Immune Responses against the Omicron Variant of SARS-CoV-2 after a Third Dose of COVID-19 Vaccine in Patients Living with Human Immunodeficiency Virus (PLWH): Comparison with Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
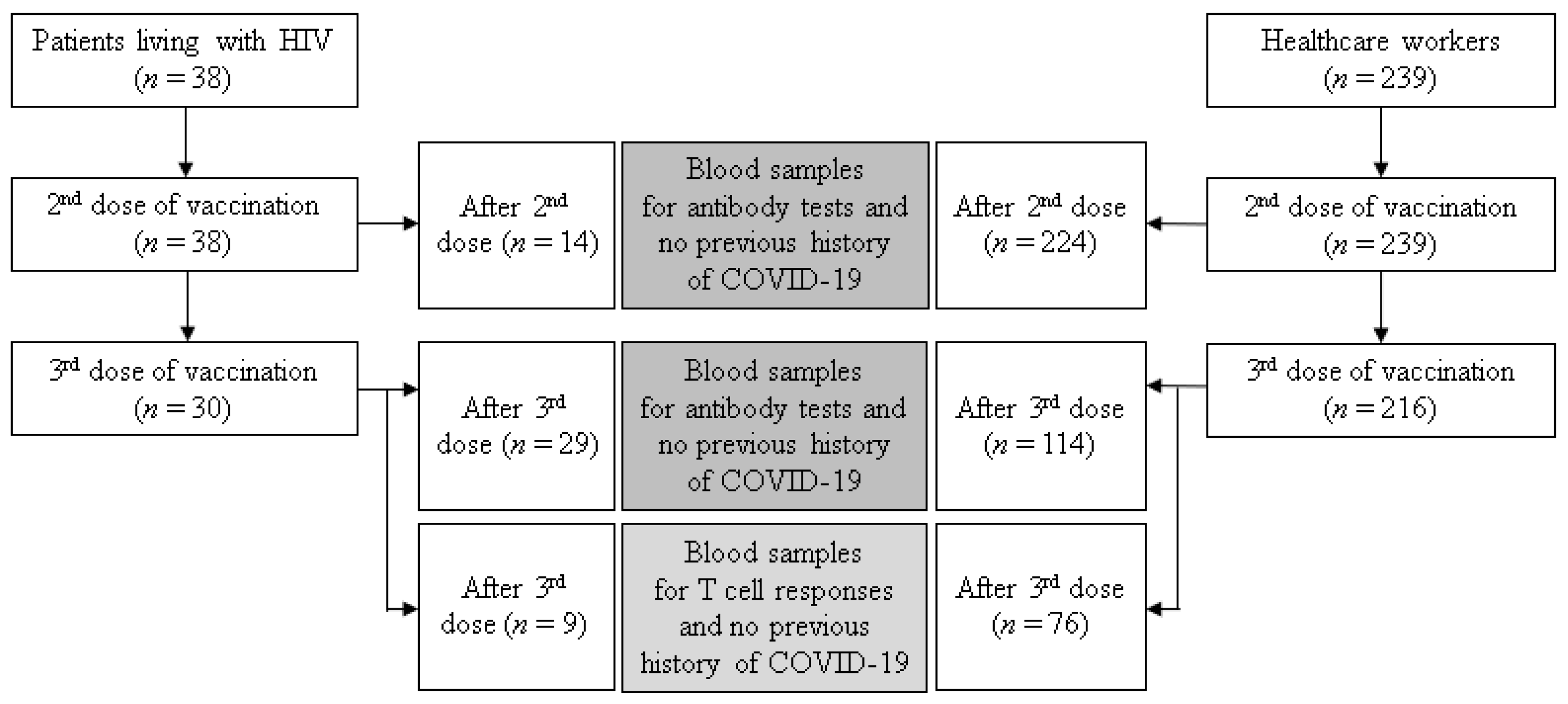
2.1. Study Population and Study Design
2.2. Assessment of SARS-CoV-2 Spike-Specific Immunoglobulin G (IgG)
2.3. Assessment of Neutralizing Antibody Responses against SARS-CoV-2
2.4. Assessment of SARS-CoV-2-Specific T Cell Responses
2.5. Statistical Analyses
3. Results
3.1. Study Population
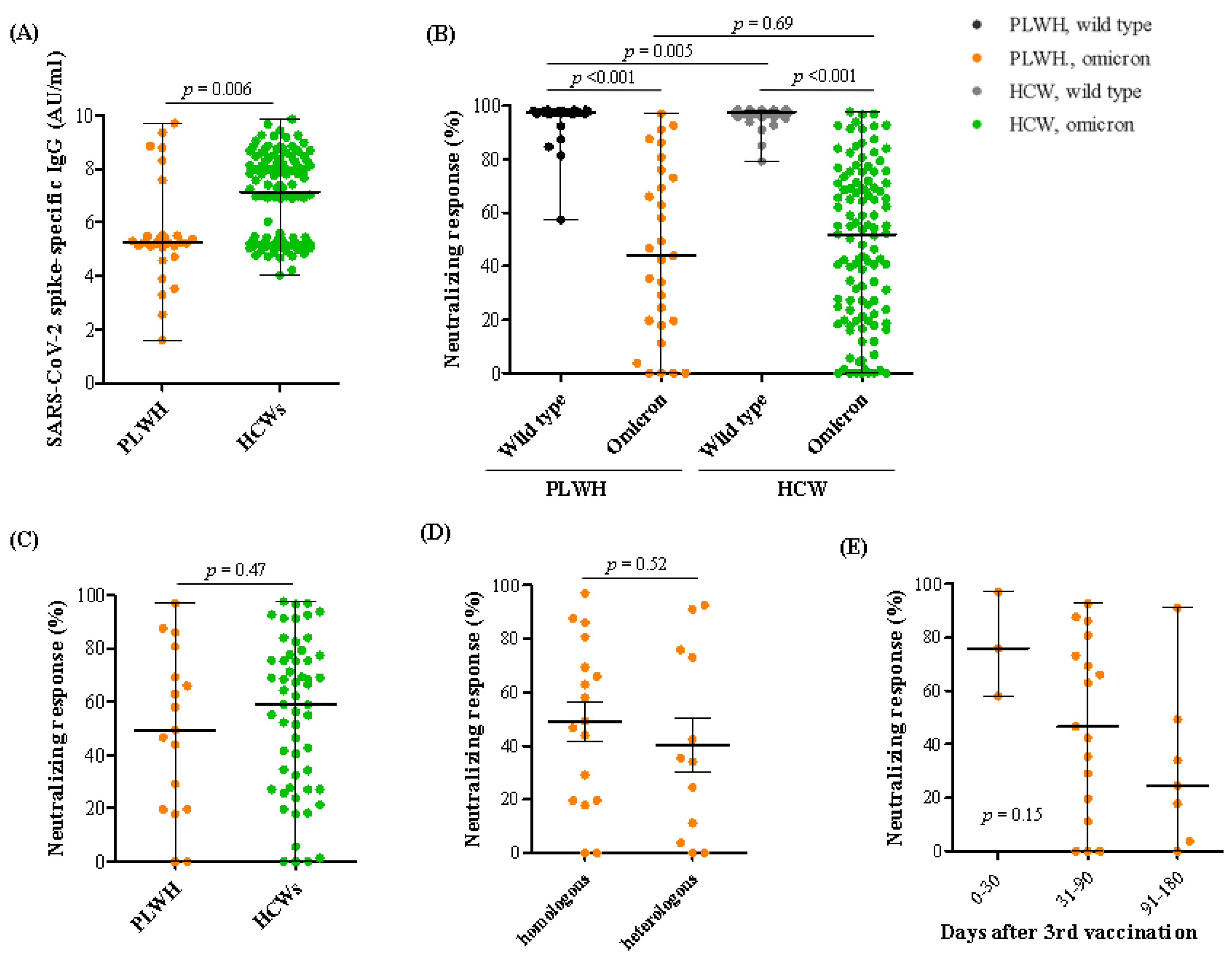
3.2. SARS-CoV-2 Spike-Specific IgG and Neutralizing Antibody Responses against SARS-CoV-2
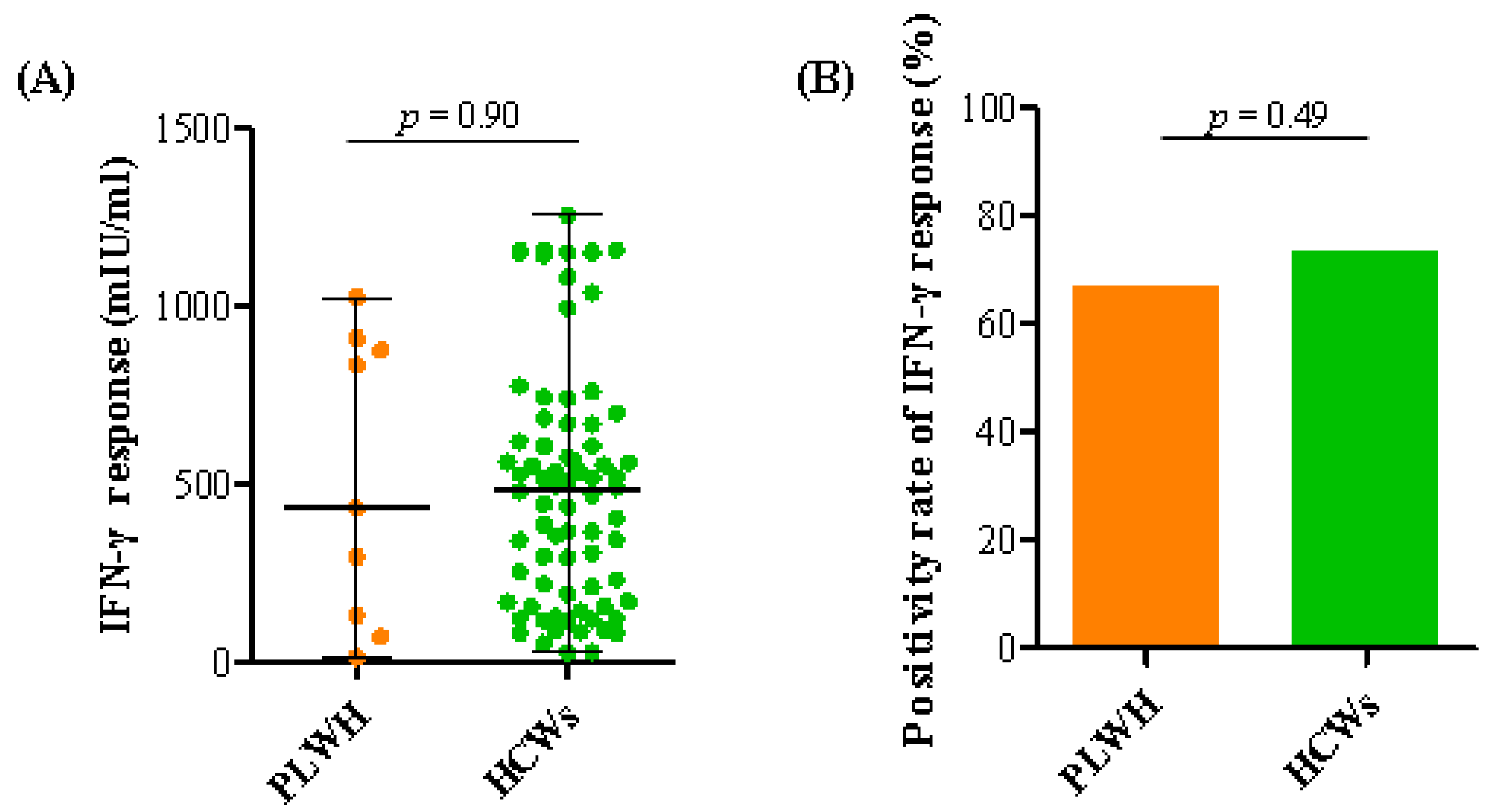
3.3. SARS-CoV-2-Specific T Cell Responses
3.4. Vaccine Breakthrough SARS-CoV-2 Infection in the Study Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhaskaran, K.; Rentsch, C.T.; MacKenna, B.; Schultze, A.; Mehrkar, A.; Bates, C.J.; Eggo, R.M.; Morton, C.E.; Bacon, S.C.J.; Inglesby, P.; et al. HIV infection and COVID-19 death: A population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021, 8, e24–e32. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, J.M.; Swain, C.-A.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open 2021, 4, e2037069. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Kernéis, S.; Launay, O.; Turbelin, C.; Batteux, F.; Hanslik, T.; Boëlle, P.Y. Long-term immune responses to vaccination in HIV-infected patients: A systematic review and meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Cicalini, S.; Meschi, S.; Bordoni, V.; Lorenzini, P.; Vergori, A.; Lanini, S.; De Pascale, L.; Matusali, G.; Mariotti, D.; et al. Humoral and cellular immune response elicited by mRNA vaccination against SARS-CoV-2 in people living with HIV (PLWH) receiving antiretroviral therapy (ART) according with current CD4 T-lymphocyte count. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, e552–e563. [Google Scholar] [CrossRef]
- Brumme, Z.L.; Mwimanzi, F.; Lapointe, H.R.; Cheung, P.K.; Sang, Y.; Duncan, M.C.; Yaseen, F.; Agafitei, O.; Ennis, S.; Ng, K.; et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines 2022, 7, 28. [Google Scholar] [CrossRef]
- Levy, I.; Wieder-Finesod, A.; Litchevsky, V.; Biber, A.; Indenbaum, V.; Olmer, L.; Huppert, A.; Mor, O.; Goldstein, M.; Levin, E.G.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1851–1855. [Google Scholar]
- El Chaer, F.; El Sahly, H.M. Vaccination in the Adult Patient Infected with HIV: A Review of Vaccine Efficacy and Immunogenicity. Am. J. Med. 2019, 132, 437–446. [Google Scholar] [CrossRef]
- Hassold, N.; Brichler, S.; Ouedraogo, E.; Leclerc, D.; Carroue, S.; Gater, Y.; Alloui, C.; Carbonnelle, E.; Bouchaud, O.; Mechai, F.; et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS 2022, 36, F1–F5. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; Kurth, F.; Sander, L.E.; Klein, F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022, 28, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Muik, A.; Lui, B.G.; Wallisch, A.K.; Bacher, M.; Mühl, J.; Reinholz, J.; Ozhelvaci, O.; Beckmann, N.; Güimil Garcia, R.D.; Poran, A.; et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 2022, 375, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Pajon, R.; Doria-Rose, N.A.; Shen, X.; Schmidt, S.D.; O’Dell, S.; McDanal, C.; Feng, W.; Tong, J.; Eaton, A.; Maglinao, M.; et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N. Engl. J. Med. 2022, 386, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, H.R.; Mwimanzi, F.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Umviligihozo, G.; Kalikawe, R.; Speckmaier, S.; Moran-Garcia, N.; Datwani, S.; et al. People with HIV receiving suppressive antiretroviral therapy show typical antibody durability after dual COVID-19 vaccination, and strong third dose responses. J. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.H.; Chung, J.W.; Hwang, M.H.; Kim, M.C. Systemic Adverse Events and Use of Antipyretics Predict the Neutralizing Antibody Positivity Early after the First Dose of ChAdOx1 Coronavirus Disease Vaccine. J. Clin. Med. 2021, 10, 2844. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.; Grundberg, E.; Selvarangan, R.; LeMaster, C.; Fraley, E.; Banerjee, D.; Banerjee, D.; Belden, B.; Louiselle, D.; Nolte, N.; et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1959–1961. [Google Scholar] [CrossRef]
- Geretti, A.M.; Doyle, T. Immunization for HIV-positive individuals. Curr. Opin. Infect. Dis. 2010, 23, 32–38. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Cox, L.S.; Bellantuono, I.; Lord, J.M.; Sapey, E.; Mannick, J.B.; Partridge, L.; Gordon, A.L.; Steves, C.J.; Witham, M.D. Tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults. Lancet Healthy Longev. 2020, 1, e55–e57. [Google Scholar] [CrossRef]
- Oyaert, M.; De Scheerder, M.A.; Van Herrewege, S.; Laureys, G.; Van Assche, S.; Cambron, M.; Naesens, L.; Hoste, L.; Claes, K.; Haerynck, F.; et al. Evaluation of Humoral and Cellular Responses in SARS-CoV-2 mRNA Vaccinated Immunocompromised Patients. Front. Immunol. 2022, 13, 858399. [Google Scholar] [CrossRef]
- Tau, L.; Turner, D.; Adler, A.; Marom, R.; Ahsanov, S.; Matus, N.; Levi, I.; Gerber, G.; Lev, S.; Ziv-Baran, T.; et al. SARS-CoV-2 Humoral and Cellular Immune Responses of Patients With HIV After Vaccination With BNT162b2 mRNA COVID-19 Vaccine in the Tel-Aviv Medical Center. Open Forum Infect. Dis. 2022, 9, ofac089. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Martínez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Reséndiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against COVID-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]

| PLWH (n = 29) | Healthcare Workers (n = 114) | p Value | |
|---|---|---|---|
| Male, n (%) | 28 (96.6) | 38 (33.3) | <0.001 |
| Age, median (IQR) | 44 (34–56) | 35 (26–45) | 0.001 |
| Vaccine regimen | 0.41 | ||
| Homologous a | 17 (58.6) | 57 (50.0) | |
| mRNA | 17 | 57 | |
| Adenovirus-vector | 0 | 0 | |
| Heterologous | 12 (41.4) | 57 (50.0) | |
| Ad-Ad-mRNA | 8 | 57 | |
| Ad-mRNA-mRNA | 4 | 0 | |
| HIV status | |||
| Duration after HIV diagnosis, years | 11.0 (7.5–13.0) | - | - |
| Duration after HIV treatment, years | 9.0 (6.5–12.0) | - | - |
| White blood cell, /μL | 6200 (5185–7245) | - | - |
| Lymphocytes, % | 36.0 (30.2–42.8) | - | - |
| CD4 lymphocytes | 670.0 (527.1–830.3) | - | - |
| <20 copies/mL of HIV RNA, n (%) | 25 (86.2) | - | - |
| Underlying diseases or conditions | |||
| Malignancy | 4 (13.8) | 0 | 0.001 |
| Chemotherapy | 1 (3.4) | 0 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Chung, H.; Kim, M.-C.; Choi, S.-H.; Chung, J.-W. Immune Responses against the Omicron Variant of SARS-CoV-2 after a Third Dose of COVID-19 Vaccine in Patients Living with Human Immunodeficiency Virus (PLWH): Comparison with Healthcare Workers. Vaccines 2022, 10, 2129. https://doi.org/10.3390/vaccines10122129
Park JH, Chung H, Kim M-C, Choi S-H, Chung J-W. Immune Responses against the Omicron Variant of SARS-CoV-2 after a Third Dose of COVID-19 Vaccine in Patients Living with Human Immunodeficiency Virus (PLWH): Comparison with Healthcare Workers. Vaccines. 2022; 10(12):2129. https://doi.org/10.3390/vaccines10122129
Chicago/Turabian StylePark, Joung Ha, Hyemin Chung, Min-Chul Kim, Seong-Ho Choi, and Jin-Won Chung. 2022. "Immune Responses against the Omicron Variant of SARS-CoV-2 after a Third Dose of COVID-19 Vaccine in Patients Living with Human Immunodeficiency Virus (PLWH): Comparison with Healthcare Workers" Vaccines 10, no. 12: 2129. https://doi.org/10.3390/vaccines10122129
APA StylePark, J. H., Chung, H., Kim, M.-C., Choi, S.-H., & Chung, J.-W. (2022). Immune Responses against the Omicron Variant of SARS-CoV-2 after a Third Dose of COVID-19 Vaccine in Patients Living with Human Immunodeficiency Virus (PLWH): Comparison with Healthcare Workers. Vaccines, 10(12), 2129. https://doi.org/10.3390/vaccines10122129








