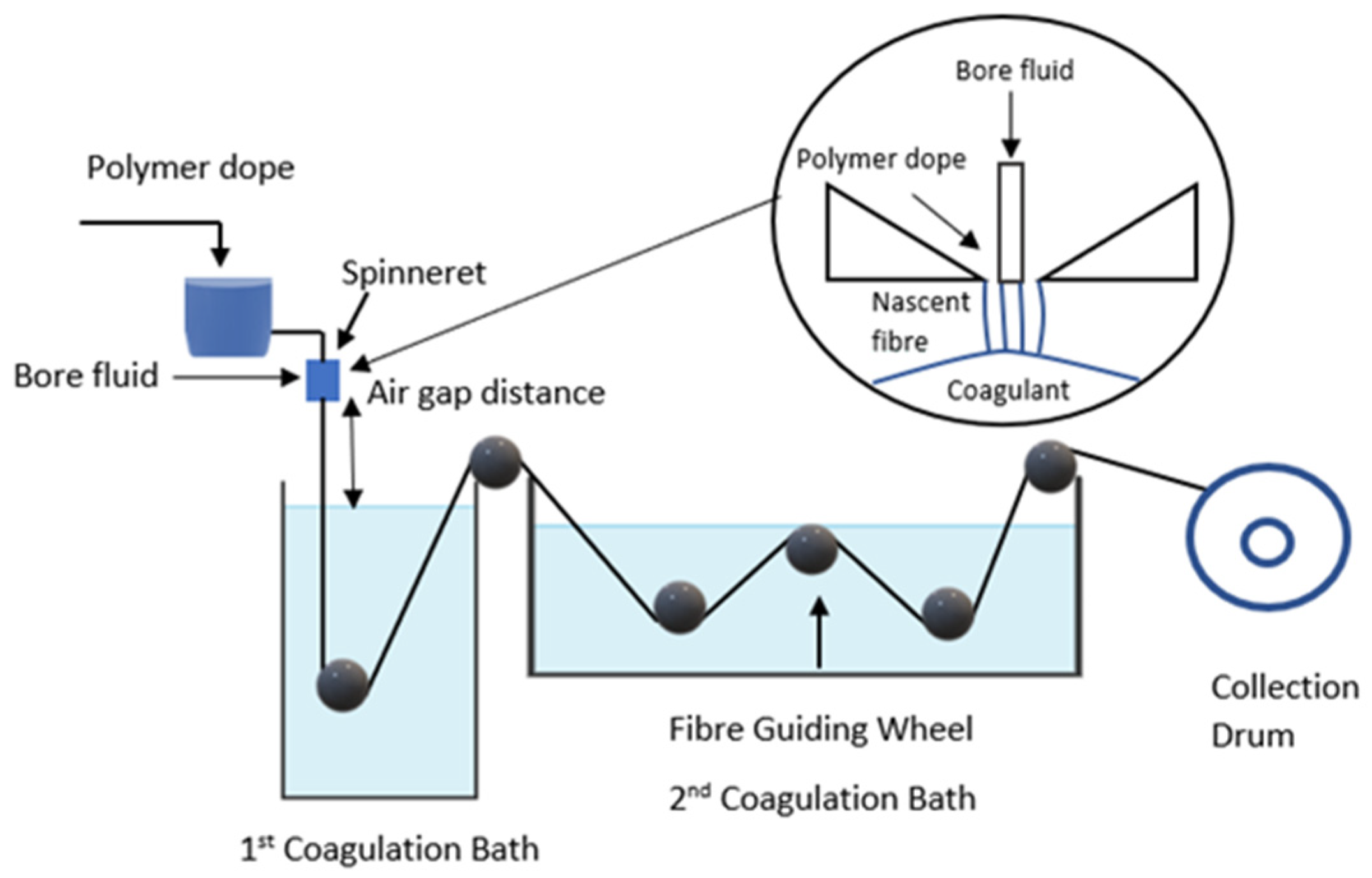
2.2.2. Dry-Wet Spinning Technique
2.3. Physical-Chemical Characterization
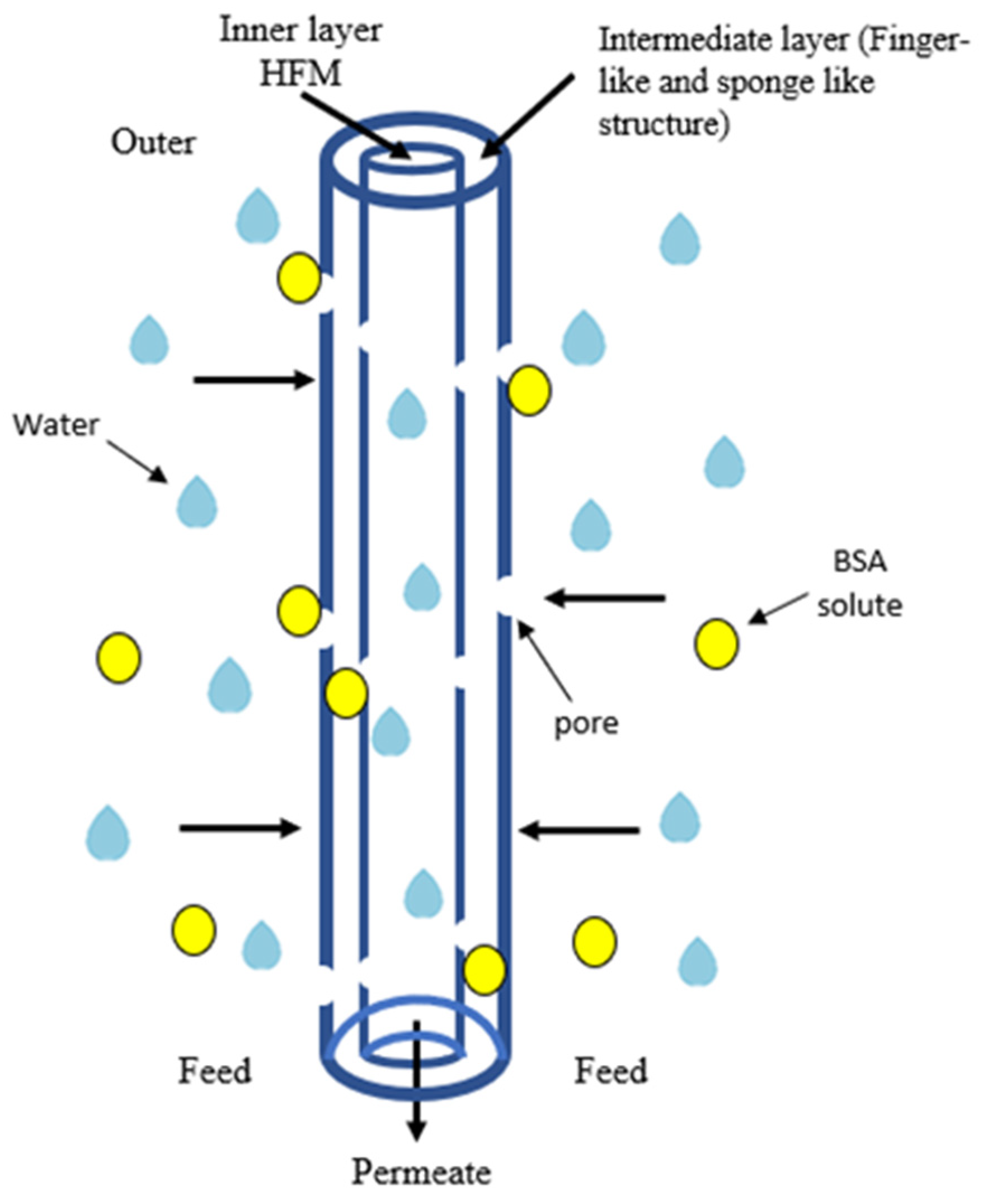
2.4. Pure Water Flux and BSA Rejection Performance
3. Results and Discussion
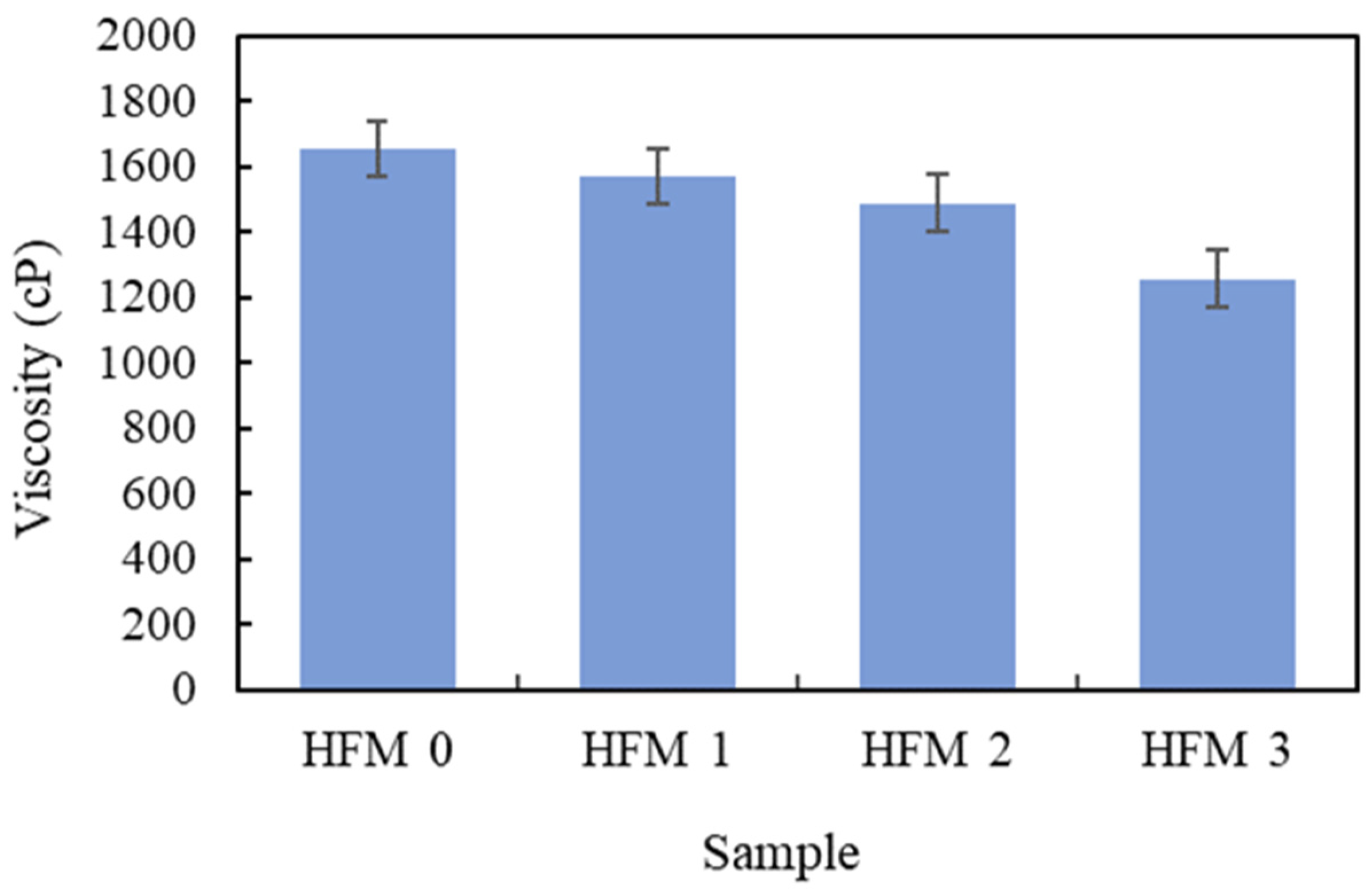
3.1. Viscosity of Dope Solution
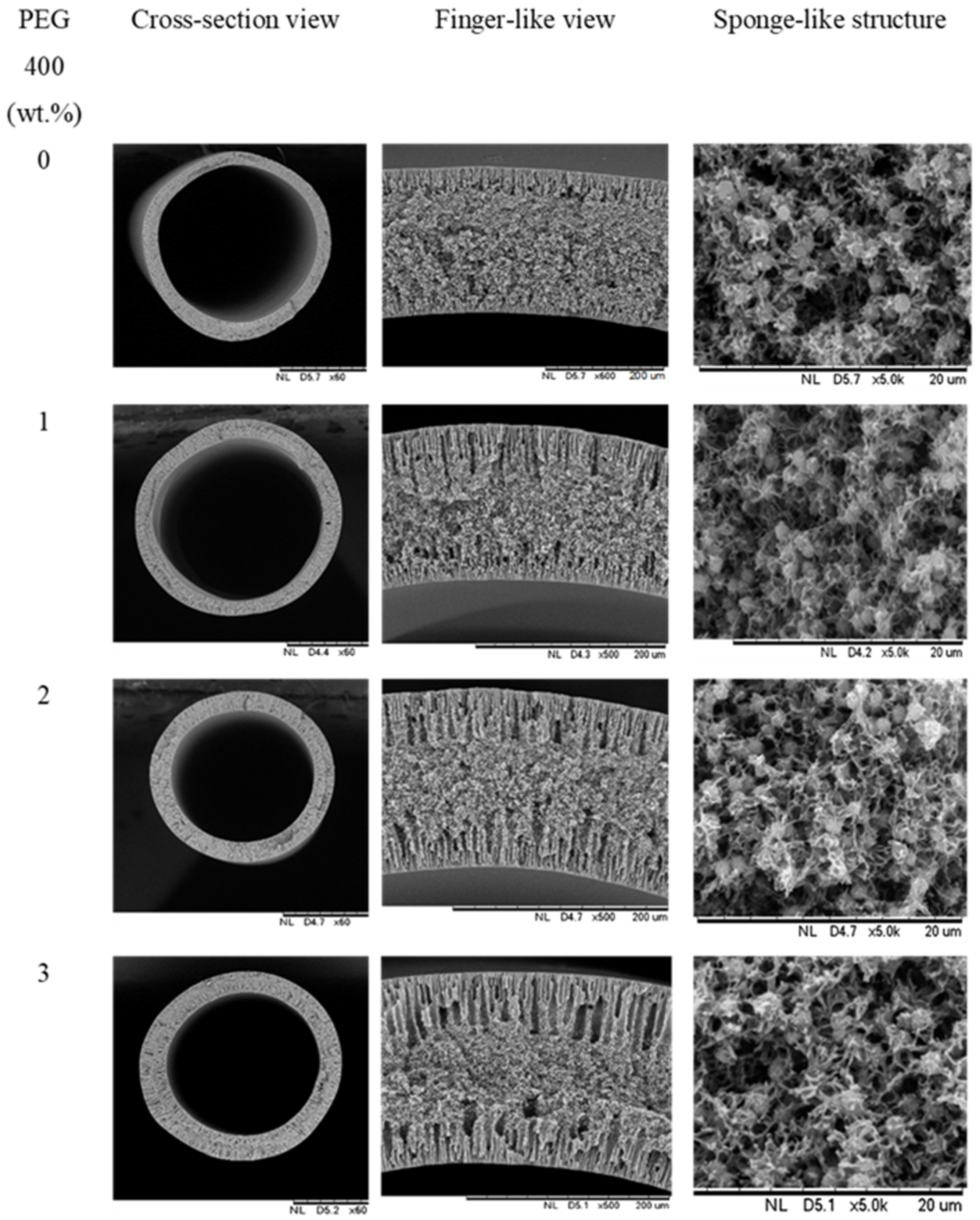
3.2. Morphology of PVDF HFM
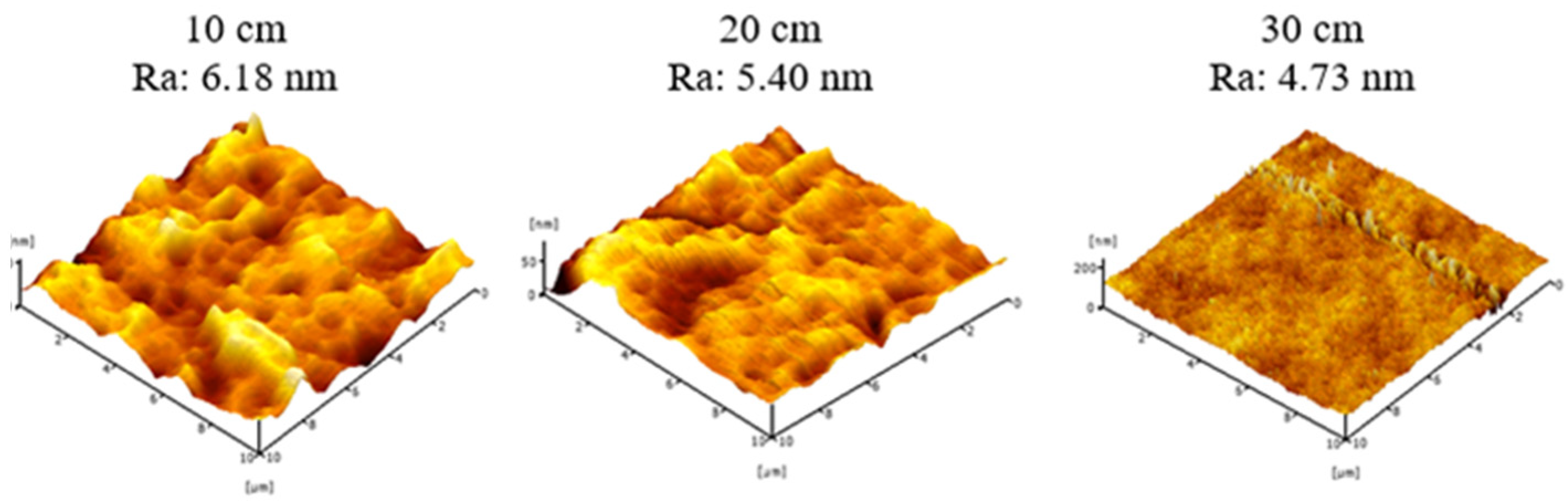
3.3. Surface Roughness of PVDF HFM
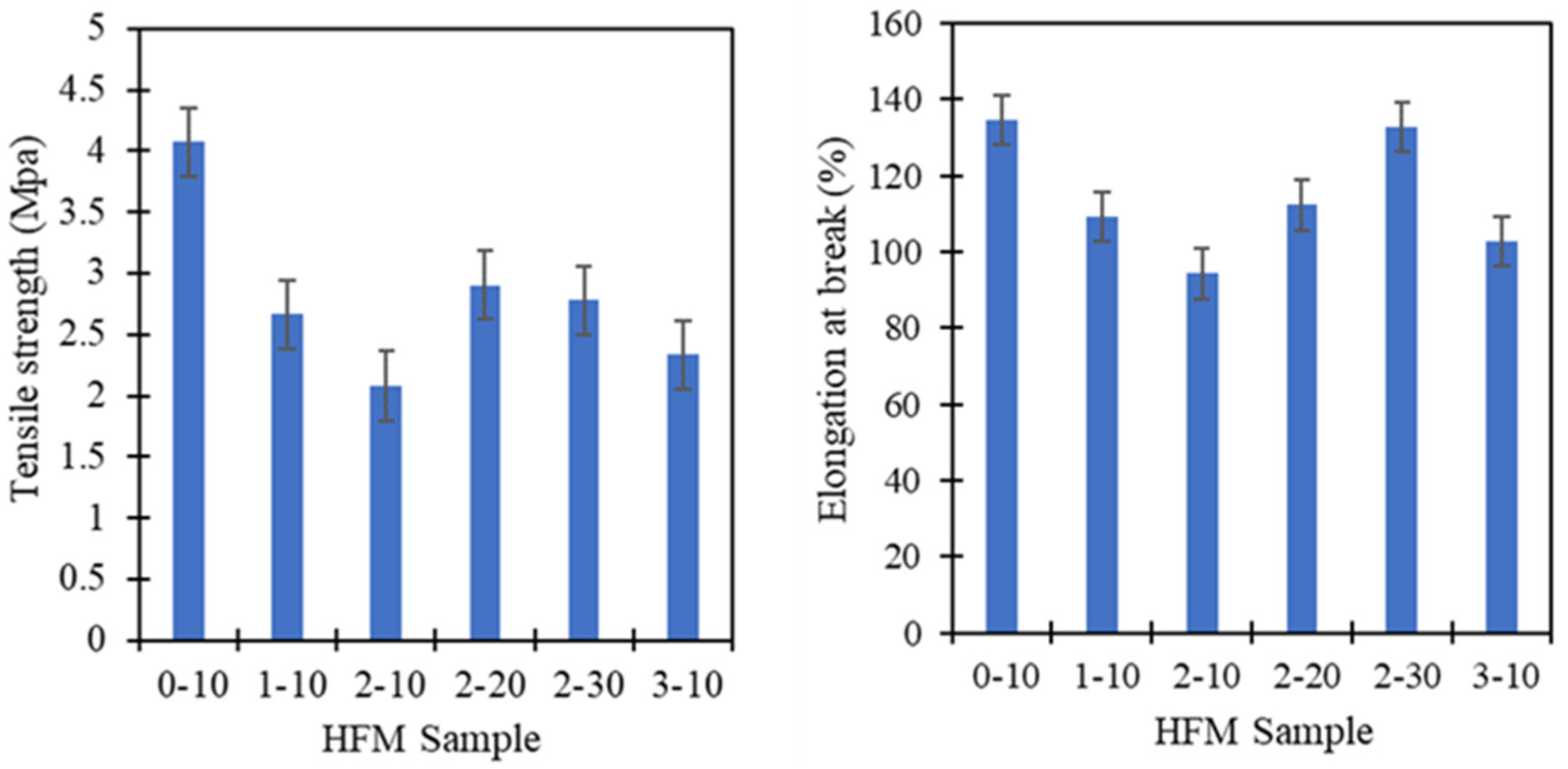
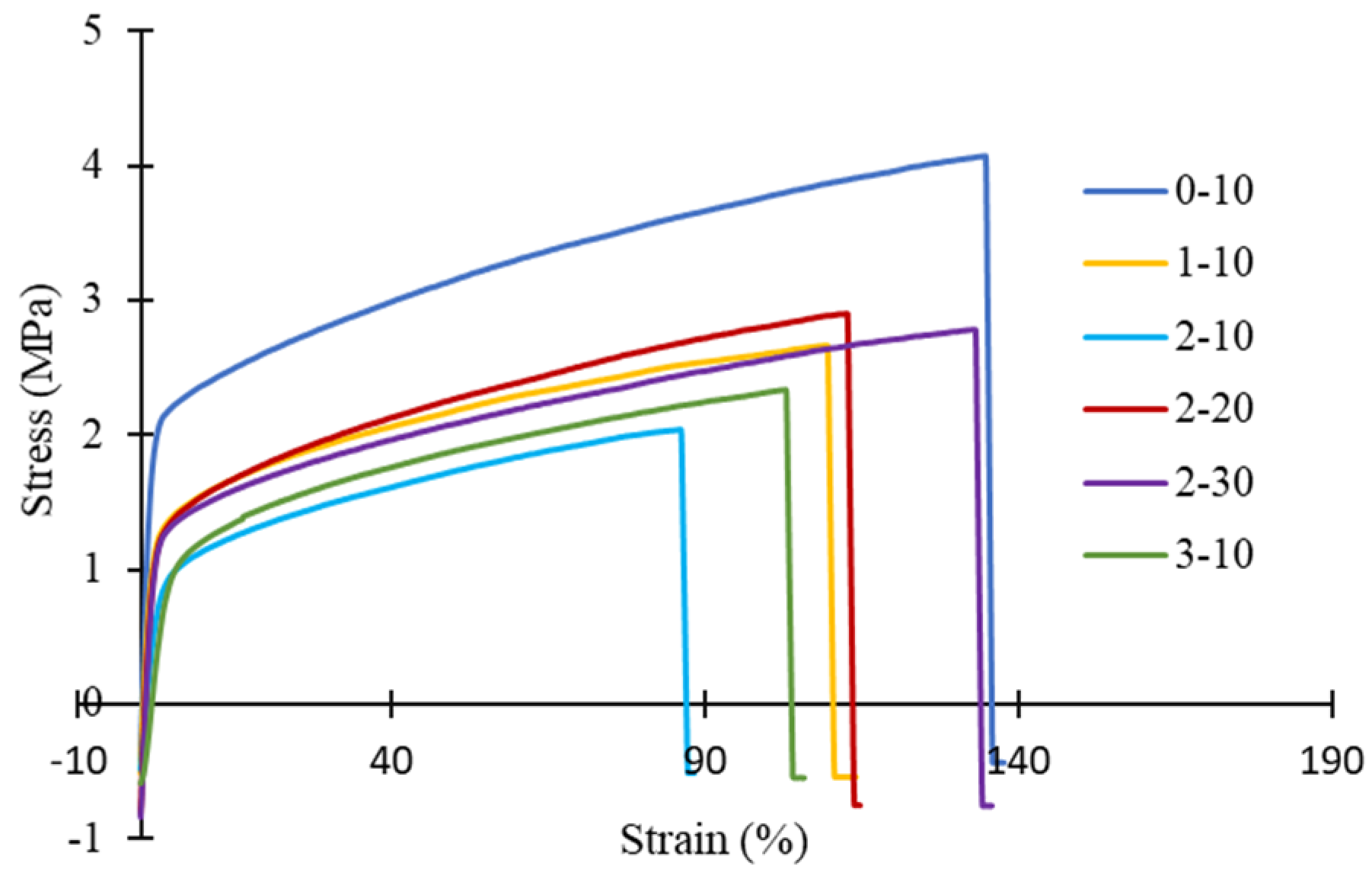
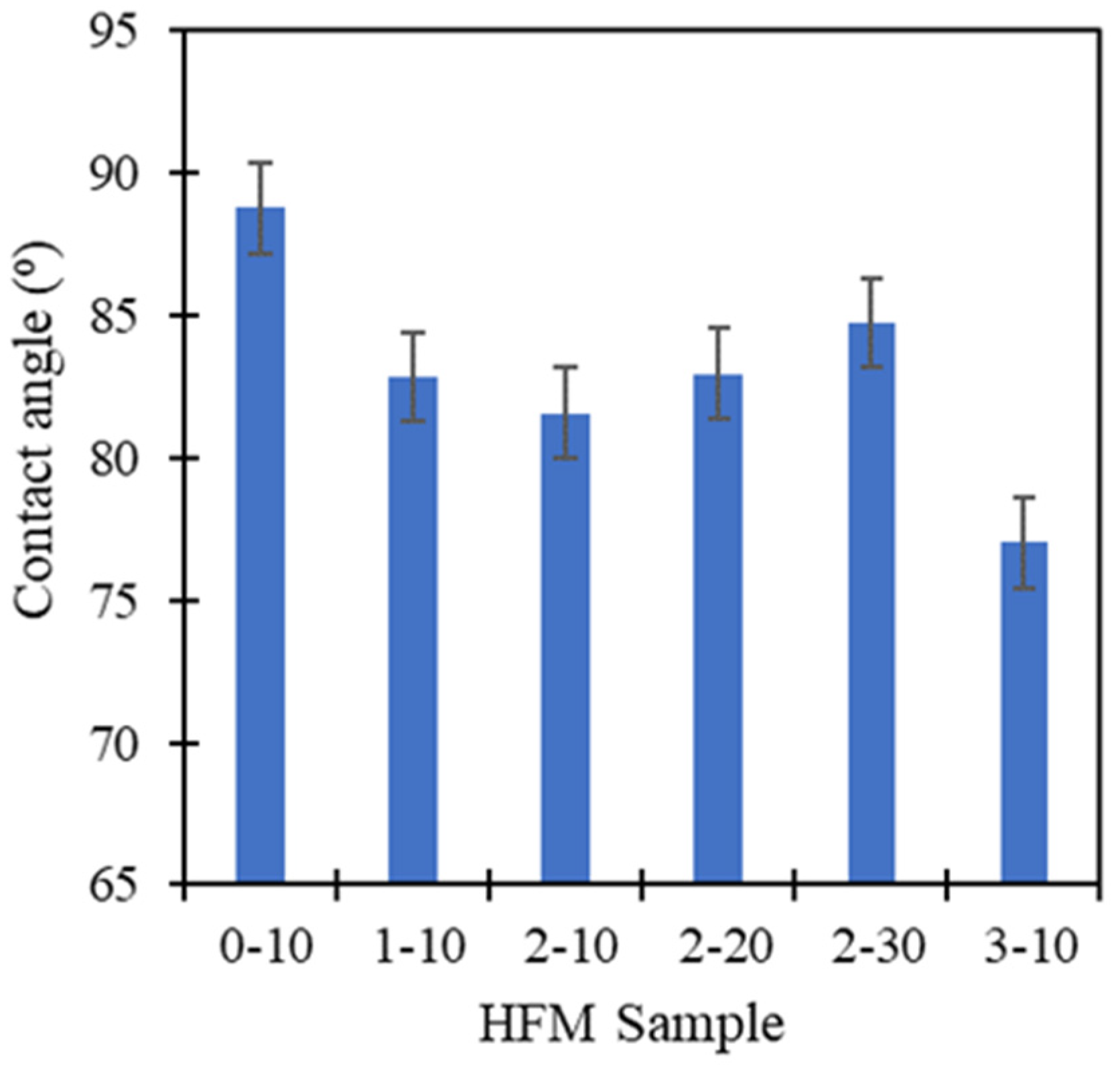
3.4. Mechanical Strength and Wettability of PVDF HFM
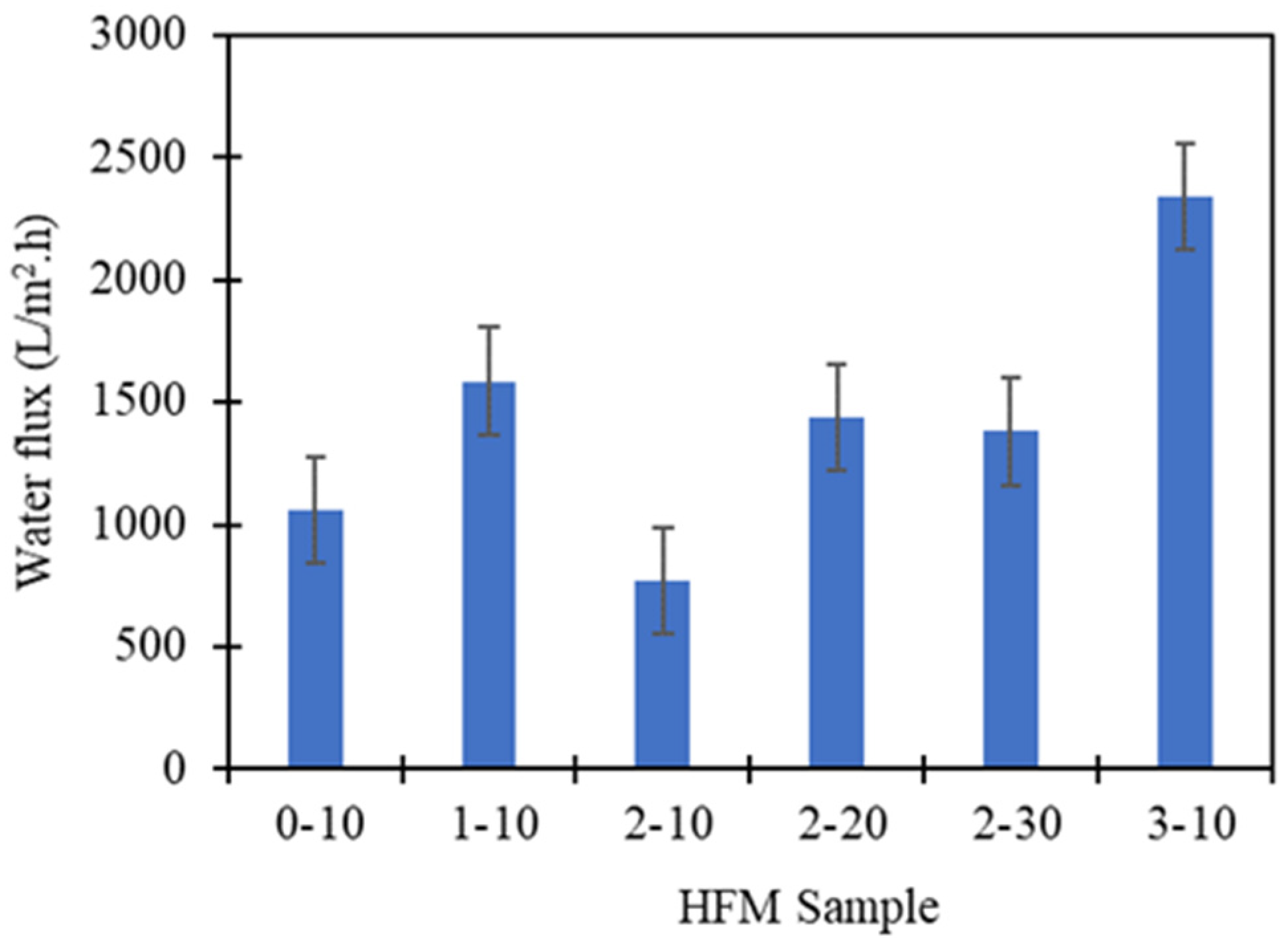
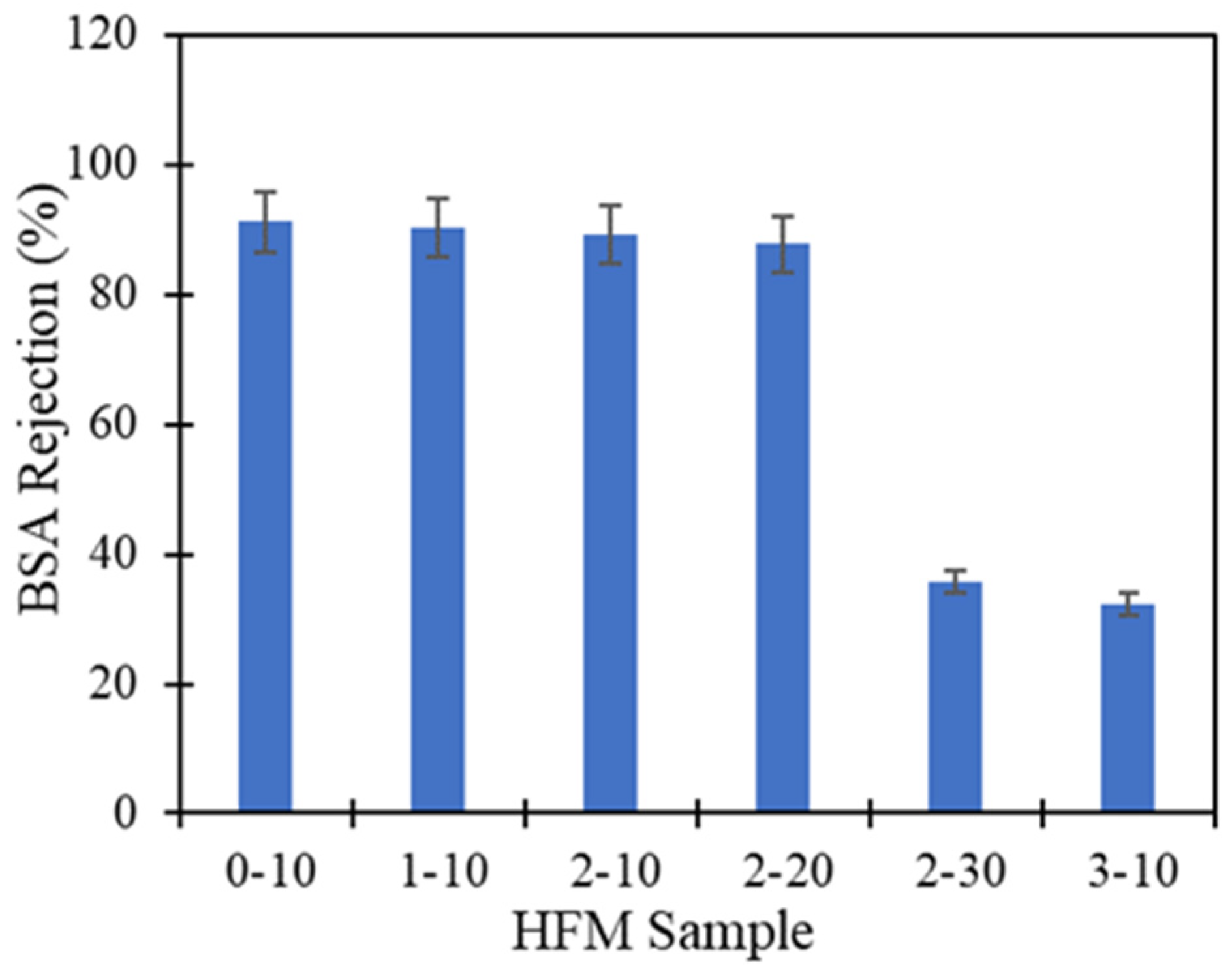
3.5. Water Flux and BSA Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, T.A.; Gupta, V.K. An Overview of Membrane Science and Technology. Nanomater. Polym. Membr. 2016, 1–23. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Koyuncu, I.; Sengur, R.; Turken, T.; Guclu, S.; Pasaoglu, M.E. Advances in water treatment by microfiltration, ultrafiltration, and nanofiltration. In Advances in Membrane Technologies for Water Treatment: Materials, Processes and Applications; Woodhead Publishing: Sawston, UK, 2015; Volume 2019, pp. 83–128. [Google Scholar] [CrossRef]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Rajender, I.; Abdullah, B.; Asiri, M. Engineering Materials Self-Standing Substrates Materials and Applications; Springer: Berlin, Germany, 2020; pp. 120–126. Available online: http://www.springer.com/series/4288 (accessed on 14 February 2020).
- Wang, L.; Huang, D.; Wang, X.; Meng, X.; Lv, Y.; Wang, X. Preparation of PVDF membranes via the low-temperature TIPS method with diluent mixtures: The role of coagulation conditions and cooling rate. Desalination 2015, 361, 25–37. [Google Scholar] [CrossRef]
- Chang, J.; Zuo, J.; Zhang, L.; O’Brien, G.S.; Chung, T.S. Using green solvent, triethyl phosphate (TEP), to fabricate highly porous PVDF hollow fiber membranes for membrane distillation. J. Membr. Sci. 2017, 539, 295–304. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Figoli, A. The formation of polyvinylidene fluoride membranes with tailored properties via vapour/non-solvent induced phase separation. Membranes 2018, 8, 71. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Fadhil, S.; Marino, T.; Makki, H.F.; Alsalhy, Q.F.; Blefari, S.; Macedonio, F. Novel PVDF-HFP flat sheet membranes prepared by triethyl phosphate (TEP) solvent for direct contact membrane distillation. Chem. Eng. Process. Process. Intensif. 2016, 102, 16–26. [Google Scholar] [CrossRef]
- Abed, M.M.R.; Kumbharkar, S.C.; Groth, A.M.; Li, K. Ultrafiltration PVDF hollow fibre membranes with interconnected bicontinuous structures produced via a single-step phase inversion technique. J. Membr. Sci. 2012, 407–408, 145–154. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Xue, L. Fabrication and characterization of antibacterial PVDF hollow fibre membrane by doping Ag-loaded zeolites. J. Membr. Sci. 2013, 437, 205–215. [Google Scholar] [CrossRef]
- Tavakolmoghadam, M.; Rekabdar, F.; Hemmati, M.; Mohammadi, T. Poly (Vinylidene Fluride) Membrane Preparation and Characterization: Effects of Mixed Solvents and PEG Molecular Weight. J. Pet. Sci. Technol. 2016, 6, 11–21. Available online: https://www.sid.ir/en/journal/ViewPaper.aspx?id=522989 (accessed on 14 February 2020).
- Liu, F.; Tao, M.; Xue, L. PVDF membranes with inter-connected pores prepared via a Nat-ips process. Desalination 2012, 298, 99–105. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Fahrina, A. Morphology and performance of PVDF membranes composed of triethyl phospate and dimethyl sulfoxide solvents. Mater. Res. Express 2019, 6, 066419. [Google Scholar] [CrossRef]
- Aminudin, N.N.; Basri, H.; Harun, Z.; Yunos, M.Z.; Sean, G.P. Comparative study on effect of PEG and PVP as additives on polysulfone (PSF) membrane structure and performance. J. Teknol. 2013, 65, 47–51. [Google Scholar] [CrossRef][Green Version]
- Song, H.; Shao, J.; Wang, J.; Zhong, X. The removal of natural organic matter with LiCl-TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. Desalination 2014, 344, 412–421. [Google Scholar] [CrossRef]
- Syawaliah, A.N.; Mukramah, M.S. Effects of PEG Molecular Weights on PVDF Membrane for Humic Acid-fed Ultrafiltration Process. IOP Conf. Ser. Mater. Sci. Eng. 2017, 180, 1–8. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Singh, M.; Sanjeev, K.; Verma, I.B.; Mehta, R. Effect of molecular weight of polyethylene glycol on the rheological properties of fumed silica-polyethylene glycol shear thickening fluid. Mater. Res. Express 2018, 1–15. [Google Scholar] [CrossRef]
- Kamaludin, R.; Puad, A.S.M.; Othman, M.H.D.; Kadir, S.H.S.A.; Harun, Z. Incorporation of N-doped TiO2 into dual layer hollow fiber (DLHF) membrane for visible light-driven photocatalytic removal of reactive black 5. Polym. Test. 2019, 78, 105939. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Lau, W.J.; Tan, Y.H.; Ng, B.C.; Ismail, A.F. Hydrophilic hollow fiber PVDF ultrafiltration membrane incorporated with titanate nanotubes for decolourization of aerobically-treated palm oil mill effluent. Chem. Eng. J. 2017, 316, 101–110. [Google Scholar] [CrossRef]
- Bellardita, M.; Camera-Roda, G.; Loddo, V.; Parrino, F.; Palmisano, L. Coupling of membrane and photocatalytic technologies for selective formation of high added value chemicals. Catal. Today 2020, 340, 128–144. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.L.; Yu, L.Y. Effects of Mixed Solvents and PVDF Types on Performances of PVDF Microporous Membranes. J. Appl. Polym. Sci. 2009, 115, 2277–2287. [Google Scholar] [CrossRef]
- Adam, M.R.; Matsuura, T.; Othman, M.H.D.; Puteh, M.H.; Pauzan, M.A.B.; Ismail, A.F. Feasibility study of the hybrid adsorptive hollow fibre ceramic membrane (HFCM) derived from natural zeolite for the removal of ammonia in wastewater. Process. Saf. Environ. Prot. 2019, 122, 378–385. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Usosky, V.V.; Volkov, V.V. Influence of the concentration and molecular weight of polyethylene glycol on the structure and permeability of polysulfone hollow fiber membranes. Pet. Chem. 2016, 56, 321–329. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar] [CrossRef]
- Srivastava, H.P.; Arthanareeswaran, G.; Anantharaman, N.; Starov, V.M. Performance and properties of modified poly (vinylidene fluoride) membranes using general purpose polystyrene (GPPS) by DIPS method. Desalination 2011, 283, 169–177. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Morphological study of poly(vinylidene fluoride) asymmetric membranes: Effects of the solvent, additive, and dope temperature. J. Appl. Polym. Sci. 2004, 92, 1782–1789. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Saljoughi, E.; Sheikhi-Kouhsar, M.R. Preparation and characterization of nanoporous polysulfone membranes with high hydrophilic property using variation in CBT and addition of tetronic-1107 surfactant. J. Appl. Polym. Sci. 2013, 127, 4177–4185. [Google Scholar] [CrossRef]
- Nikooe, N.; Saljoughi, E. Preparation and characterization of novel PVDF nanofiltration membranes with hydrophilic property for filtration of dye aqueous solution. Appl. Surf. Sci. 2017, 413, 41–49. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Lau, V.J.; Ji, Y.L.; Huang, S.H.; An, Q.F.; Caparanga, A.R. Correlating PSf support physicochemical properties with the formation of piperazine-based polyamide and evaluating the resultant nanofiltration membrane performance. Polymers 2017, 9, 505. [Google Scholar] [CrossRef]
- Feng, F.; Han, G.; Chung, T.S.; Weber, M.; Widjojo, N.; Maletzko, C. Effects of polyethylene glycol on membrane formation and properties of hydrophilic sulfonated polyphenylenesulfone (sPPSU) membranes. J. Membr. Sci. 2017, 531, 27–35. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Kadir, S.H.S.A.; Sokri, M.N.M.; Tai, Z.S.; Iwamoto, Y. Influence of the natural zeolite particle size toward the ammonia adsorption activity in ceramic hollow fiber membrane. Membranes 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, C.; Liu, H.; Huang, Q.; Hao, J.; Fu, H. Poly(vinylidene Fluoride-Hexafluoropropylene) Porous Membrane with Controllable Structure and Applications in Efficient Oil/Water Separation. Materials 2018, 11, 443. [Google Scholar] [CrossRef]
- Milescu, R.A.; McElroy, C.R.; Farmer, T.J.; Williams, P.M.; Walters, M.J.; Clark, J.H. Fabrication of PES/PVP water filtration membranes using cyrene®, a safer bio-based polar aprotic solvent. Adv. Polym. Technol. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Feng, C.Y.; Matsuura, T. Membrane Characterization. In Water and Wastewater Treatment Technologies; Eolss: Oxford, UK, 2010; Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.639.7419&rep=rep1&type=pdf (accessed on 22 June 2021).
- Tang, Y.; Li, N.; Liu, A.; Ding, S.; Yi, C.; Liu, H. Effect of spinning conditions on the structure and performance of hydrophobic PVDF hollow fiber membranes for membrane distillation. Desalination 2012, 287, 326–339. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Shafie, Z.M.H.M. Effect of air gap distance on PES/PVA hollow fibre membrane’s morphology and performance. J. Phys. Sci. 2017, 28, 185–199. [Google Scholar] [CrossRef]
- Paxton, N.C.; Wong, C.S.; Desselle, M.R.; Allenby, M.C.; Woodruff, M.A. Bone morphogenetic protein–assisted bone regeneration and applications in biofabrication. In Biomaterials for Organ and Tissue Regeneration; Woodhead Publishing: Sawston, UK, 2020; pp. 363–391. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N,Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Sai Guru Srinivasan, S.; Govardhanan, B.; Aabel, P.; Ashok, M.; Santhosh Kumar, M.C. Effect of oxygen partial pressure on the tuning of copper oxide thin films by reactive sputtering for solar light driven photocatalysis. Sol. Energy 2019, 187, 368–378. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and characterization of poly(ether-block-amide)/polyethylene glycol composite films with temperature- dependent permeation. Polymers 2018, 10, 225. [Google Scholar] [CrossRef]
- Slimane, F.Z.; Ellouze, F.; Amar, N.B. Fouling mechanism and screening of backwash parameters: Seawater ultrafiltration case. Environ. Eng. Res. 2019, 24, 298–308. [Google Scholar] [CrossRef]
- Pauzan, M.A.B.; Hubadillah, S.K.; Othman, M.H.D.; Ismail, N.J.; Puteh, M.H.; Abdullah, H.; Bakar, S.A.; Kadir, S.H.S.A.; Yinn, W.K. Fabrication and characterization of robust zirconia-kaolin hollow fiber membrane: Alkaline dissolution study in ammonia solution. Korean J. Chem. Eng. 2021, 1–15. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, H.; Feng, L.; Zhang, L. Effects of polyethylene glycol on the structure and filtration performance of thin-film PA-Psf composite forward osmosis membranes. Sep. Sci. Technol. 2016, 51, 862–873. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Chean, H.M.; Shamsuddin, N.; Bilad, M.R.; Narkkun, T.; Faungnawakij, K.; Khan, A.L. Development of Hydrophilic PVDF Membrane Using Vapour Induced Phase Separation Method for Produced Water Treatment. Membranes 2020, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, Z.; Wang, Y.; Ding, L.; Wang, Y. Role of pH-induced structural change in protein aggregation in foam fractionation of bovine serum albumin. Biotechnol. Rep. 2016, 9, 46–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, R.; Yuan, M.; Yue, X. Effects of pore-forming additives on structures and properties of PVDF/Fe3+/Cu2+ hollow fiber membranes. Desalination Water Treat. 2013, 51, 3903–3908. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Ren, Z.; Li, L. Preparation and characterization of PVDF-PFSA flat sheet ultrafiltration membranes. Front. Chem. Sci. Eng. 2012, 6, 301–310. [Google Scholar] [CrossRef]
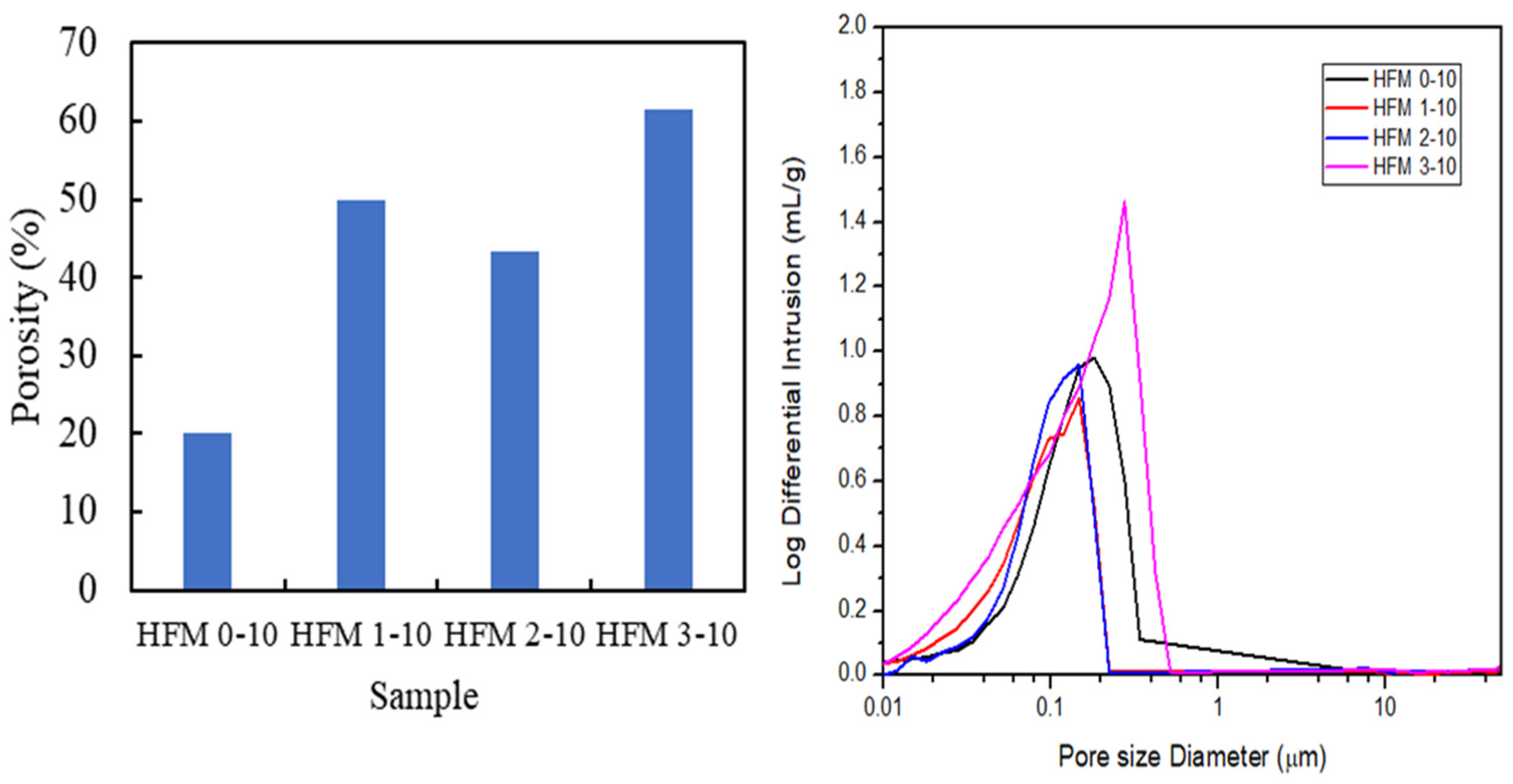
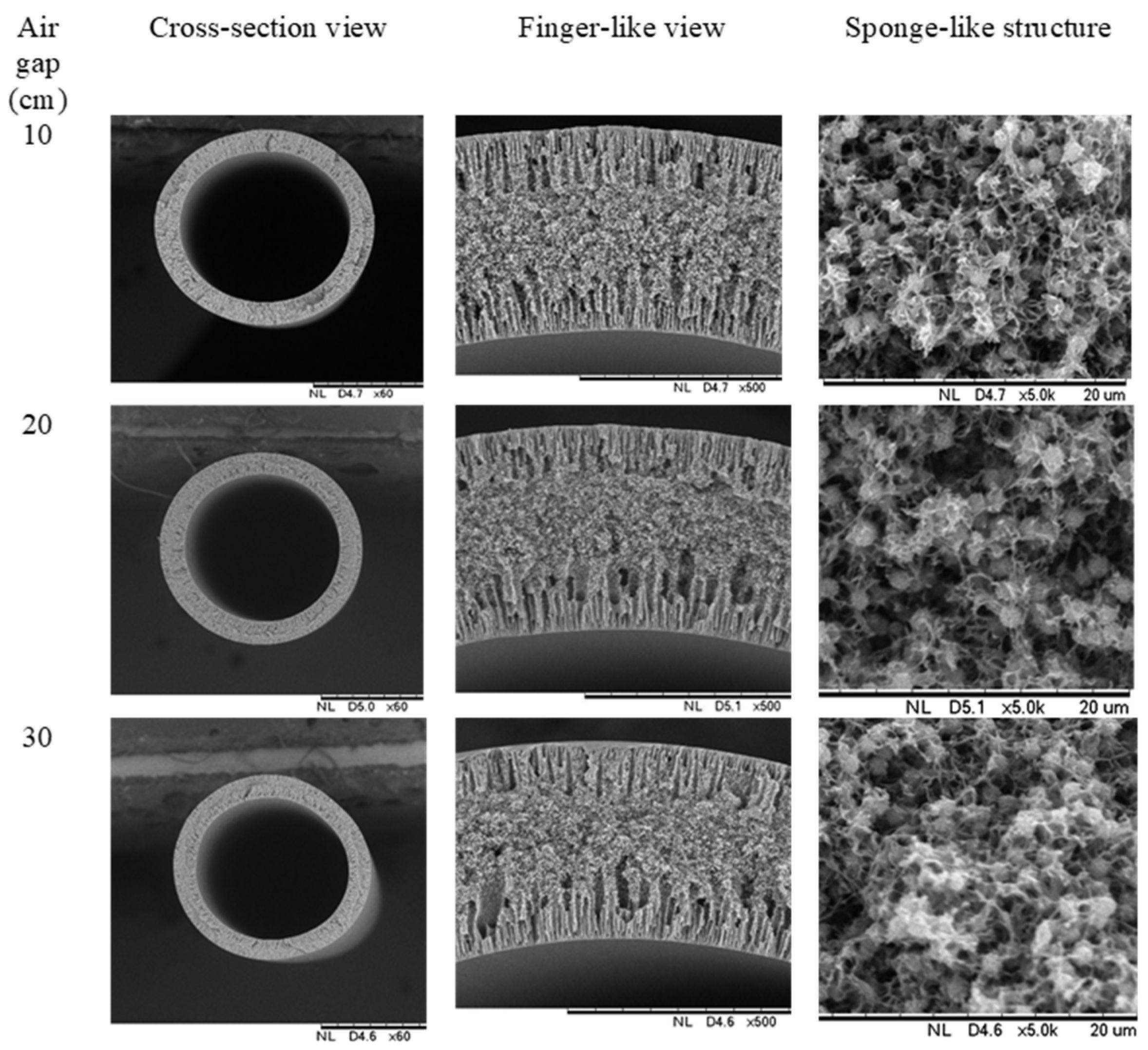
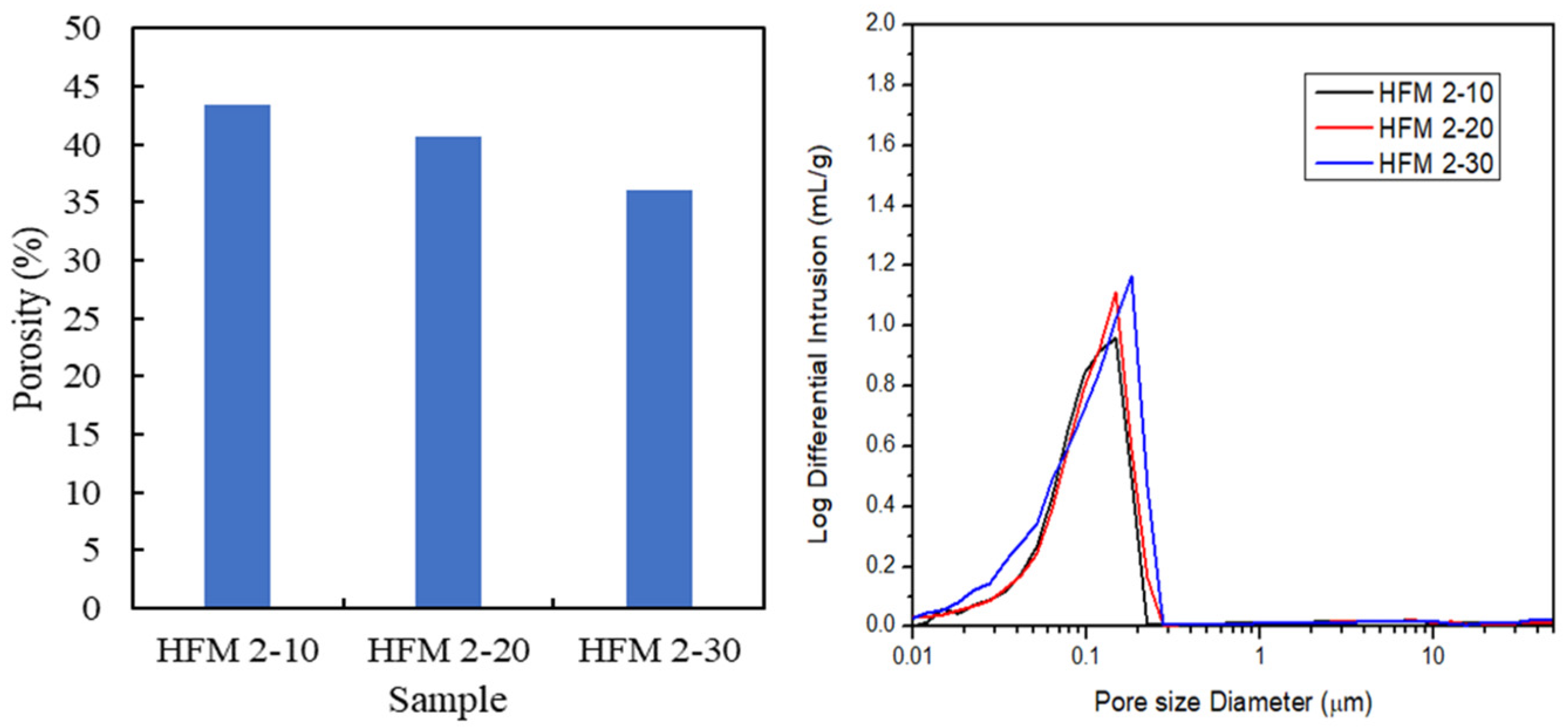
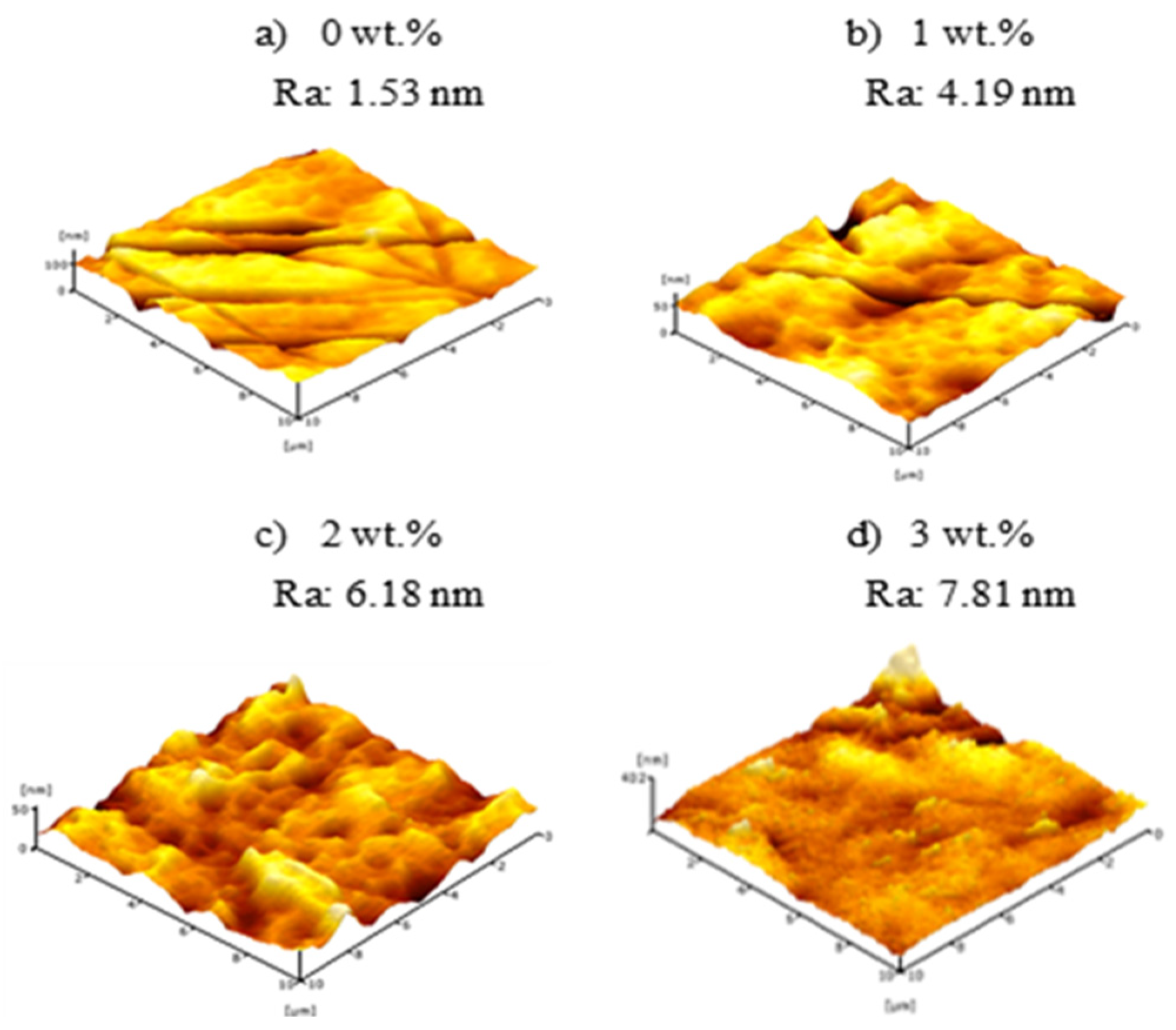
| Sample | PVDF (wt.%) | TEP (wt.%) | DMAc (wt.%) | PEG 400 (wt.%) |
|---|---|---|---|---|
| HFM 0 | 15 | 50 | 35 | 0 |
| HFM 1 | 15 | 50 | 34 | 1 |
| HFM 2 | 15 | 50 | 33 | 2 |
| HFM 3 | 15 | 50 | 32 | 3 |
| Sample Name | Dope Flow Rate (rpm) | Bore Fluid Type | Bore Fluid Flow Rate (mL/min) | Coagulation Bath | Spinneret Dimension (mm) | Air Gap (cm) |
|---|---|---|---|---|---|---|
| HFM 0-10 | 26 | Water | 8 | water | 0.8/1.2 | 10 |
| HFM 1-10 | 10 | |||||
| HFM 2-10 | 10 | |||||
| HFM 2-20 | 20 | |||||
| HFM 2-30 | 30 | |||||
| HFM 3-10 | 10 |
| Sample | Air-Gap (cm) | Outer Diameter (µm) | Thickness (µm) |
|---|---|---|---|
| HFM 2-10 | 10 | 1510 | 360 |
| HFM 2-20 | 20 | 1300 | 305 |
| HFM 2-30 | 30 | 1230 | 280 |
| PVDF Sample | σmax Tensile Strength (MPa) | εb Elongation at Break (%) |
|---|---|---|
| 0-10 | 4.08 ± 0.1 | 134.65 ± 0.8 |
| 1-10 | 2.66 ± 0.8 | 109.18 ± 1.0 |
| 2-10 | 2.08 ± 0.1 | 94.3 ± 4.3 |
| 2-20 | 2.91 ± 0.3 | 112.28 ± 2.8 |
| 2-30 | 2.78 ± 0.1 | 132.74 ± 1.0 |
| 3-10 | 2.34 ± 0.3 | 102.74 ± 1.1 |
| Sample | Type of Solvent | PEG 400 Loading (wt.%) | Air Gap (cm) | Water Flux (L/m2 h) | BSA Rejection (%) | Reference |
|---|---|---|---|---|---|---|
| HFM 0-10 | TEP/DMAc | 0 | 10 | 1060.54 | 91.2 | This study |
| HFM 1-10 | TEP/DMAc | 1 | 10 | 1585.85 | 90.3 | This study |
| Flat sheet PA/PSF | NMP/DMF | 0 | - | 28.6 | - | [45] |
| Flat sheet PA/PSF | NMP/DMF | 3 | - | 36.5 | - | [45] |
| Flat sheet PA/PSF | NMP/DMF | 6 | - | 47.4 | - | [45] |
| Flat sheet PA/PSF | NMP/DMF | 9 | - | 39.9 | - | [45] |
| Flat sheet PVDF | DMAc | 2 | - | 24 ± 1.3 | - | [31] |
| Flat sheet PVDF | DMAc | 4 | - | 30 ± 2.5 | - | [31] |
| Hollow PVDF/Fe3+/Cu2+ | DMAc | 1.5 | 12 | 35 | 91.6 | [47] |
| Flat sheet PVDF | DMAc | 5 | - | - | 80 | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakria, H.S.; Othman, M.H.D.; Kadir, S.H.S.A.; Kamaludin, R.; Jilani, A.; Omar, M.F.; Bakar, S.A.; Jaafar, J.; Rahman, M.A.; Abdullah, H.; et al. Fabrication of High Performance PVDF Hollow Fiber Membrane Using Less Toxic Solvent at Different Additive Loading and Air Gap. Membranes 2021, 11, 843. https://doi.org/10.3390/membranes11110843
Zakria HS, Othman MHD, Kadir SHSA, Kamaludin R, Jilani A, Omar MF, Bakar SA, Jaafar J, Rahman MA, Abdullah H, et al. Fabrication of High Performance PVDF Hollow Fiber Membrane Using Less Toxic Solvent at Different Additive Loading and Air Gap. Membranes. 2021; 11(11):843. https://doi.org/10.3390/membranes11110843
Chicago/Turabian StyleZakria, Hazirah Syahirah, Mohd Hafiz Dzarfan Othman, Siti Hamimah Sheikh Abdul Kadir, Roziana Kamaludin, Asim Jilani, Muhammad Firdaus Omar, Suriani Abu Bakar, Juhana Jaafar, Mukhlis A. Rahman, Huda Abdullah, and et al. 2021. "Fabrication of High Performance PVDF Hollow Fiber Membrane Using Less Toxic Solvent at Different Additive Loading and Air Gap" Membranes 11, no. 11: 843. https://doi.org/10.3390/membranes11110843
APA StyleZakria, H. S., Othman, M. H. D., Kadir, S. H. S. A., Kamaludin, R., Jilani, A., Omar, M. F., Bakar, S. A., Jaafar, J., Rahman, M. A., Abdullah, H., Puteh, M. H., Sinsamphanh, O., & Ayub, M. (2021). Fabrication of High Performance PVDF Hollow Fiber Membrane Using Less Toxic Solvent at Different Additive Loading and Air Gap. Membranes, 11(11), 843. https://doi.org/10.3390/membranes11110843