Qualitative Aspects of Some Traditional Landraces of the Tomato “Piennolo” (Solanum lycopersicum L.) of the Campania Region, Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Chemical Analysis
2.3.1. Total Polyphenols
2.3.2. β-Carotene Content
2.3.3. Lycopene Content
2.3.4. Dosage of Ascorbic Acid
2.3.5. Antioxidant Activity
2.3.6. Polyphenols Chromatographic Analysis
2.4. Statistical Analysis
3. Results
3.1. Chemical Parameters
3.2. Biochemical Aspects
3.2.1. Total Polyphenols
3.2.2. Carotenoids
3.2.3. Ascorbic Acid
3.2.4. Polyphenol Profile
3.2.5. Antioxidant Activity
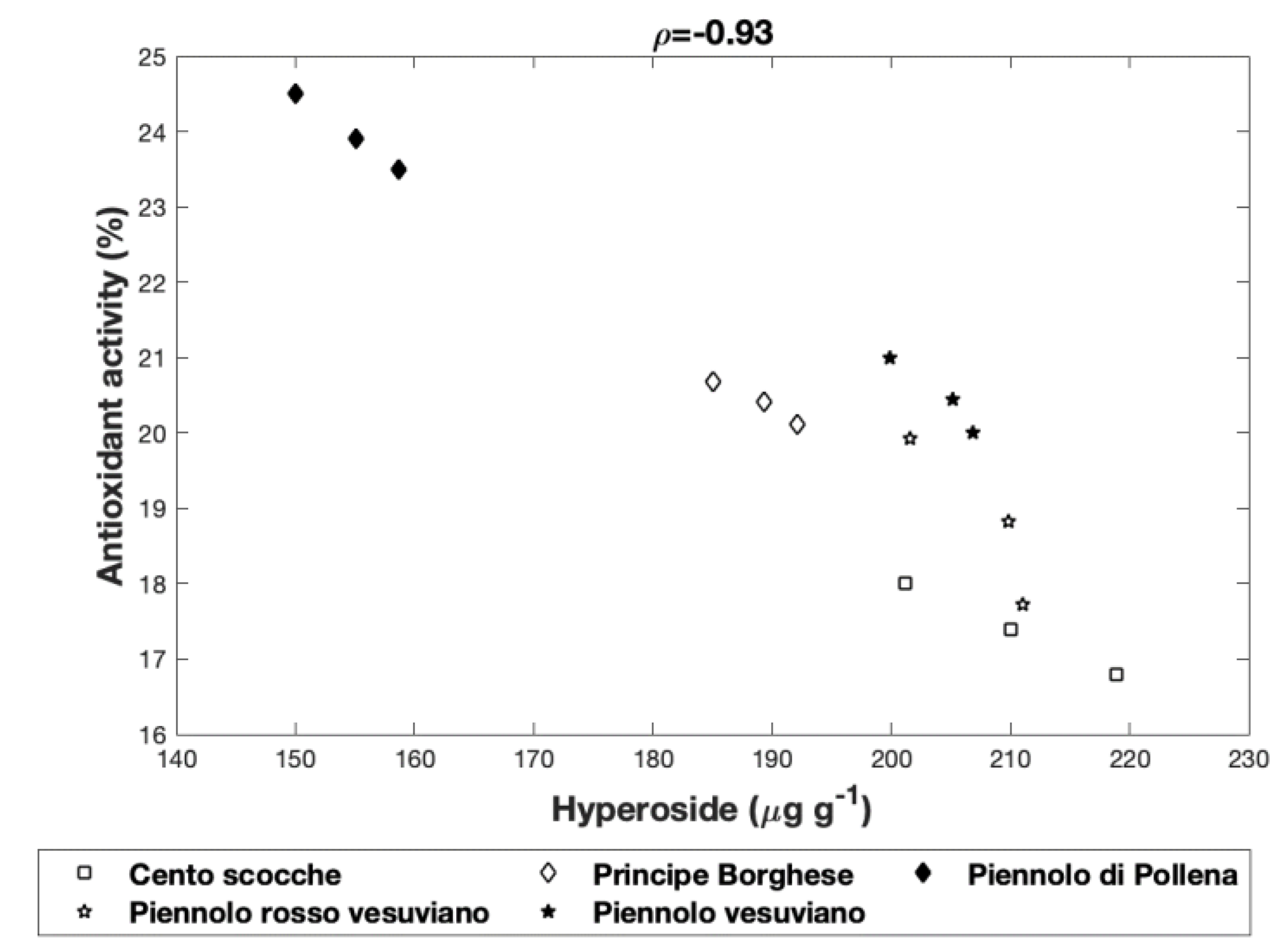
3.2.6. Interrelationships Among the Antioxidant Activity and Singular Polyphenols
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xaba, B.G. Factors affecting the productivity and profitability of vegetables production in Swaziland. J. Agric. Stud. 2013, 1, 1–16. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Conesa, M.A.; Fullana-Pericàs, M.; Granell, A.; Galmés, J. Mediterranean long shelf-life landraces: An untapped genetic resource for tomato improvement. Front. Plant Sci. 2020. [Google Scholar] [CrossRef]
- Passaro, P.; Salomone, S. Consumer Innovativeness in Food Industry: From Literature Review Some Indications for Business Practices. Int. J. Bus. Adm. 2017, 8, 10–24. [Google Scholar] [CrossRef][Green Version]
- Papa, S.; Bartoli, G.; Alvarez-Romero, M. Nutrient elements in a bean ecotype “Lenzariello” from the Alto Casertano area, Campania Region, Italy. In Proceedings of the “Capitale Naturale: La Gestione per la Conservazione” XXIX National Congress S.It.E of the Italian Society of Ecology, Ferrara, Italy, 10–12 September 2019. [Google Scholar]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Pepe, S.; Riccardi, R.; Spigno, P.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of two autochthonous brassicaceae of the Campania Region, Southern Italy. Food Nutr. Sci. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Blasi, C.; Boitani, L.; La Posta, S.; Manes, F.; Marchetti, M. Stato Della Biodiversità in Italia—Contributo Alla Strategia Nazionale per la Biodiversità; Palombi Editori: Rome, Italy, 2005; pp. 1–99. ISBN 88-7621-514-X. [Google Scholar]
- Barbera, G.; Cullotta, S. The traditional Mediterranean poly-cultural landscape as cultural heritage: Its origin and hystorical importance, its agro-silvo- pastoral complexity and the necessity for its identification and inventory. In Biocultural Diversity in Europe; Agnoletti, M., Emanueli, F., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 21–48. [Google Scholar]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef]
- Szabo, K.; Vasile Dulf, F.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. Lebensm. Wiss. Und Technol. Food Sci. Technol. 2019, 116, 108558. [Google Scholar] [CrossRef]
- Mazzucato, A.; Papa, R.; Bitocchi, E.; Mosconi, P.; Nanni, L.; Negri, V.; Picarella, M.E.; Siligato, F.; Soressi, G.P.; Tiranti, B.; et al. Genetic diversity, structure and marker-trait associations in a collection of Italian tomato (Solanum lycopersicum L.) landraces. Theor. Appl. Genet. 2008, 116, 657–669. [Google Scholar] [CrossRef]
- Available online: http://www.agricoltura.regione.campania.it/biodiversita/doc/DRD_08-29-05-17.pdf (accessed on 12 May 2020).
- Rao, R.; Corrado, G.; Bianchi, M.; Di Mauro, A. (GATA)4 DNA fingerprinting identifies morphologically characterized ‘San Marzano’ tomato plants. Plant Breed. 2006, 125, 173–176. [Google Scholar] [CrossRef]
- Carillo, P.; Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; dell’Aversana, E.; D’Amelia, L.; Colla, G.; Caruso, G.; De Pascale, S.; Rouphael, Y. Sensory and functional quality characterization of protected designation of origin ‘Piennolo del Vesuvio’ cherry tomato landraces from Campania-Italy. Food Chem. 2019, 292, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Tranchida-Lombardo, V.; Aiese Cigliano, R.; Anzar, I.; Landi, S.; Palombieri, S.; Colantuono, C.; Bostan, H.; Termolino, P.; Aversano, R.; Batelli, G.; et al. Whole-genome re-sequencing of two Italian tomato landraces reveals sequence variations in genes associated with stress tolerance, fruit quality and long shelf-life traits. DNA Res. 2018, 25, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, S.S.; Figas Moreno, M.R.; Pereira Dias, L.; Casanova Calancha, C.; Soler Calabuig, E.; Tomas, J.T. Morphological, agronomic and quality characterization of ‘de penjar’ traditional tomato varieties in different growing systems. In Proceedings of the Abstracts of the XIV Solanaceae and 3rd Cucurbitaceae Joint Conference, Valencia, Spain, 3–4 september 2017; p. 220. [Google Scholar]
- Bisson, T.N. The Medieval Crown of Aragon: A Short History; Oxford University Press: Oxford, UK, 1986. [Google Scholar]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and nutritional quality of Vesuvian tomato PDO as affected by farming system and biostimulant application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef]
- Archivio Meteo Storico. Available online: http://www.ilmeteo.it/portale/archivio-meteo (accessed on 12 May 2020).
- Available online: https://www.gazzettaufficiale.it/eli/gu/1989/07/20/168/so/51/sg/pdf (accessed on 12 May 2020).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Fratianni, F.; Ombra, M.N.; Cozzolino, A.; Riccardi, R.; Spigno, P.; Tremonte, P.; Coppola, R.; Nazzaro, F. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.). J. Funct. Foods 2016, 21, 240–248. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology and Technology; Wiley: Chicago, IL, USA, 2015; pp. 1–328. ISBN 978-1-118-73330-1. [Google Scholar]
- Nazzaro, F.; Caliendo, G.; Arnesi, G.; Veronesi, A.; Sarzi, P.; Fratianni, F. Comparative content of some bioactive compounds in two varieties of Capsicum annuum L. Sweet pepper and evaluation of their antimicrobial and mutagenic activities. J. Food Biochem. 2009, 33, 852–868. [Google Scholar] [CrossRef]
- Ombra, M.N.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxidative Med. Cell. Longev. 2016, 1–12. [Google Scholar] [CrossRef]
- Pane, C.; Fratianni, F.; Parisi, M.; Nazzaro, F.; Zaccardelli, M. Control of Alternaria post-harvest infections on cherry tomato fruits by wild pepper phenolic-rich extracts. Crop Prot. 2016, 84, 81–87. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; d’Acierno, A.; De Feo, V.; Da Cruz, A.G.; Nazzaro, F. Biochemical composition and antioxidant activity of three extra virgin olive oils from the Irpinia Province, Southern Italy. Food Sci. Nutr. 2019, 7, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Raffo, A.; Leonardi, C.; Fogliano, V.; Ambrosino, P.; Salucci, M.; Gennaro, L.; Bugianesi, R.; Giuffrida, F.; Quaglia, G. Nutritional value of cherry tomatoes (Lycopersicon esculentum cv. Naomi F1) harvested at different ripening stages. J. Agric. Food Chem. 2002, 50, 6550–6556. [Google Scholar] [CrossRef]
- Hiraga, M.; Shirasawa, K.; Nishiyama, M.; Kanahama, K.; Kanayama, Y. Analysis of a tomato introgression line, IL8-3, with increased Brix content. Sci. Hortic. 2013, 153, 103–108. [Google Scholar]
- Raiola, A.; Pizzolongo, F.; Manzo, N.; Montefusco, I.; Spigno, P.; Romano, R.; Barone, A. A comparative study of the physico-chemical properties affecting the organoleptic quality of fresh and thermally treated yellow tomato ecotype fruit. Int. J. Food Sci. Technol. 2018, 53, 1219–1226. [Google Scholar] [CrossRef]
- Helyes, L.; Varga, G.; Pek, Z.; Dimeny, J. The simultaneous effect of variety, irrigation and weather on tomato yield. Acta Hortic. 1999, 487, 499–505. [Google Scholar] [CrossRef]
- Helyes, L.; Dimény, J.; Pék, Z.; Lugasi, A. Effect of the variety and growing methods as well as cultivation conditions on the composition of tomato (Lycopersicon lycopersicum (l.) Karsten) fruit. Acta Hortic. 2006, 712, 511–516. [Google Scholar] [CrossRef]
- Blaszczak, W.; Je, M.; Szwengiel, A. Polyphenols and inhibitory effects of crude and purified extracts from tomato varieties on the formation of advanced glycation end products and the activity of angiotensin-converting and acetylcholinesterase enzymes. Food Chem. 2020, 314. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato. (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar]
- Siracusa, L.; Patanè, C.; Avola, G.; Ruberto, G. Polyphenols as chemotaxonomic markers in Italian “long-storage” tomato genotypes. J. Agric. Food Chem. 2012, 60, 309–314. [Google Scholar] [CrossRef]
- KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.kegg.jp (accessed on 1 June 2020).
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino Vesuviano” tomatoes. J. Food Compos. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Manzo, N.; Pizzolongo, F.; Meca, G.; Aiello, A.; Marchetti, N.; Romano, R. Comparative chemical compositions of fresh and stored vesuvian PDO “Pomodorino Del Piennolo” tomato and the ciliegino variety. Molecules 2018, 23, 2871. [Google Scholar] [CrossRef]
- Boileau, T.W.; Boileau, A.C.; Erdman, J.W., Jr. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med. 2002, 227, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, N.S.; Bhagya, L.K.; Chandy, V. Lycopene properties and its benefits in human health: A brief review. World J. Pharma Pharm. Sci. 2016, 5, 424–436. [Google Scholar]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J. 2000, 163, 739–744. [Google Scholar]
- Giovannucci, E. Tomatoes, Tomato-Based Products, Lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Lima, M.J.R.; Oliveria, J.; Teixeira Lemos, E. Tomato Lycopene: Functional Properties and Health Benefits. World Acad. Sci. Eng. Technol. 2015, 9, 458–468. [Google Scholar]
- Fraser, G.E.; Jacobsen, B.K.; Knutsen, S.F.; Mashchak, A.; Lloren, J.I. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: The Adventist Health Study 2. Cancer Causes Control 2020, 31, 341–351. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Malami, D.I. Effect of heat treatment on the lycopene content of tomato puree. ChemSearch J. 2013, 4, 18–21. [Google Scholar]
- Wu, T.; Gao, Y.; Hao, J.; Yin, J.; Li, W.; Geng, J.; Liu, R.; Sui, W.; Zhang, M. Lycopene, amaranth, and sorghum red pigments counteract obesity and modulate the gut microbiota in high-fat diet fed C57BL/6 mice. J. Funct. Foods 2019, 60. [Google Scholar] [CrossRef]
- Wiese, M.; Bashmakov, Y.; Chalyk, N.; Nielsen, D.S.; Krych, L.; Kot, W.; Klochkov, V.; Pristensky, D.; Bandaletova, T.; Chernyshova, M.; et al. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons. Biomed Res. Int. 2019, 2019, 4625279. [Google Scholar] [CrossRef]
- Cano, A.; Costa, M.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activity changes during on-vine ripening of tomatoes (Lycopersicon esculentum Mill.). Postharvest Biol. Technol. 2003, 28, 59–65. [Google Scholar] [CrossRef]
- García-Valverde, V.; Navarro-González, I.; García-Alonso, J.; Periago, M.J. Antioxidant Bioactive Compounds in Selected Industrial Processing and Fresh Consumption Tomato Cultivars. Food Bioprocess Technol. 2013, 6, 391–402. [Google Scholar] [CrossRef]
- Del Giudice, R.; Raiola, A.; Tenore, G.C.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Medina-Remon, A.; Martinez-Huelamo, M.; Jauregui, O.; Andres-Lacueva, C.; Lamuela-Raventos, R. Phenolic profile and hydrophilic antioxidant capacity as chemotaxonomic markers of tomato varieties. J. Agric. Food Chem. 2011, 59, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G. Nutrient content of tomatoes and tomato products. Proc. Soc. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Martin-Diana, A.B.; Rico, D.; Barry-Ryan, C. Quality and nutritional status of fresh-cut tomato as affected by spraying of delactosed whey permeate compared to industrial washing treatment. Food Bioprocess Technol. 2012, 5, 3103–3114. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of minimal processing on bioactive compounds and color attributes of fresh-cut tomatoes. Lebensm. Wiss. Und Technol. Food Sci. Technol. 2008, 41, 217–226. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; De Feo, V.; Cruz, A.G.; d’Acierno, A. Antibacterial activity of three extra virgin olive oils of the Campania region, Southern Italy, related to their polyphenol content and composition. Microorganisms 2019, 7, 321. [Google Scholar] [CrossRef]
- Patanè, C.; Malvuccio, A.; Saita, A.; Rizzarelli, P.; Siracusa, L.; Rizzo, V.; Muratore, G. Nutritional changes during storage in fresh-cut long storage tomato as affected by biocompostable polylactide and cellulose based packaging. Lebensm. Wiss. Und Technol. Food Sci. Technol. 2019, 101, 618–624. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.O.; Moon, H.Y.; Kang, H.G.; Lee, C.Y. Contribution of Individual Polyphenolics to Total Antioxidant Capacity of Plums. J. Agric. Food Chem. 2003, 51, 7240–7245. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Demirata, B.; Ozyurek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Borguini, R.; Torres, E. Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
| Landraces | °Brix | pH | Titratable Acidity (% Citric Acid) | Total Reducing Sugars (%) |
|---|---|---|---|---|
| Centoscocche | 6.9 (±0.1) | 4.23 (±0.13) | 0.56 (±0.01) | 4.22 (±0.2) |
| Principe Borghese | 6.4 (±0.12) | 4.36 (±0.14) | 0.58 (0.01) | 4.12 (±0.12) |
| Piennolo di Pollena | 7.6 (±0.38) | 4.29 (±0.20) | 0.58 (0.02) | 4.37 (±0.21) |
| Piennolo Rosso Vesuviano | 6.9 (±0.10) | 4.27 (±0.20) | 0.55 (±0.04) | 4.72 (±0.34) |
| Piennolo Vesuviano | 7.1 (±0.12) | 4.28 (±0.15) | 0.60 (±0.03) | 5.19 (±0.15) |
| Variety | TPs (μg g−1) | β-C (μg g−1) | Lyc (μg g−1) | ASA (μg g−1) | AA (%) |
|---|---|---|---|---|---|
| PDC | 230.17 (±15.09) | 1.34 (±0.023) | 218.89 (±25.20) | 240 (±17.0) | 17.39 (±0.66) |
| PDB | 278.12 (±13.76) | 1.07 (±0.013) | 192.11 (±10.99) | 270 (±16.0) | 20.41 (±0.27) |
| PDP | 320.75 (±23.76) | 1.65 (±0.018) | 158.71 (±2.13) | 190 (±2.0) | 23.91 (±0.51) |
| PRS | 296.26 (±17.03) | 1.36 (±0.024) | 209.84 (±14.31) | 230 (±25.0) | 18.82 (±1.18) |
| PDV | 320.99 (±16.33) | 1.94 (±0.02) | 206.87 (±12.64) | 250 (±3.0) | 20.45 (±0.40) |
| Variety | CHL μg g−1 | FER μg/g−1 | GAL μg g−1 | CAT μg g−1 | HYP μg g−1 | RUT μg g−1 |
|---|---|---|---|---|---|---|
| PDC | 98.65 (±6.12) | 15.25 (±3.38) | 45.22 (±5.00) | 1.82 (±0.71) | 210.01 (±8.88) | 6.41 (±1.23) |
| PDB | 125.78 (±5.01) | 9.13 (±0.39) | 49.97 (±5.21) | 2.66 (±0.86) | 188.84 (±3.53) | 33.51 (±3.63) |
| PDP | 128.19 (±11.97) | 25.14 (±5.22) | 56.82 (±4.27) | 4.42 (±0.49) | 154.57 (±4.39) | 15.28 (±1.55) |
| PRS | 146.27 (±9.53) | 18.12 (±3.35) | 61.35 (±4.51) | 4.37 (±0.46) | 207.48 (±5.16) | 0.00 (±0.00) |
| PDV | 154.83 (±5.30) | 11.86 (±3.62) | 45.14 (±3.50) | 2.83 (±0.63) | 203.95 (±3.65) | 19.51 (±1.53) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fratianni, F.; Cozzolino, A.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P. Qualitative Aspects of Some Traditional Landraces of the Tomato “Piennolo” (Solanum lycopersicum L.) of the Campania Region, Southern Italy. Antioxidants 2020, 9, 565. https://doi.org/10.3390/antiox9070565
Fratianni F, Cozzolino A, d’Acierno A, Nazzaro F, Riccardi R, Spigno P. Qualitative Aspects of Some Traditional Landraces of the Tomato “Piennolo” (Solanum lycopersicum L.) of the Campania Region, Southern Italy. Antioxidants. 2020; 9(7):565. https://doi.org/10.3390/antiox9070565
Chicago/Turabian StyleFratianni, Florinda, Autilia Cozzolino, Antonio d’Acierno, Filomena Nazzaro, Riccardo Riccardi, and Patrizia Spigno. 2020. "Qualitative Aspects of Some Traditional Landraces of the Tomato “Piennolo” (Solanum lycopersicum L.) of the Campania Region, Southern Italy" Antioxidants 9, no. 7: 565. https://doi.org/10.3390/antiox9070565
APA StyleFratianni, F., Cozzolino, A., d’Acierno, A., Nazzaro, F., Riccardi, R., & Spigno, P. (2020). Qualitative Aspects of Some Traditional Landraces of the Tomato “Piennolo” (Solanum lycopersicum L.) of the Campania Region, Southern Italy. Antioxidants, 9(7), 565. https://doi.org/10.3390/antiox9070565






