Antioxidant Properties of Agri-Food Byproducts and Specific Boosting Effects of Hydrolytic Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Methods
2.2. Preparation of Agri-Food Byproducts
2.3. Preparation of Polymers from Caffeic Acid (PolyCAF) and Ferulic Acid (PolyFER)
2.4. Hydrolytic Treatment
2.5. DPPH Assay
2.6. Ferric Reducing/Antioxidant Power (FRAP) Assay
2.7. Total Phenolic Content (TPC) Assay
2.8. Alkali Fusion
2.9. Alkaline Hydrogen Peroxide Degradation
2.10. Acid Degradation
2.11. Extraction of Phenolic Compounds
3. Results and Discussion
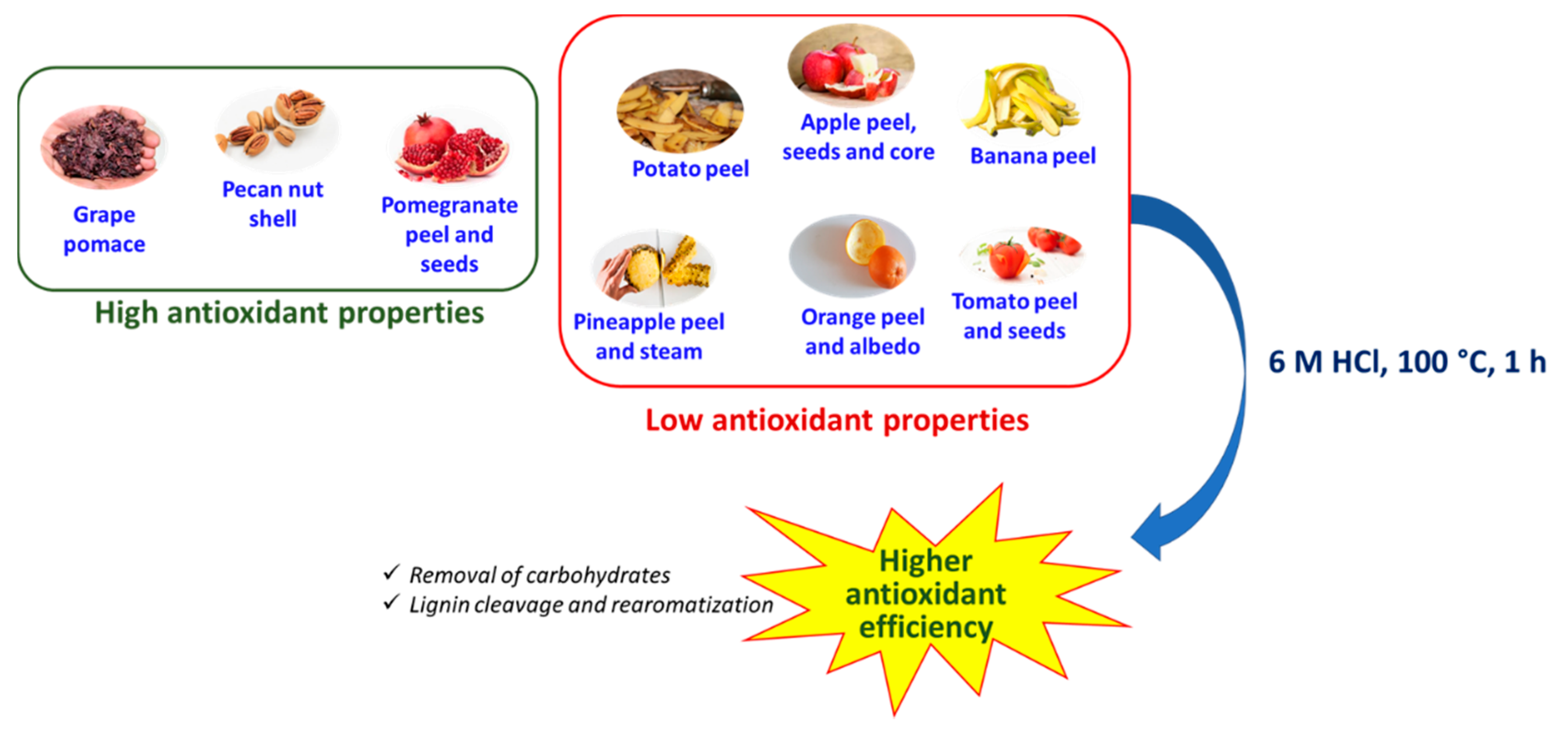
3.1. Antioxidant Properties of the Agri-Food Byproducts
3.2. Effects of the Hydrolytic Treatment on the Antioxidant Properties of the Agri-Food Byproducts
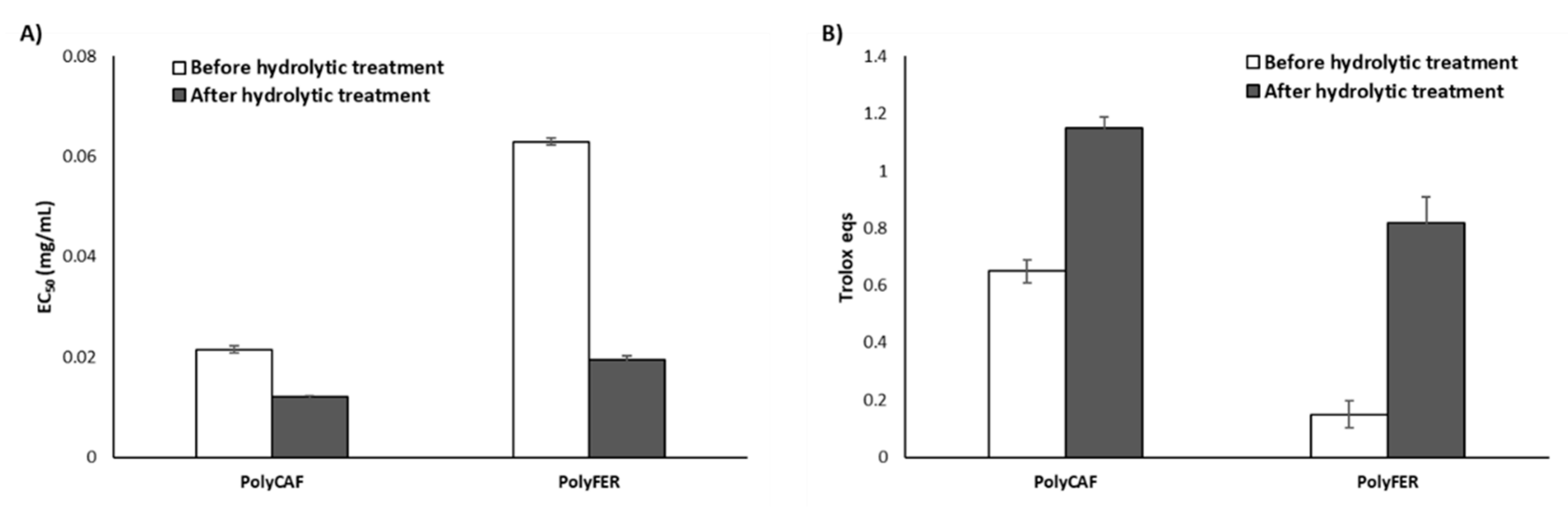
3.3. Antioxidant Properties of Natural Tannins and Lignin-Mimicking Phenolic Polymers before and after the Hydrolytic Treatment
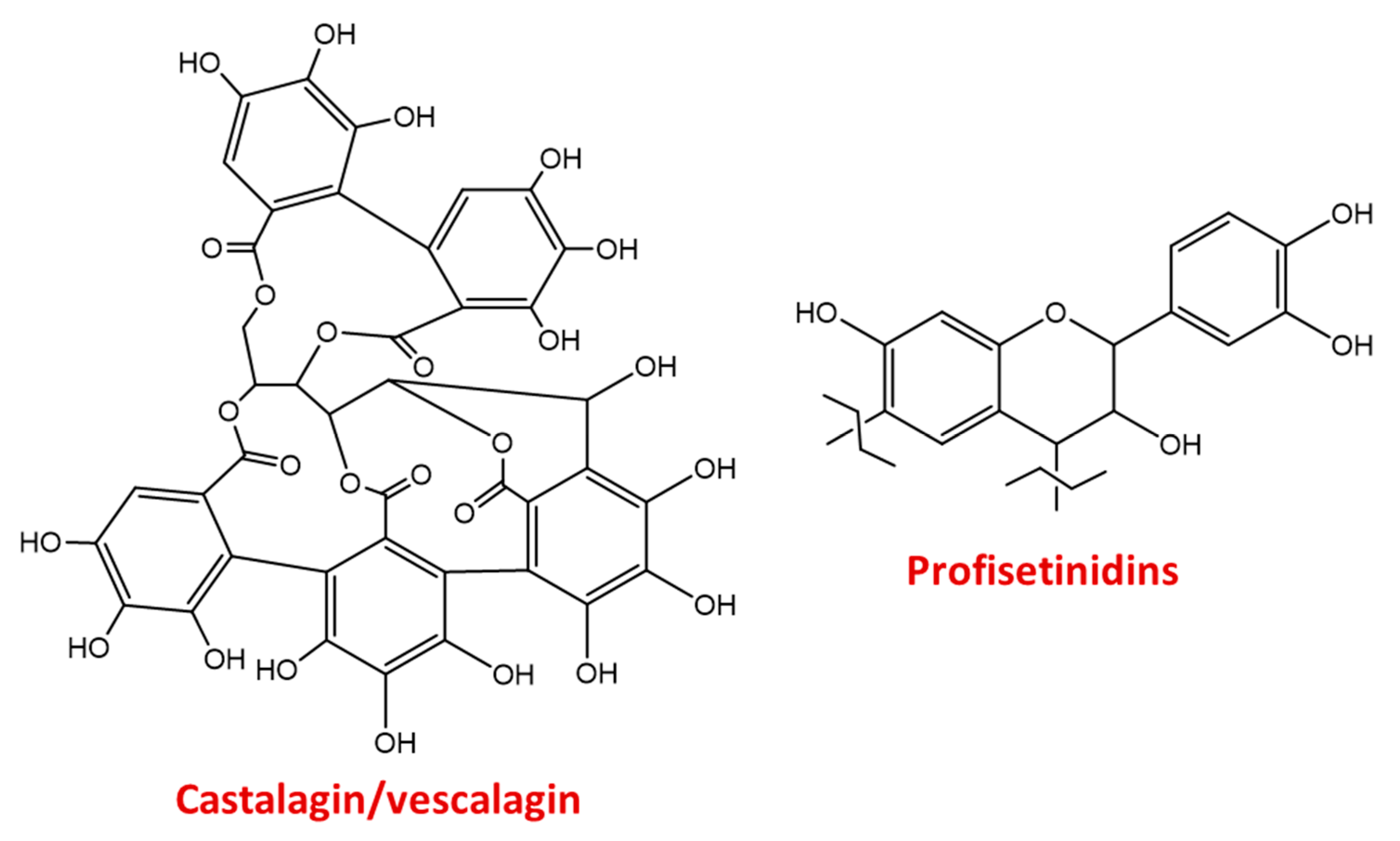
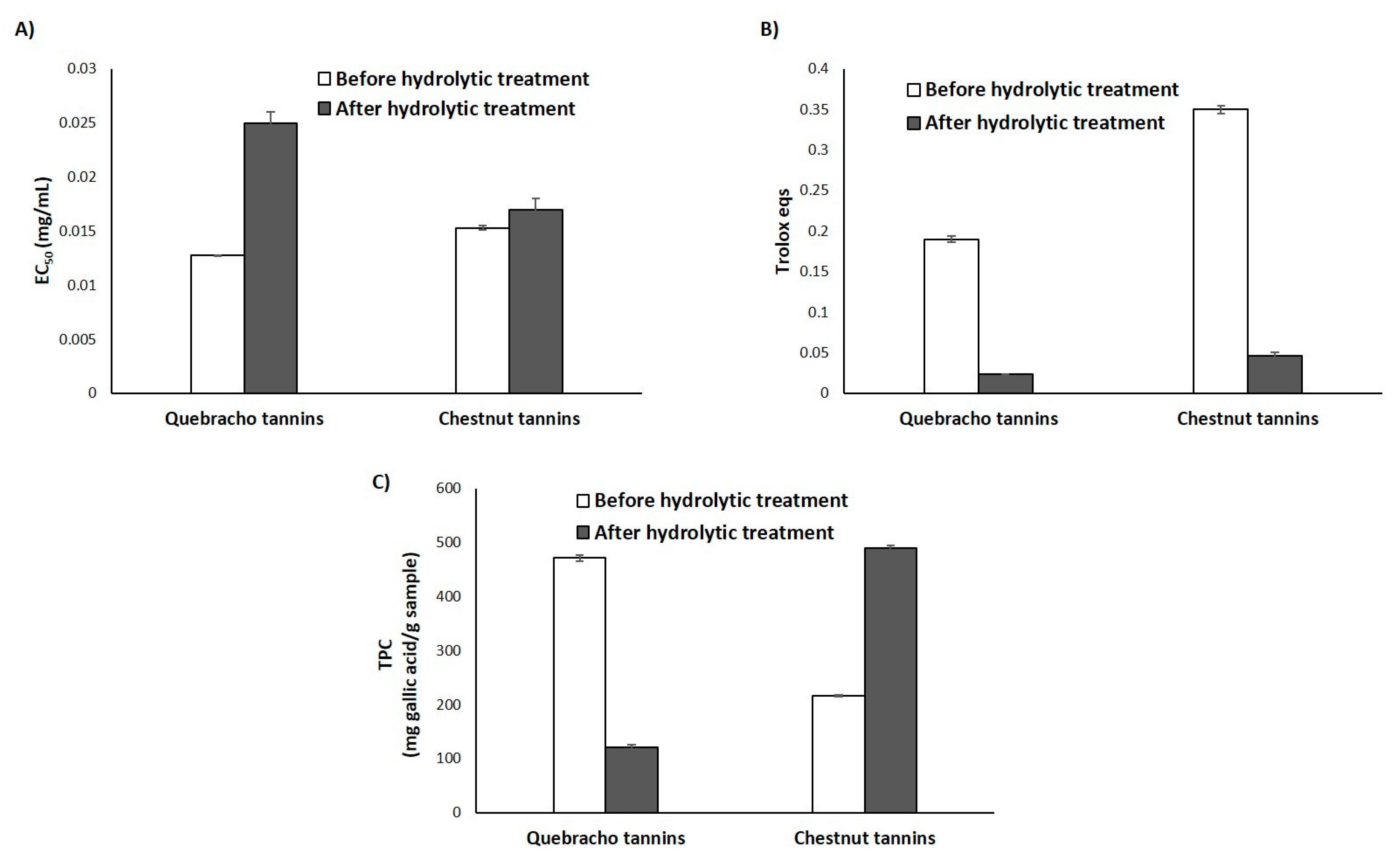
3.3.1. Natural Tannins
3.3.2. Lignin-Mimicking Phenolic Polymers
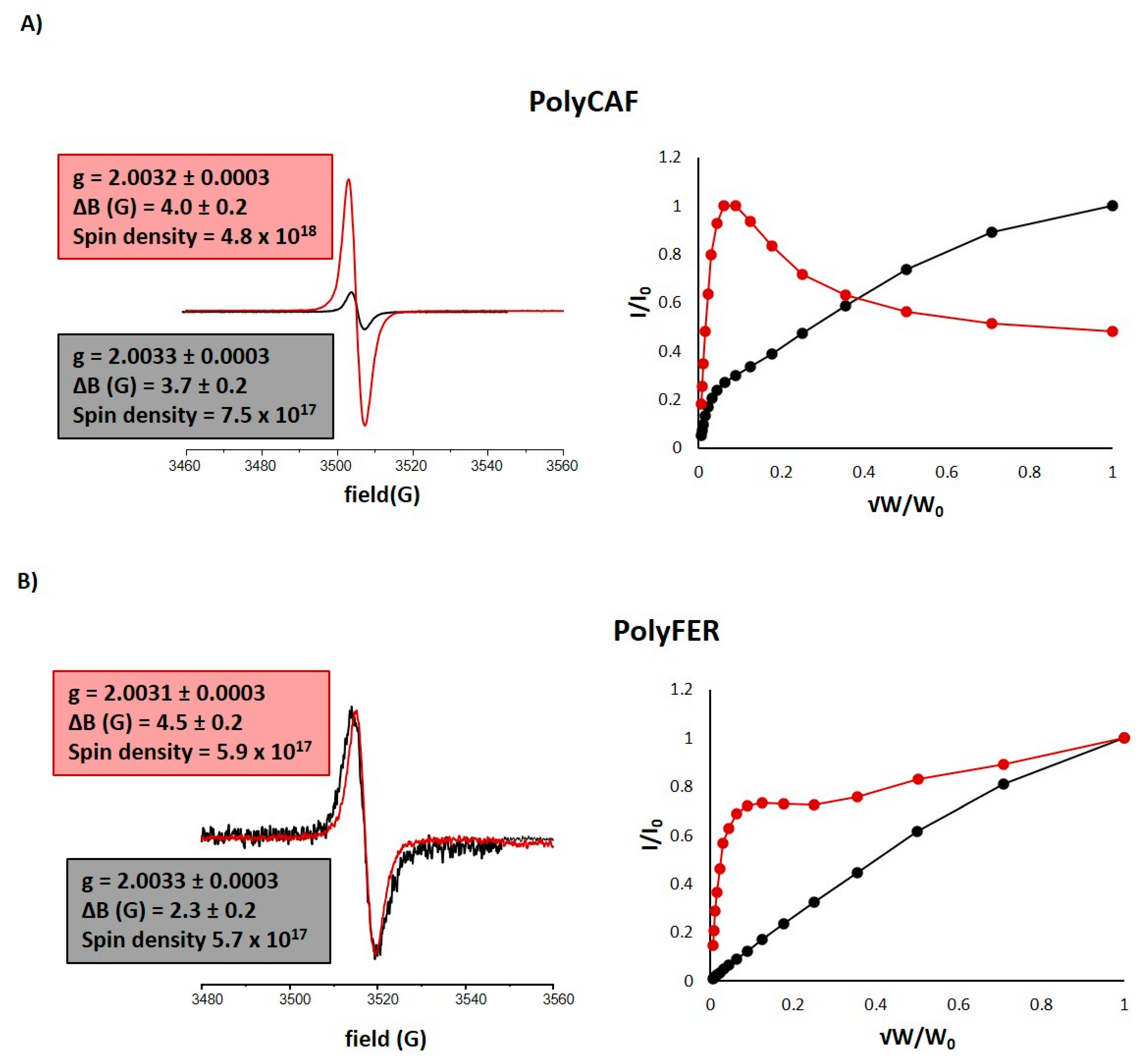
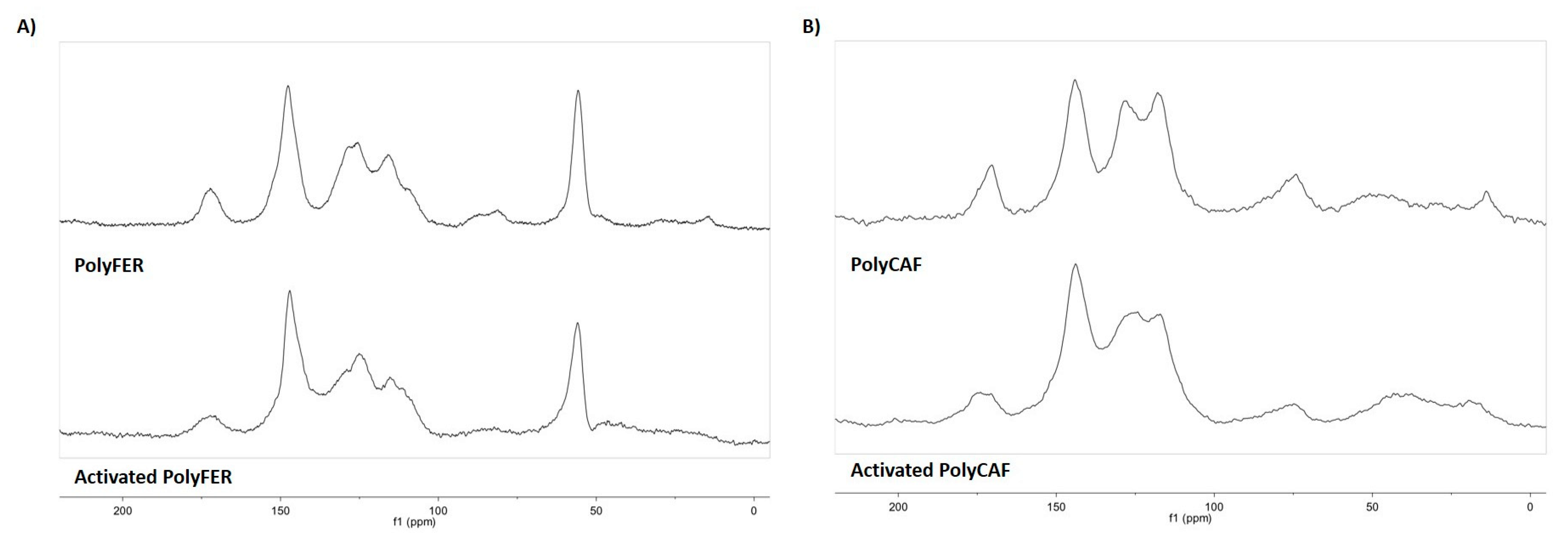
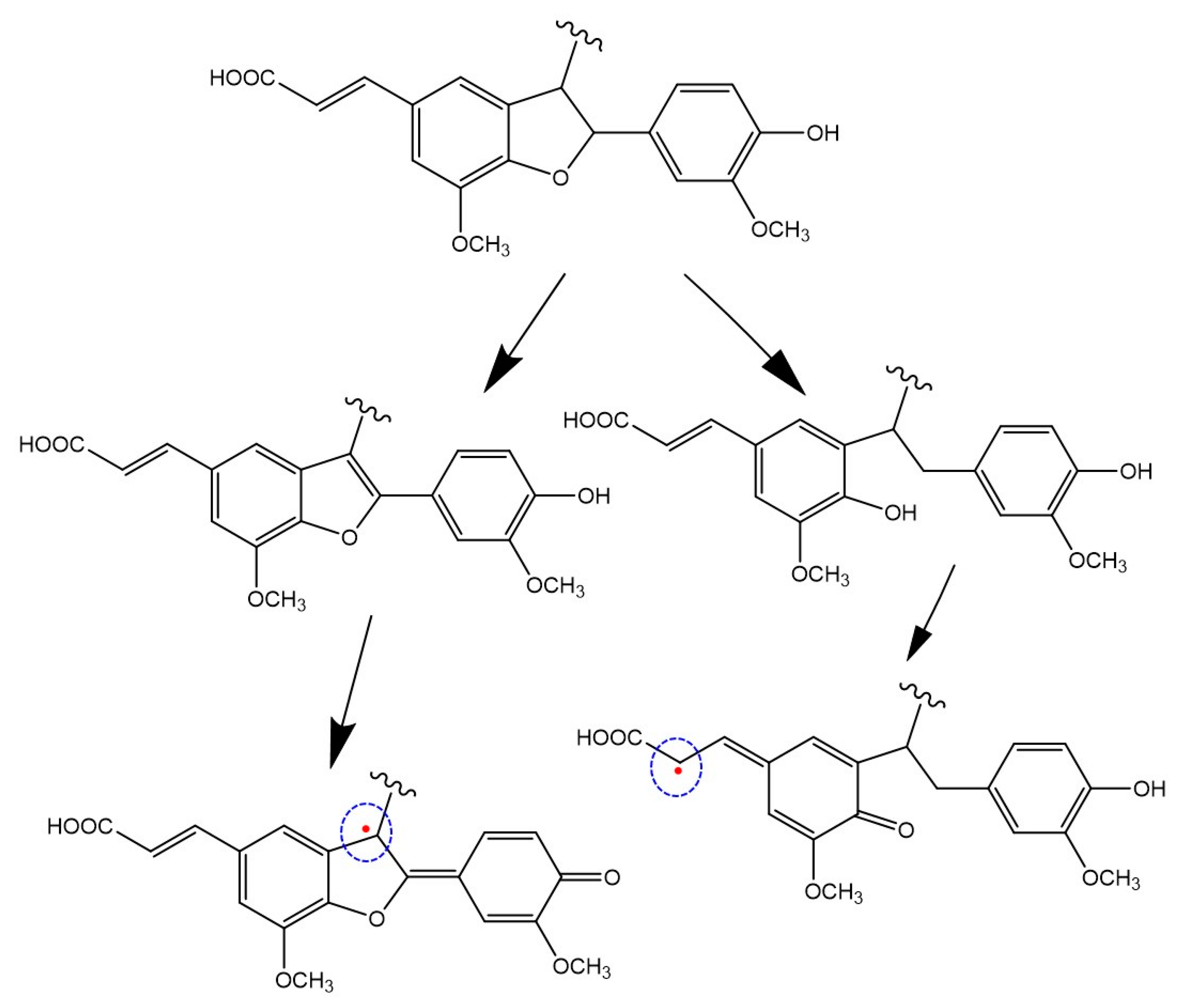
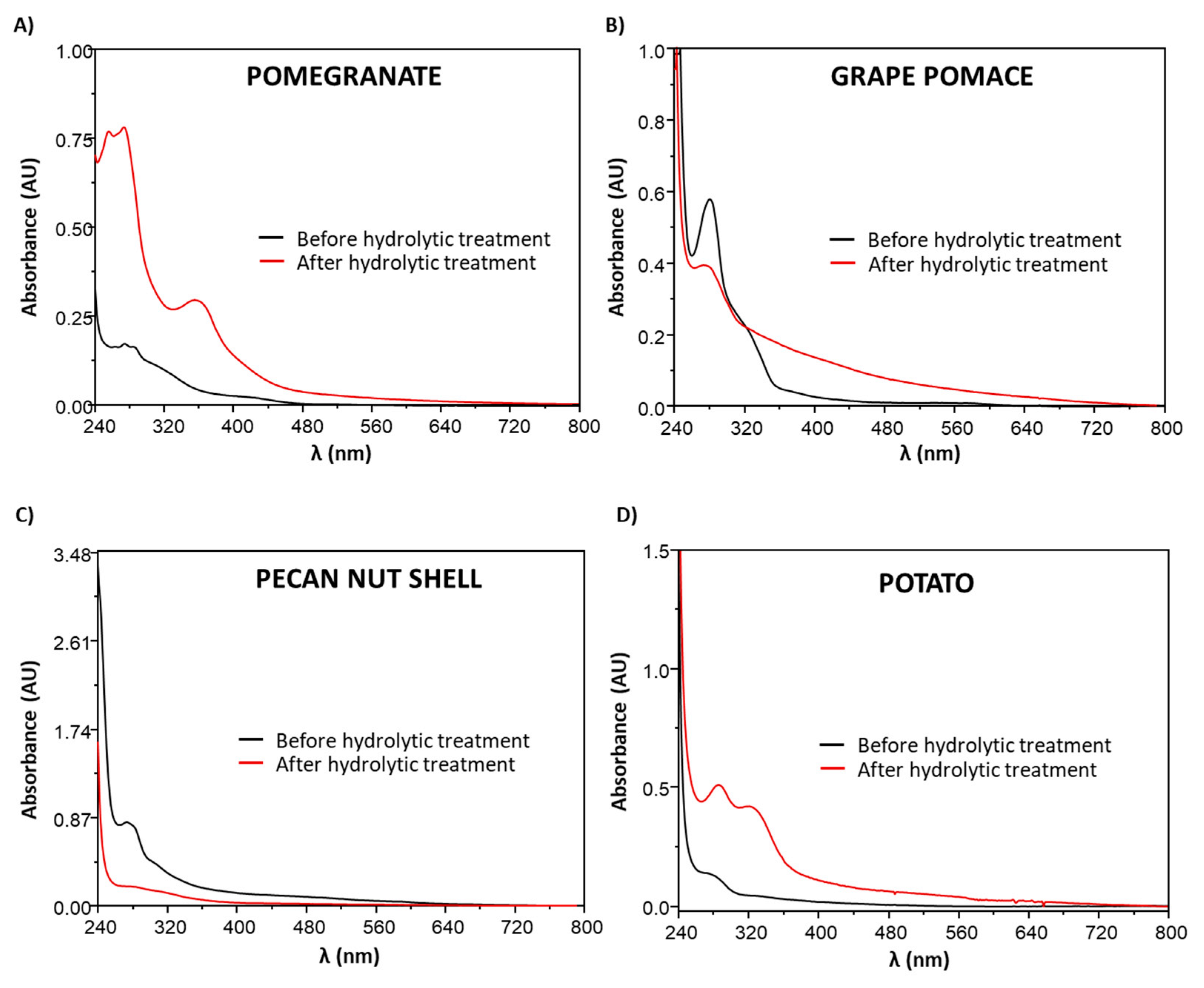
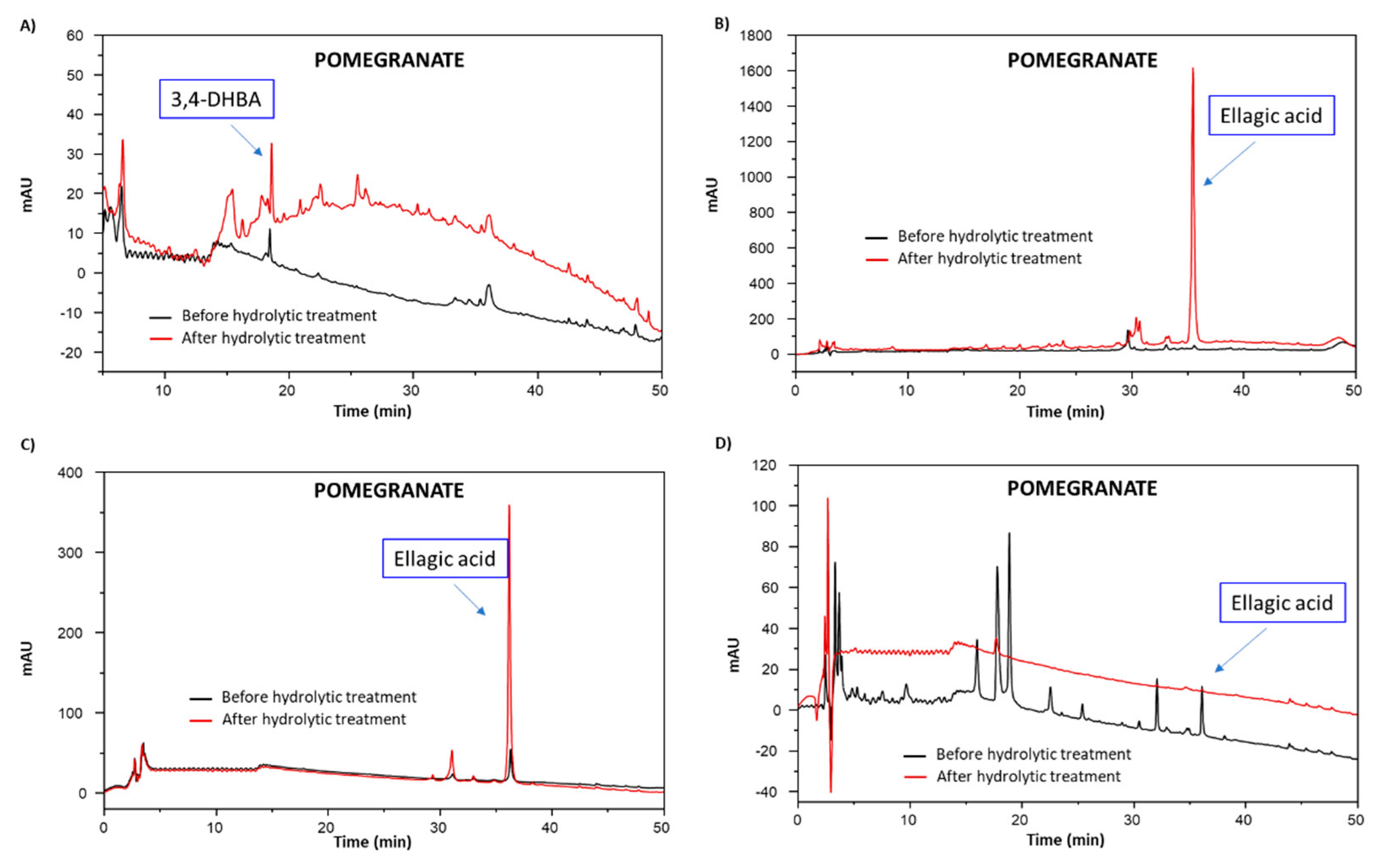
3.4. Chemical Modifications Induced by the Hydrolytic Treatment on the Agri-Food Byproducts
3.4.1. ATR-FTIR Analysis
3.4.2. Analysis of the Extractable Fraction
3.4.3. Chemical Degradation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, H.; Houghton, J.A.; Clark, J.H.; Matharu, A.S. Potential utilization of unavoidable food supply chain wastes-valorization of pea vine wastes. ACS Sustain. Chem. Eng. 2016, 4, 6002–6009. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and sustainable separation of natural products from agro-industrial waste: Challenges, potentialities, and perspectives on emerging approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinos, I.; Goula, A. Polyphenols in agricultural byproducts and food waste. In Polyphenols in Plants; Watson, R.R., Ed.; Elsevier Inc.: London, UK, 2019; pp. 23–44. ISBN 9780128137680. [Google Scholar]
- Djilas, S.; Čanadanović-Brunet, J.; Ćetković, G. By-products of fruits processing as a source of phytochemicals. Chem. Ind. Chem. Eng. Q 2009, 15, 191–202. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De Bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Sukruansuwan, V.; Napathorn, S.C. Use of agro-industrial residue from the canned pineapple industry for polyhydroxybutyrate production by Cupriavidus necator strain A-04. Biotechnol. Biofuels 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Tres, M. V Potential applications of pecan residual biomasses: A review. Biointerface Res. Appl. Chem. 2020, 10, 5524–5531. [Google Scholar]
- Sepelev, I.; Galoburda, R. Industrial potato peel waste application in food production: A review. Res. Rural Dev. 2015, 1, 130–136. [Google Scholar]
- Løvdal, T.; Van Droogenbroeck, B.; Eroglu, E.C.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of tomato surplus and waste fractions: A case study using Norway, Belgium, Poland, and Turkey as examples. Foods 2019, 8, 229. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiovanni, F.; Mukhopadhya, A.; Lacetera, N.; Ryan, M.T.; Romani, A.; Bernini, R.; Sweeney, T. Anti-inflammatory effects of pomegranate peel extracts on in vitro human intestinal caco-2 cells and ex vivo porcine colonic tissue explants. Nutrients 2019, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, F.; Møller, S.G.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Vilaplana-Pérez, C.; Auñón, D.; Garcí-a-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 1–11. [Google Scholar]
- Austermann, K.; Baecker, N.; Stehle, P.; Heer, M. Putative effects of nutritive polyphenols on bone metabolism in vivo-evidence from human studies. Nutrients 2019, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Moulaoui, K.; Caddeo, C.; Manca, M.L.; Castangia, I.; Valenti, D.; Escribano, E.; Atmani, D.; Fadda, A.M.; Manconi, M. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: In vitro and in vivo wound healing potential. Eur. J. Med. Chem. 2015, 89, 179–188. [Google Scholar] [CrossRef] [PubMed]
- De la Ossa, J.G.; Felice, F.; Azimi, B.; Salsano, J.E.; Digiacomo, M.; Macchia, M.; Danti, S.; Di Stefano, R. Waste autochthonous tuscan olive leaves (Olea europaea var. olivastra seggianese) as antioxidant source for biomedicine. Int. J. Mol. Sci. 2019, 20, 5918. [Google Scholar] [CrossRef]
- Rahim, M.A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 2019, 58, 1904–1927. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Pilotta, G.; Panzella, L.; Cipolla, L.; Napolitano, A. Gelatin-based hydrogels for the controlled release of 5,6-dihydroxyindole-2-carboxylic acid, a melanin-related metabolite with potent antioxidant activity. Antioxidants 2020, 9, 295. [Google Scholar] [CrossRef]
- Liberti, D.; Alfieri, M.L.; Monti, D.M.; Panzella, L.; Napolitano, A. A melanin-related phenolic polymer with potent photoprotective and antioxidant activities for dermo-cosmetic applications. Antioxidants 2020, 9, 270. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Milinˇ, D.D.; Levi, S.M.; Kosti, A.Ž. Application of polyphenol-loaded nanoparticles in food industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Agustin-salazar, S.; Berg, A.; Setaro, B.; Micillo, R.; Pizzo, E.; Weber, F.; Gamez-Meza, N.; Cerruti, P.; Panzella, L.; et al. Pecan (Carya illinoinensis (Wagenh.) K. Koch) nut shell as an accessible polyphenol source for active packaging and food colorant stabilization. ACS Sustain. Chem. Eng. 2020, 8, 6700–6712. [Google Scholar] [CrossRef]
- Socaci, S.A.; Rugină, D.O.; Diaconeasa, Z.M.; Pop, O.L.; Fărcaș, A.C.; Păucean, A.; Tofană, M.; Pintea, A. Antioxidant compounds recovered from food wastes. In Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; InTech: London, UK, 2017. [Google Scholar]
- Chacar, S.; Tarighi, M.; Fares, N.; Faivre, J.F.; Louka, N.; Maroun, R.G. Identification of phenolic compounds-rich grape pomace extracts urine metabolites and correlation with gut microbiota modulation. Antioxidants 2018, 7, 5–10. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Serrano, A.; Alonso-Fariñas, B.; Fernández-Bolaños, J.; Borja, R.; Rodríguez-Gutiérrez, G. Valuable compound extraction, anaerobic digestion, and composting: A leading biorefinery approach for agricultural wastes. J. Agric. Food Chem. 2018, 66, 8451–8468. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018, 111, 148–152. [Google Scholar] [CrossRef]
- Makris, D.P.; Şahin, S. Polyphenolic antioxidants from agri-food waste biomass. Antioxidants 2019, 8, 624. [Google Scholar] [CrossRef]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; Da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef]
- Barba, F.J.; Gavahian, M.; Es, I.; Zhu, Z.; Chemat, F.; Lorenzo, J.M.; Mousavi Khaneghah, A. Solar radiation as a prospective energy source for green and economic processes in the food industry: From waste biomass valorization to dehydration, cooking, and baking. J. Clean. Prod. 2019, 220, 1121–1130. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.N.; Volobuff, C.R.F.; Santiago, M.; Cardoso, C.A.L.; Vieira, M.D.C.; Pereira, Z.V. Evaluation of antioxidant activity, total flavonoids, tannins and phenolic compounds in Psychotria leaf extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. BioResources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Lignins and their derivatives with beneficial effects on human health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Toscanesi, M.; Trifuoggi, M.; Giovando, S.; Napolitano, A. Exhausted woods from tannin extraction as an unexplored waste biomass: Evaluation of the antioxidant and pollutant adsorption properties and activating e ects of hydrolytic treatments. Antioxidants 2019, 8, 84. [Google Scholar] [CrossRef]
- Olejar, K.J.; Ricci, A.; Swift, S.; Zujovic, Z.; Gordon, K.C.; Fedrizzi, B.; Versari, A.; Kilmartin, P.A. Characterization of an antioxidant and antimicrobial extract from cool climate, white grape marc. Antioxidants 2019, 8, 1–13. [Google Scholar] [CrossRef]
- Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J. Lignins from agroindustrial by-products as natural ingredients for cosmetics: Chemical structure and in vitro sunscreen and cytotoxic activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hansen, B.E.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-derived biomaterials for drug release and tissue engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/poly (butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; Ferreira, D. de F.; Magalhães, W.L.E.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Zadeh, E.M.; O’Keefe, S.F.; Kim, Y.T. Utilization of lignin in biopolymeric packaging films. ACS Omega 2018, 3, 7388–7398. [Google Scholar] [CrossRef] [PubMed]
- Missio, A.L.; Mattos, B.D.; Otoni, C.G.; Gentil, M.; Coldebella, R.; Khakalo, A.; Gatto, D.A.; Rojas, O.J. Cogrinding wood fibers and tannins: Surfactant effects on the interactions and properties of functional films for sustainable packaging materials. Biomacromolecules 2020, 21, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural tannin wood extracts as a potential food ingredient in the food industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Rukmanikrishnan, B.; Ramalingam, S.; Rajasekharan, S.K.; Lee, J.; Lee, J. Binary and ternary sustainable composites of gellan gum, hydroxyethyl cellulose and lignin for food packaging applications: Biocompatibility, antioxidant activity, UV and water barrier properties. Int. J. Biol. Macromol. 2020, 153, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Olejar, K.J.; Ray, S.; Ricci, A.; Kilmartin, P.A. Superior antioxidant polymer films created through the incorporation of grape tannins in ethyl cellulose. Cellulose 2014, 21, 4545–4556. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Gamez-Meza, N.; Medina-Juárez, L.Á.; Malinconico, M.; Cerruti, P. Stabilization of polylactic acid and polyethylene with nutshell extract: Efficiency assessment and economic evaluation. ACS Sustain. Chem. Eng. 2017, 5, 4607–4618. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, J.; Wang, H.; Song, T.; Hu, W.; Li, S. Preparation and characterization of antioxidative and UV-protective larch bark tannin/PVA composite membranes. Molecules 2018, 23, 2073. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Regina Da Silva Farias, F.; Arunatat, S.; Griffin, D.; Gerometta, M.; Rocca-Smith, J.R.; Weber, G.; Sok, N.; Karbowiak, T. Functionalization of chitosan with lignin to produce active materials by waste valorization. Green Chem. 2019, 21, 4633–4641. [Google Scholar] [CrossRef]
- Li, P.; Sirviö, J.A.; Haapala, A.; Khakalo, A.; Liimatainen, H. Anti-oxidative and UV-absorbing biohybrid film of cellulose nanofibrils and tannin extract. Food Hydrocoll. 2019, 92, 208–217. [Google Scholar] [CrossRef]
- Tedeschi, G.; Guzman-Puyol, S.; Ceseracciu, L.; Paul, U.C.; Picone, P.; Di Carlo, M.; Athanassiou, A.; Heredia-Guerrero, J.A. Multifunctional bioplastics inspired by wood composition: Effect of hydrolyzed lignin addition to xylan-cellulose matrices. Biomacromolecules 2020, 21, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Vibha, K.; Negi, S. Enzymatic plasticising of lignin and styrene with adipic acid to synthesize a biopolymer with high antioxidant and thermostability. Polym. Degrad. Stab. 2020, 174, 109081. [Google Scholar] [CrossRef]
- Panzella, L.; Eidenberger, T.; Napolitano, A.; d’Ischia, M. Black sesame pigment: DPPH assay-guided purification, antioxidant/antinitrosating properties, and identification of a degradative structural marker. J. Agric. Food Chem. 2012, 60, 8895–8901. [Google Scholar] [CrossRef]
- Manini, P.; Panzella, L.; Eidenberger, T.; Giarra, A.; Cerruti, P.; Trifuoggi, M.; Napolitano, A. Efficient binding of heavy metals by black sesame pigment: Toward innovative dietary strategies to prevent bioaccumulation. J. Agric. Food Chem. 2016, 64, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Cerruti, P.; Ambrogi, V.; Agustin-Salazar, S.; D’Errico, G.; Carfagna, C.; Goya, L.; Ramos, S.; Martín, M.A.; Napolitano, A.; et al. A superior all-natural antioxidant biomaterial from spent coffee grounds for polymer stabilization, cell protection, and food lipid preservation. ACS Sustain. Chem. Eng. 2016, 4, 1169–1179. [Google Scholar] [CrossRef]
- Panzella, L.; Pérez-Burillo, S.; Pastoriza, S.; Martín, M.Á.; Cerruti, P.; Goya, L.; Ramos, S.; Rufián-Henares, J.Á.; Napolitano, A.; d’Ischia, M. High antioxidant action and prebiotic activity of hydrolyzed spent coffee grounds (HSCG) in a simulated digestion-fermentation model: Toward the development of a novel food supplement. J. Agric. Food Chem. 2017, 65, 6452–6459. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Sannino, F.; Vitiello, G.; Pirozzi, D.; Minieri, L.; Aronne, A.; Pernice, P.; Pavone, M.; D’Errico, G. Origin and electronic features of reactive oxygen species at hybrid zirconia-acetylacetonate interfaces. ACS Appl. Mater. Interfaces 2015, 7, 21662–21667. [Google Scholar] [CrossRef]
- Mostert, A.B.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Powell, B.J.; Meredith, P. Hydration-controlled X-band EPR spectroscopy: A tool for unravelling the complexities of the solid-state free radical in eumelanin. J. Phys. Chem. B 2013, 117, 4965–4972. [Google Scholar] [CrossRef]
- Ambrogi, V.; Panzella, L.; Persico, P.; Cerruti, P.; Lonz, C.A.; Carfagna, C.; Verotta, L.; Caneva, E.; Napolitano, A.; d’Ischia, M. An antioxidant bioinspired phenolic polymer for efficient stabilization of polyethylene. Biomacromolecules 2014, 15, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Antenucci, S.; Panzella, L.; Farina, H.; Ortenzi, M.A.; Caneva, E.; Martinotti, S.; Ranzato, E.; Burlando, B.; d’Ischia, M.; Napolitano, A.; et al. Powering tyrosol antioxidant capacity and osteogenic activity by biocatalytic polymerization. RSC Adv. 2016, 6, 2993–3002. [Google Scholar] [CrossRef]
- Panzella, L.; D’Errico, G.; Vitiello, G.; Perfetti, M.; Alfieri, M.L.; Napolitano, A.; d’Ischia, M. Disentangling structure-dependent antioxidant mechanisms in phenolic polymers by multiparametric EPR analysis. Chem. Commun. 2018, 54, 9426–9429. [Google Scholar] [CrossRef] [PubMed]
- Goupy, P.; Dufour, C.; Loonis, M.; Dangles, O. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem. 2003, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The “QUENCHER” approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Seifzadeh, N.; Ali Sahari, M.; Barzegar, M.; Ahmadi Gavlighi, H.; Calani, L.; Del Rio, D.; Galaverna, G. Evaluation of polyphenolic compounds in membrane concentrated pistachio hull extract. Food Chem. 2019, 277, 398–406. [Google Scholar] [CrossRef]
- Panzella, L.; Eidenberger, T.; Napolitano, A. Anti-amyloid aggregation activity of black sesame pigment: Toward a novel Alzheimer’s disease preventive agent. Molecules 2018, 23, 676. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Espín, J.C.; Kroon, P.A.; Alasalvar, C.; Heinonen, M.; Voorspoels, S.; Tomas-Barberan, F. A validated method for the characterization and quantification of extractable and non-extractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef]
- Moccia, F.; Flores-Gallegos, A.C.; Chávez-González, M.L.; Sepúlveda, L.; Marzorati, S.; Verotta, L.; Panzella, L.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Napolitano, A. Ellagic acid recovery by solid state fermentation of pomegranate wastes by Aspergillus niger and Saccharomyces cerevisiae: A comparison. Molecules 2019, 24, 3689. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, J.; Boschet, J.; Kammerer, D.R.; Carle, R. Enrichment and fractionation of major apple flavonoids, phenolic acids and dihydrochalcones using anion exchange resins. LWT Food Sci. Technol. 2011, 44, 1079–1087. [Google Scholar] [CrossRef]
- Singh, A.; Nair, G.R.; Liplap, P.; Gariepy, Y.; Orsat, V.; Raghavan, V. Effect of dielectric properties of a solvent-water mixture used in microwave-assisted extraction of antioxidants from potato peels. Antioxidants 2014, 3, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Schieber, A. Reactions of quinones—Mechanisms, structures, and prospects for food research. J. Agric. Food Chem. 2018, 66, 13051–13055. [Google Scholar] [CrossRef]
- Reid, D.G.; Bonnet, S.L.; Kemp, G.; Van Der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 4: Solid state 13C NMR as a tool for in situ analysis of proanthocyanidin tannins, in heartwood and bark of quebracho and acacia, and related species. Phytochemistry 2013, 94, 243–248. [Google Scholar] [CrossRef]
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin analysis of chestnut bark samples (Castanea sativa Mill.) by HPLC-DAD-MS. Food Chem. 2014, 157, 290–295. [Google Scholar] [CrossRef]
- Funaoka, M.; Shibata, M.; Abe, I. Structure and depolymerization of acid-condensed lignin. Holzforschung 1990, 44, 357–366. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; Kim, S.; Lawrence, K.; Paton, R.S.; Chmely, S.C.; Nimlos, M.; Foust, T.D.; Beckham, G.T. A mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkages: Implications for lignin depolymerization in acidic environments. ACS Sustain. Chem. Eng. 2014, 2, 472–485. [Google Scholar] [CrossRef]
- Liu, Q.; Matsushita, Y.; Aoki, D.; Yagami, S.; Fukushima, K. Effects of hydrothermal reaction of sulfuric acid lignin from Cryptomeria japonica for industrial utilization. BioResources 2018, 13, 7805–7825. [Google Scholar] [CrossRef]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; d’Ischia, M. Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties. Angew. Chem. 2013, 52, 12684–12687. [Google Scholar] [CrossRef] [PubMed]
- Moussouni, S.; Saru, M.L.; Ioannou, E.; Mansour, M.; Detsi, A.; Roussis, V.; Kefalas, P. Crude peroxidase from onion solid waste as a tool for organic synthesis. Part II: Oxidative dimerization-cyclization of methyl p-coumarate, methyl caffeate and methyl ferulate. Tetrahedron Lett. 2011, 52, 1165–1168. [Google Scholar] [CrossRef]
- Capriotti, K.; Capriotti, J.A. Dimethyl sulfoxide: History, chemistry, and clinical utility in dermatology. J. Clin. Aesthet. Dermatol. 2012, 5, 24–26. [Google Scholar] [PubMed]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-assisted green solvent extraction of high-added value compounds from microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Cronje, P.J.R.; Landahl, S.; Ortiz, J.O.; Terry, L.A. Rapid methods for extracting and quantifying phenolic compounds in citrus rinds. Food Sci. Nutr. 2016, 4, 4–10. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Sun, R.C.; Fowler, P.; Baird, M.S. Inhomogeneities in the chemical structure of sugarcane bagasse lignin. J. Agric. Food Chem. 2003, 51, 6719–6725. [Google Scholar] [CrossRef]
- Oliveira, L.; Evtuguin, D.V.; Cordeiro, N.; Silvestre, A.J.D.; Silva, A.M.S.; Torres, I.C. Structural characterization of lignin from leaf sheaths of “dwarf cavendish” banana plant. J. Agric. Food Chem. 2006, 54, 2598–2605. [Google Scholar] [CrossRef]
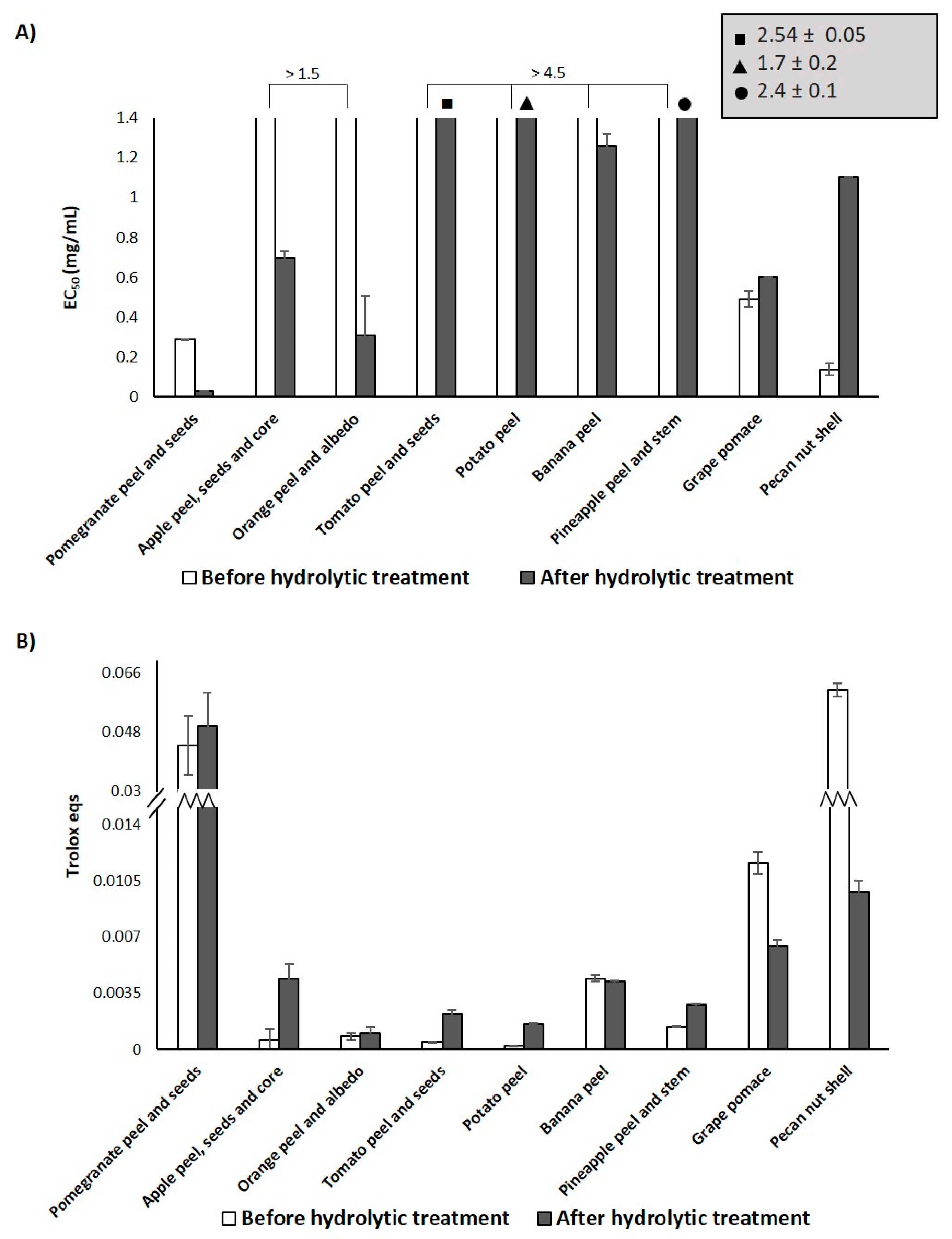
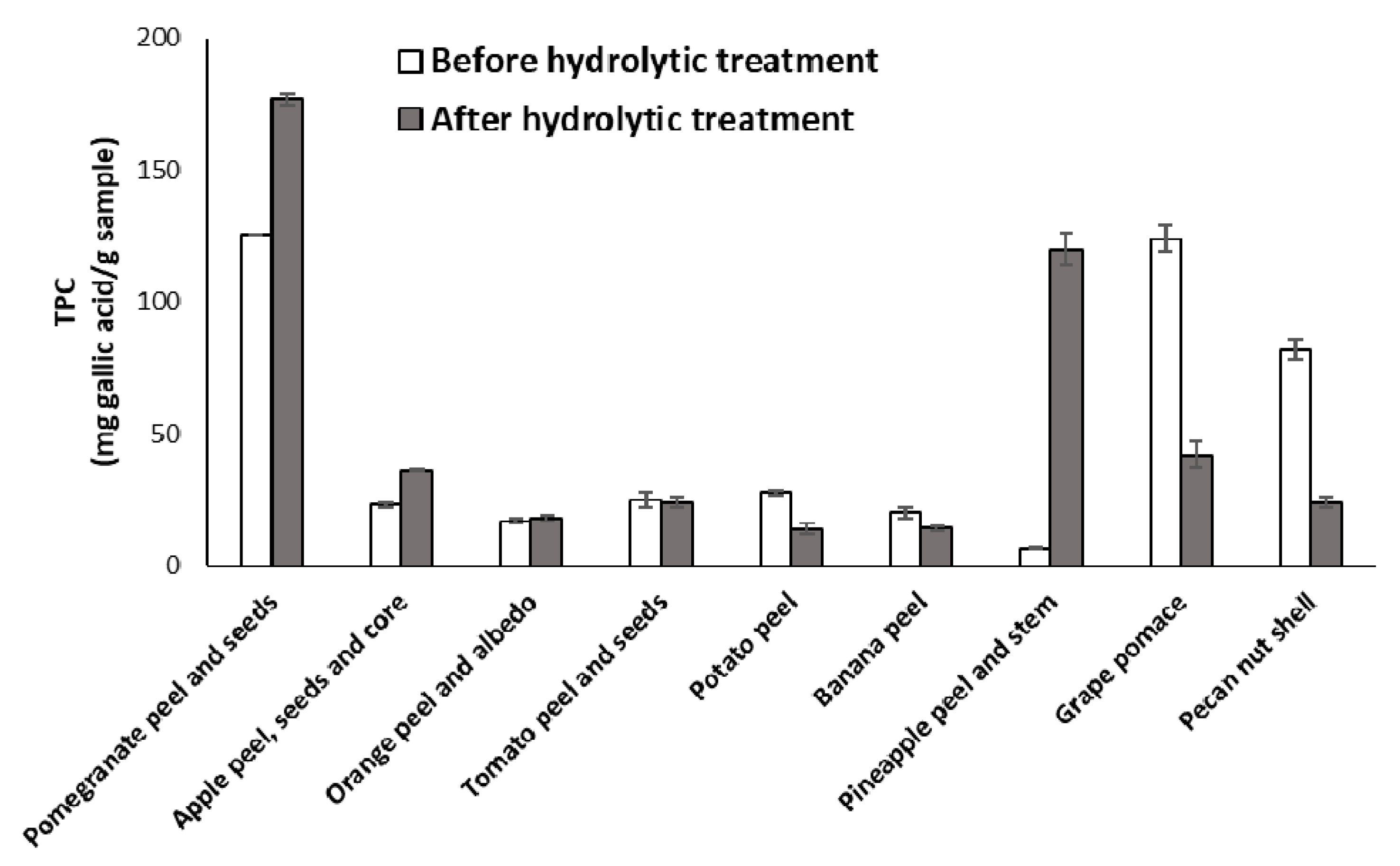

| Sample | EC50 (mg/mL) 2 (DPPH Assay) | Trolox Eqs (FRAP Assay) | TPC (mg of Gallic Acid/g of Sample) (Folin-Ciocalteu Assay) |
|---|---|---|---|
| Pomegranate peel and seeds | 0.29 ± 0.04 | 0.044 ± 0.009 | 125.6 ± 0. 9 |
| Apple peel, seeds and core | >1.5 | 0.0006 ± 0.0007 | 23 ± 1 |
| Orange peel and albedo | >1.5 | 0.0008 ± 0.0002 | 17 ± 1 |
| Tomato peel and seeds | > 1.5 | 0.00046 ± 0.00001 | 25 ± 3 |
| Potato peel | > 1.5 | 0.00023 ± 0.00001 | 27.7 ± 0.8 |
| Banana peel | > 1.5 | 0.0044 ± 0.0002 | 20 ± 2 |
| Pineapple peel and stem | > 1.5 | 0.00141 ± 0.00004 | 6.4 ± 0.3 |
| Grape pomace | 0.49 ± 0.05 | 0.0116 ± 0.0007 | 124 ± 5 |
| Pecan nut shell | 0.137 ± 0.005 | 0.061 ± 0.002 | 82 ± 4 |
| Trolox | 0.011 ± 0.001 | - |
| Sample | Yields (w/w) |
|---|---|
| Pomegranate peel and seeds | 10% |
| Apple peel, seeds and core | 2% |
| Orange peel and albedo | 9% |
| Tomato peel and seeds | 12% |
| Potato peel | 2% |
| Banana peel | 7% |
| Pineapple peel and stem | 20% |
| Grape pomace | 30% |
| Pecan nut shell | 45% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, F.; Agustin-Salazar, S.; Verotta, L.; Caneva, E.; Giovando, S.; D’Errico, G.; Panzella, L.; d’Ischia, M.; Napolitano, A. Antioxidant Properties of Agri-Food Byproducts and Specific Boosting Effects of Hydrolytic Treatments. Antioxidants 2020, 9, 438. https://doi.org/10.3390/antiox9050438
Moccia F, Agustin-Salazar S, Verotta L, Caneva E, Giovando S, D’Errico G, Panzella L, d’Ischia M, Napolitano A. Antioxidant Properties of Agri-Food Byproducts and Specific Boosting Effects of Hydrolytic Treatments. Antioxidants. 2020; 9(5):438. https://doi.org/10.3390/antiox9050438
Chicago/Turabian StyleMoccia, Federica, Sarai Agustin-Salazar, Luisella Verotta, Enrico Caneva, Samuele Giovando, Gerardino D’Errico, Lucia Panzella, Marco d’Ischia, and Alessandra Napolitano. 2020. "Antioxidant Properties of Agri-Food Byproducts and Specific Boosting Effects of Hydrolytic Treatments" Antioxidants 9, no. 5: 438. https://doi.org/10.3390/antiox9050438
APA StyleMoccia, F., Agustin-Salazar, S., Verotta, L., Caneva, E., Giovando, S., D’Errico, G., Panzella, L., d’Ischia, M., & Napolitano, A. (2020). Antioxidant Properties of Agri-Food Byproducts and Specific Boosting Effects of Hydrolytic Treatments. Antioxidants, 9(5), 438. https://doi.org/10.3390/antiox9050438










