Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds
Abstract
1. Introduction
2. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
3. Preparation Methods and Functionalization Approaches
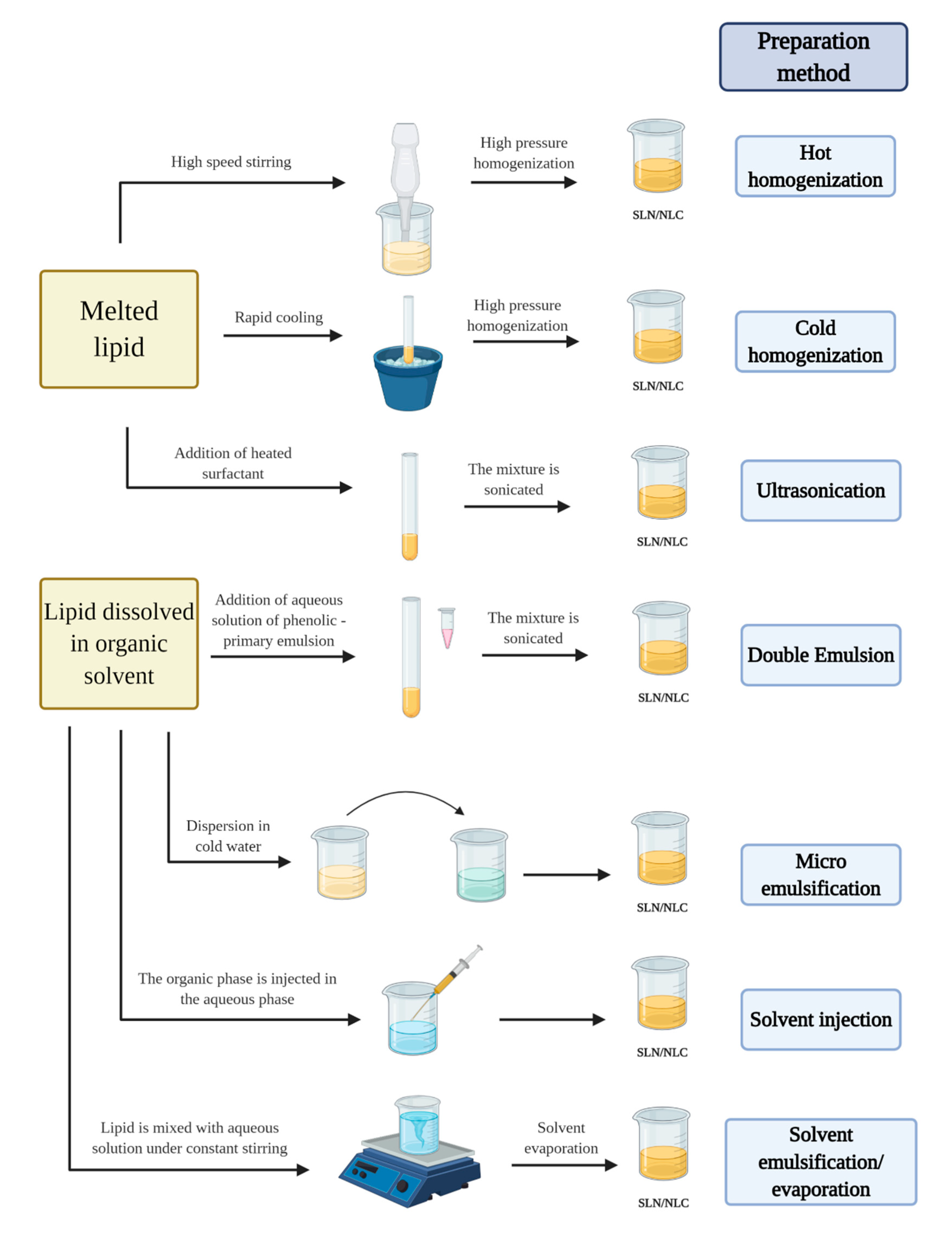
3.1. Preparation Methods
3.1.1. High Pressure Homogenization (HPH)
3.1.2. Solvent Emulsification/Evaporation
3.1.3. Micro-emulsion
3.1.4. Solvent Diffusion and Solvent Injection
3.1.5. Ultrasonication
3.1.6. Double-emulsion
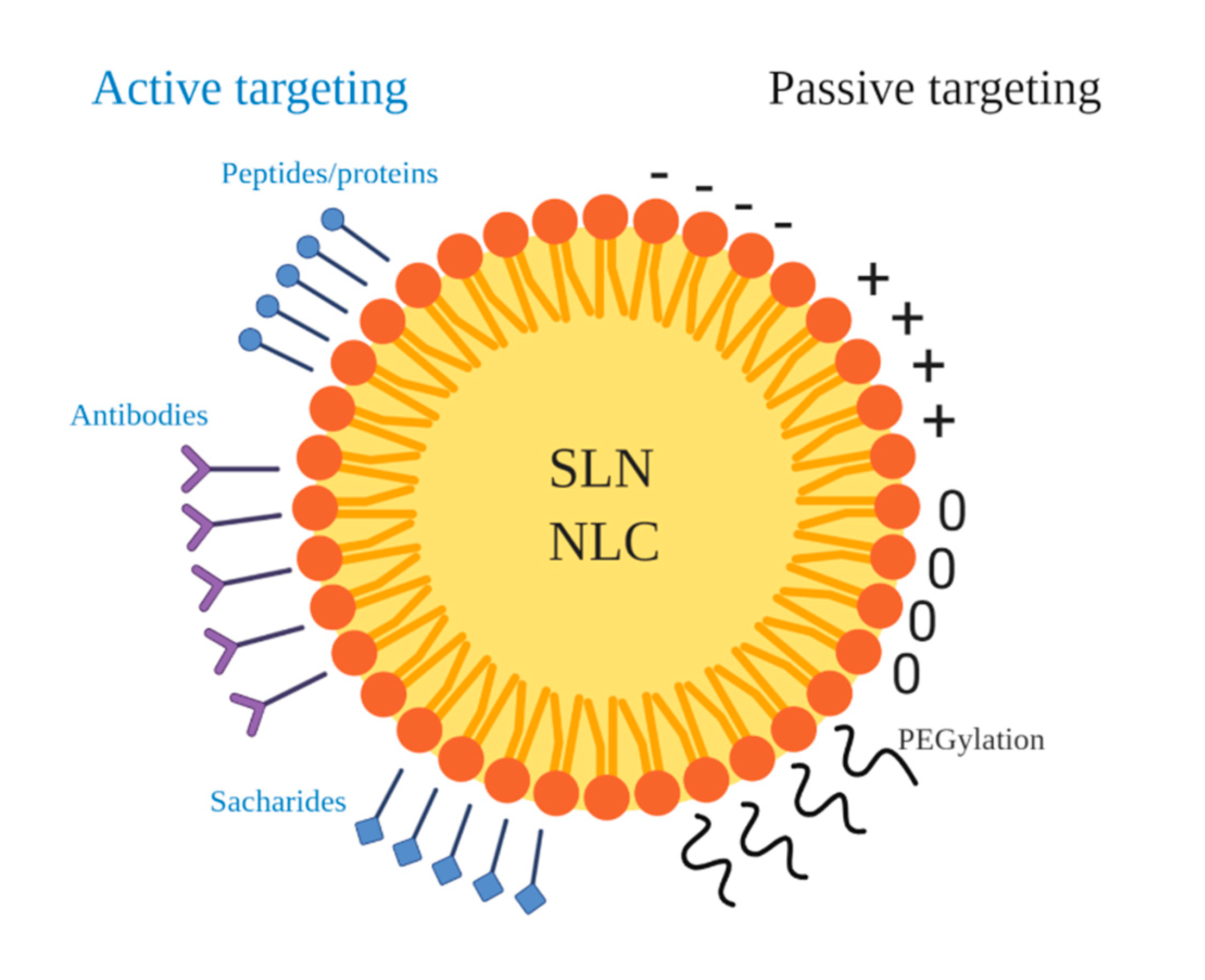
3.2. Functionalization Approaches
4. Applications of SLNs/NLCs Loaded with Phenolic Compounds
4.1. Cancer Applications
4.2. Oral Bioavailability
4.3. Skin Applications
4.4. Neurological Diseases
5. Conclusions and Current Challenges
Funding
Acknowledgments
Conflicts of Interest
References
- Billot, J. Evolution des composes phénoliques au cours de la maturation de la Poire Passe-Crassane. Physiol. Vég. 1983, 21. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.C.; Sumpio, B.E. Polyphenols are medicine: Is it time to prescribe red wine for our patients? Int. J. Angiol. 2009, 18, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Beaulieu, É.; Labbé, D.P.; Bédard, V.; Moghrabi, A.; Barrette, S.; Gingras, D.; Béliveau, R. Delphinidin, a dietary anthocyanidin, inhibits platelet-derived growth factor ligand/receptor (PDGF/PDGFR) signaling. Carcinogenesis 2008, 29, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Shipp, J.; Abdel-Aal, E.-S. Food Applications and physiological effects of anthocyanins as functional food ingredients. Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef]
- Nunes, S.; Madureira, R.; Campos, D.A.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F.; Madureira, A.R.; Pintado, M.M. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: Overcoming pharmacokinetic limitations for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 1863–1873. [Google Scholar] [CrossRef]
- McDougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. Biofactors 2005, 23, 189–195. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef] [PubMed]
- Sloley, B.D.; Urichuk, L.J.; Morley, P.; Durkin, J.; Shan, J.J.; Pang, P.K.T.; Coutts, R.T. Identification of kaempferol as a monoamine oxidase inhibitor and potential neuroprotectant in extracts of Ginkgo Biloba leaves. J. Pharm. Pharmacol. 2000, 52, 451–459. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Cho, W.C.S.; Kręgiel, D.; Cho, W.C.S.; Durazzo, A.; Lucarini, M.; Santini, A.; Santini, A.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef]
- Omenn, G.S. What accounts for the association of vegetables and fruits with lower incidence of cancers and coronary heart disease? Ann. Epidemiol. 1995, 5, 333–335. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Azevedo, J.; Soares, S.; Calhau, C.; De Freitas, V.; Mateus, N. Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. J. Agric. Food Chem. 2010, 58, 3785–3792. [Google Scholar] [CrossRef]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin confers neuroprotection by regulating Nrf2/TLR4/NF-κB signaling in an Aβ mouse model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef]
- Song, T.T.; Hendrich, S.; Murphy, P.A. Estrogenic activity of glycitein, a soy isoflavone. J. Agric. Food Chem. 1999, 47, 1607–1610. [Google Scholar] [CrossRef]
- Bensaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complementary Altern. Med. 2017, 17, 47. [Google Scholar]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Ndiaye, M.; Philippe, C.; Mukhtar, H.; Ahmad, N. The grape antioxidant resveratrol for skin disorders: Promise, prospects, and challenges. Arch. Biochem. Biophys. 2011, 508, 164–170. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Lucks, J.S.; Muller, R.H. Medication Vehicles Made of Solid Lipid Nanoparticles (Solid Lipid Nanospheres). WO Patent No. EP0000605497, 18 September 1991. [Google Scholar]
- Han, S.B.; Kwon, S.S.; Jeong, Y.M.; Yu, E.R.; Park, S.N. Physical characterization and in vitro skin permeation of solid lipid nanoparticles for transdermal delivery of quercetin. Int. J. Cosmet. Sci. 2014, 36, 588–597. [Google Scholar] [CrossRef]
- Reis, S.; Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef]
- Gregoriadis, G. (Ed.) Liposome Techonology; CRC Press Inc.: Boca Raton, FL, USA, 1984. [Google Scholar]
- Gregoriadis, G.; Florence, A.T.; Patel, H.M. (Eds.) Liposomes in Drug Delivery; Harwood Academic Publishers: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Chapter 3-Nanotechnology applications in drug controlled release. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 81–116. [Google Scholar]
- Livney, Y.D. Nanostructured delivery systems in food: Latest developments and potential future directions. Curr. Opin. Food Sci. 2015, 3, 125–135. [Google Scholar] [CrossRef]
- Wretlind, A. Development of fat emulsions. JPEN J. Parenter. Enteral. Nutr. 1981, 5, 230–235. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Fangueiro, J.F.; Andreani, T.; Souto, E.B. Comparison of antiproliferative effect of epigallocatechin gallate when loaded into cationic solid lipid nanoparticles against different cell lines. Pharm. Dev. Technol. 2019, 24, 1243–1249. [Google Scholar] [CrossRef]
- Kondel, R.; Shafiq, N.; Kaur, I.P.; Singh, M.P.; Pandey, A.K.; Ratho, R.K.; Malhotra, S. Effect of acyclovir solid lipid nanoparticles for the treatment of Herpes Simplex Virus (HSV) infection in an animal model of HSV-1 infection. Pharm. Nanotechnol. 2019, 7, 389–403. [Google Scholar] [CrossRef]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Valacchi, G.; Cortesi, R. Lipid nanostructures for antioxidant delivery: A comparative preformulation study. Beilstein J. Nanotechnol. 2019, 10, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, V.; Ribeiro, A.P.B.; Santana, M.H.A. Solid lipid nanoparticles as carriers for lipophilic compounds for applications in foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.R.; Severino, P.; Santini, A.; Silva, A.M.; Shegokar, R.; Souto, S.B.; Souto, E.B. Chapter 1-Solid lipid nanoparticles (SLN): Prediction of toxicity, metabolism, fate and physicochemical properties. In Nanopharmaceuticals; Shegokar, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Zhai, Y.; Zhai, G. Advances in lipid-based colloid systems as drug carrier for topic delivery. J. Control. Release 2014, 193, 90–99. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W.; Lucks, J.; Müller, R. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release 1994, 30, 83–96. [Google Scholar] [CrossRef]
- Eiras, F.; Amaral, M.H.; Silva, R.; Martins, E.; Lobo, J.M.S.; Silva, A. Characterization and biocompatibility evaluation of cutaneous formulations containing lipid nanoparticles. Int. J. Pharm. 2017, 519, 373–380. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Wu, P.-C.; Huang, Y.-B.; Chang, J.-S.; Lin, C.-L.; Tsai, Y.-H.; Fang, J.-Y. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int. J. Pharm. 2012, 423, 461–470. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Huang, Y.-B.; Wu, P.-C.; Fu, Y.-S.; Kao, Y.-R.; Fang, J.-Y.; Tsai, Y.-H. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: Pharmacokinetic and behavioral evaluations. J. Pharm. Sci. 2011, 100, 547–557. [Google Scholar] [CrossRef]
- Li, J.; Guo, X.; Liu, Z.; Okeke, C.I.; Li, N.; Zhao, H.; Aggrey, M.O.; Pan, W.; Wu, T. Preparation and evaluation of charged solid lipid nanoparticles of tetrandrine for ocular drug delivery system: Pharmacokinetics, cytotoxicity and cellular uptake studies. Drug Dev. Ind. Pharm. 2014, 40, 980–987. [Google Scholar] [CrossRef]
- Zhou, W.; Xie, S.; Zhu, L.; Dong, Z.; Wang, Y.; Wang, X. Preparation and evaluation of ofloxacin-loaded palmitic acid solid lipid nanoparticles. Int. J. Nanomed. 2011, 6, 547–555. [Google Scholar] [CrossRef][Green Version]
- Puglia, C.; Frasca, G.; Musumeci, T.; Rizza, L.; Puglisi, G.; Bonina, F.; Chiechio, S. Curcumin loaded NLC induces histone hypoacetylation in the CNS after intraperitoneal administration in mice. Eur. J. Pharm. Biopharm. 2012, 81, 288–293. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Huang, Z.-R.; Zhuo, R.-Z.; Fang, J.-Y. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int. J. Nanomed. 2010, 5, 117–128. [Google Scholar]
- Yang, X.-Y.; Li, Y.-X.; Li, M.; Zhang, L.; Feng, L.-X.; Zhang, N. Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett. 2013, 334, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.P.; Creusat, G.; Frochot, C.; Moussaron, A.; Verhille, M.; Vanderesse, R.; Thomann, J.-S.; Boisseau, P.; Texier, I.; Couffin, A.-C.; et al. Preparation and characterization of mTHPC-loaded solid lipid nanoparticles for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2014, 130, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Teixeira, M.D.C.; Sousa, M.D.C.; Martins-Gomes, C.; Silva, A.M.; Souto, E.B.; Musumeci, T. Clotrimazole-loaded mediterranean essential oils NLC: A synergic treatment of Candida skin infections. Pharmaceutics 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN and NLC): Present state of development and industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Almeida, A.; Müller, R.H. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: Structure, protection and skin effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef]
- Campos, D.A.; Madureira, A.R.; Sarmento, B.; Pintado, M.M.; Gomes, A.M. Technological stability of solid lipid nanoparticles loaded with phenolic compounds: Drying process and stability along storage. J. Food Eng. 2017, 196, 1–10. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Muzzalupo, R.; Pingitore, A.; Cione, E.; Picci, N. Stearyl ferulate-based solid lipid nanoparticles for the encapsulation and stabilization of β-carotene and α-tocopherol. Colloids Surf. B Biointerfaces 2009, 72, 181–187. [Google Scholar] [CrossRef]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Shishikura, Y.; Khokhar, S.; Murray, B.S. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J. Agric. Food Chem. 2006, 54, 1906–1913. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Müller, R.H. Stability determination of solid lipid nanoparticles (SLN) in aqueous dispersion after addition of electrolyte. J. Microencapsul. 1999, 16, 59–71. [Google Scholar] [CrossRef]
- Erni, C.; Suard, C.; Freitas, S.; Dreher, N.; Merkle, H.P.; Walter, E. Evaluation of cationic solid lipid microparticles as synthetic carriers for the targeted delivery of macromolecules to phagocytic antigen-presenting cells. Biomaterials 2003, 23, 4667–4676. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schrader, K.; Schwarz, K. Encapsulation of (─)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2019, 244, 91–100. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef]
- Cassano, R.; Trombino, S.; Muzzalupo, R.; Tavano, L.; Picci, N. A novel dextran hydrogel linking trans-ferulic acid for the stabilization and transdermal delivery of vitamin E. Eur. J. Pharm. Biopharm. 2009, 72, 232–238. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Bloise, E.; Muzzalupo, R.; Tavano, L.; Picci, N. Synthesis and antioxidant activity evaluation of a novel cellulose hydrogel containing trans-ferulic acid. Carbohydr. Polym. 2009, 75, 184–188. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Lander, R.; Manger, W.; Scouloudis, M.; Ku, A.; Davis, C.; Lee, A. Gaulin homogenization: A mechanistic study. Biotechnol. Prog. 2000, 16, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, L.; Nielson, H.M. (Eds.) Delivery Technologies for Biopharmaceuticals: Peptides, Proteins, Nucleic Acids and Vaccines; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Muller, R.; Runge, S.; Ravelli, V.; Mehnert, W.; Thunemann, A.; Souto, E. Oral bioavailability of cyclosporine: Solid lipid nanoparticles (SLN®) versus drug nanocrystals. Int. J. Pharm. 2006, 317, 82–89. [Google Scholar] [CrossRef]
- Almeida, A.J.; Runge, S.; Müller, R.H. Peptide-loaded solid lipid nanoparticles (SLN): Influence of production parameters. Int. J. Pharm. 1997, 149, 255–265. [Google Scholar] [CrossRef]
- Santonocito, D.; Sarpietro, M.G.; Carbone, C.; Panico, A.; Campisi, A.; Siciliano, E.A.; Sposito, G.; Castelli, F.; Puglia, C. Curcumin containing PEGylated solid lipid nanoparticles for systemic administration: A preliminary study. Molecules 2020, 25, 2991. [Google Scholar] [CrossRef]
- Sjöström, B.; Bergenståhl, B. Preparation of submicron drug particles in lecithin-stabilized o/w emulsions I. Model studies of the precipitation of cholesteryl acetate. Int. J. Pharm. 1992, 88, 53–62. [Google Scholar] [CrossRef]
- Picone, P.; Bondì, M.L.; Montana, G.; Bruno, A.; Pitarresi, G.; Giammona, G.; Di Carlo, M. Ferulic acid inhibits oxidative stress and cell death induced by Ab oligomers: Improved delivery by solid lipid nanoparticles. Free Radic. Res. 2009, 43, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.P.; Tiyaboonchai, W.; Patankar, S.; Madhusudhan, B.; Souto, E.B. Curcuminoids-loaded lipid nanoparticles: Novel approach towards malaria treatment. Colloids Surf. B Biointerfaces 2010, 81, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- Trotta, M.; Debernardi, F.; Caputo, O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int. J. Pharm. 2003, 257, 153–160. [Google Scholar] [CrossRef]
- Schubert, M.A.; Müller-Goymann, C.C. Solvent injection as a new approach for manufacturing lipid nanoparticles—Evaluation of the method and process parameters. Eur. J. Pharm. Biopharm. 2003, 55, 125–131. [Google Scholar] [CrossRef]
- Ferreira, M.; Chaves, L.L.; Lima, S.A.C.; Reis, S. Optimization of nanostructured lipid carriers loaded with methotrexate: A tool for inflammatory and cancer therapy. Int. J. Pharm. 2015, 492, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gao, R.; Li, F.; He, H.; Tang, X. The influence of lipid characteristics on the formation, in vitro release, and in vivo absorption of protein-loaded SLN prepared by the double emulsion process. Drug Dev. Ind. Pharm. 2011, 37, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Moral, S.; Teixeira, M.; Fernandes, A.; Borrás-Linares, I.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Polyphenols-enriched Hibiscus sabdariffa extract-loaded nanostructured lipid carriers (NLC): Optimization by multi-response surface methodology. J. Drug Deliv. Sci. Technol. 2019, 49, 660–667. [Google Scholar] [CrossRef]
- Ravanfar, R.; Tamaddon, A.M.; Niakousari, M.; Moein, M.R. Preservation of anthocyanins in solid lipid nanoparticles: Optimization of a microemulsion dilution method using the Placket–Burman and Box–Behnken designs. Food Chem. 2016, 199, 573–580. [Google Scholar] [CrossRef]
- Bunjes, H.; Siekmann, B.; Westesen, K. Chapter 7-Emulsions of supercooled melts—A novel drug delivery system. In Submicron Emulsions in Drug Targeting and Delivery; Benita, S., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1998; pp. 175–218. [Google Scholar]
- Weyhers, H.; Mehnert, W.; Hahn, H.; Müller, R.H. Solid lipid nanoparticles-determination of in vivo toxicity. In Proceedings of the 1st World Meeting on Pharmaceutics. Biopharmaceutics, Pharmaceutical Technology, Budapest, Hungary, 9–11 May 1995. [Google Scholar]
- Jenning, V.; Gysler, A.; Schäfer-Korting, M.; Gohla, S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Correlation between long-term stability of solid lipid nanoparticles (SLN™) and crystallinity of the lipid phase. Eur. J. Pharm. Biopharm. 1999, 47, 125–132. [Google Scholar] [CrossRef]
- Müller, R.H.; Heinemann, S. Fat emulsions for parenteral nutrition. III: Lipofundin MCT/LCT regimens for total parenteral nutrition (TPN) with low electrolyte load. Int. J. Pharm. 1994, 101, 175–189. [Google Scholar] [CrossRef]
- Huang, C.-C.; Wu, P.-C.; Tsai, T.-H.; Fang, Y.-P.; Tsai, Y.-H.; Cheng, T.-C.; Huang, M.-Y.; Chen, F.-M.; Hsieh, Y.-C.; Lin, W.-W.; et al. Development of pH-sensitive cationic PEGylated solid lipid nanoparticles for selective cancer-targeted therapy. J. Biomed. Nanotechnol. 2017, 13, 192–203. [Google Scholar]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid based nanocarriers: An overview. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.Y.; Ming, K.; Chan, W.C. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Gaspar, D.; Almeida, A. Surface-functionalized lipid nanoparticles for site-specific drug delivery. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer: Cham, Switzerland, 2019; pp. 73–98. [Google Scholar]
- Saha, K.; Bajaj, A.; Duncan, B.; Rotello, V.M. Beauty is skin deep: A surface monolayer perspective on nanoparticle interactions with cells and bio-macromolecules. Small 2011, 7, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Gascón, A.R.; del Pozo-Rodríguez, A.; des Rieux, A.; Préat, V. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J. Control. Release 2013, 166, 115–123. [Google Scholar] [CrossRef]
- Mancini, G.; Lopes, R.M.; Clemente, P.; Raposo, S.; Gonçalves, L.M.; Bica, A.; Ribeiro, H.; Almeida, A.J. Lecithin and parabens play a crucial role in tripalmitin-based lipid nanoparticle stabilization throughout moist heat sterilization and freeze-drying: Physical stability of tripalmitin solid lipid nanoparticles. Eur. J. Lipid Sci. Technol. 2015, 117, 1947–1959. [Google Scholar] [CrossRef]
- Lim, S.-J.; Kim, C.-K. Formulation parameters determining the physicochemical characteristics of solid lipid nanoparticles loaded with all-trans retinoic acid. Int. J. Pharm. 2002, 243, 135–146. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Faria, V.; Quintas, J.P.; Almeida, A.J.; Gaspar, V.F.D.P. Targeted delivery of lipid nanoparticles by means of surface chemical modification. Curr. Org. Chem. 2017, 21. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.U.; Nie, S.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Maaben, S.; Weyhers, H.; Mehnert, W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J. Drug Target. 1996, 4, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef]
- Xu, H.; Deng, Y.-H.; Chen, D.; Hong, W.; Lu, Y.; Dong, X. Esterase-catalyzed dePEGylation of pH-sensitive vesicles modified with cleavable PEG-lipid derivatives. J. Control. Release 2008, 130, 238–245. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Arranja, A.; Gouveia, L.F.; Gener, P.; Rafael, D.F.; Pereira, C.; Schwartz, S.; Videira, M.A. Self-assembly PEGylation assists SLN-paclitaxel delivery inducing cancer cell apoptosis upon internalization. Int. J. Pharm. 2016, 501, 180–189. [Google Scholar] [CrossRef]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J.Y. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Chapman, A.P. PEGylated antibodies and antibody fragments for improved therapy: A review. Adv. Drug Deliv. Rev. 2002, 54, 531–545. [Google Scholar] [CrossRef]
- Michaelis, K.; Hoffmann, M.M.; Dreis, S.; Herbert, E.; Alyautdin, R.N.; Kreuter, J.; Langer, K. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J. Pharmacol. Exp. Ther. 2006, 317, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef]
- Jose, J.; Netto, G. Role of solid lipid nanoparticles as photoprotective agents in cosmetics. J. Cosmet. Dermatol. 2019, 18, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Chaturvedi, M.M.; Aggarwal, B.B. Role of curcumin in cancer therapy. Curr. Probl. Cancer 2007, 31, 243–305. [Google Scholar] [CrossRef]
- Farabegoli, F.; Papi, A.; Orlandi, M. (-)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci. Rep. 2011, 31, 99–108. [Google Scholar] [CrossRef]
- Santos, I.S.; Ponte, B.M.; Boonme, P.; Silva, A.M.; Souto, E.B. Nanoencapsulation of polyphenols for protective effect against colon–rectal cancer. Biotechnol. Adv. 2013, 31, 514–523. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Ramakrishna, S. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.J. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008, 269, 243–261. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
- Mori, H.; Kawabata, K.; Yoshimi, N.; Tanaka, T.; Murakami, T.; Okada, T.; Murai, H. Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999, 19, 3775–3778. [Google Scholar]
- Coradini, K.; Lima, F.; Oliveira, C.; Chaves, P.; Athayde, M.; Carvalho, L.; Beck, R.C.R. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur. J. Pharm. Biopharm. 2014, 88, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef]
- Granja, A.; Vieira, A.C.; Chaves, L.L.; Nunes, C.; Neves, A.R.; Pinheiro, M.; Reis, S. Folate-targeted nanostructured lipid carriers for enhanced oral delivery of epigallocatechin-3-gallate. Food Chem. 2017, 237, 803–810. [Google Scholar] [CrossRef]
- Bose, S.; Du, Y.; Takhistov, P.; Michniak, B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int. J. Pharm. 2013, 441, 56–66. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A.; Dorta, D.J.; Dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B Biol. 2006, 84, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Plianbangchang, P.; Tungpradit, W.; Tiyaboonchai, W. Efficacy and safety of curcuminoids loaded solid lipid nanoparticles facial cream as an anti-aging agent. Naresuan Univ. J. Sci. Technol. 2013, 15, 73–81. [Google Scholar]
- Friedrich, R.B.; Kann, B.; Coradini, K.; Offerhaus, H.L.; Beck, R.C.; Windbergs, M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur. J. Pharm. Sci. 2015, 78, 204–213. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Cho, J.-Y.; Kim, D.H.; Yan, J.-J.; Lee, H.-K.; Suh, H.-W.; Song, D.-K. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.; Montana, G.; Craparo, E.; Picone, P.; Capuano, G.; Carlo, M.; Giammona, G. Ferulic acid-loaded lipid nanostructures as drug delivery systems for Alzheimers disease: Preparation, characterization and cytotoxicity studies. Curr. Nanosci. 2009, 5, 26–32. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Li, X.; Jasti, P.D.B.R. Design of Controlled Release Drug Delivery Systems; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 2016, 8, 307. [Google Scholar] [CrossRef]
- Liang, T.; Guan, R.; Shen, H.; Xia, Q.; Liu, M.-Q. Optimization of conditions for cyanidin-3-O-glucoside (C3G) nanoliposome production by response surface methodology and cellular uptake studies in Caco-2 cells. Molecules 2017, 22, 457. [Google Scholar] [CrossRef]
- Desnita, R.; Veronika, M.; Wahdaningsih, S. Topical microemulsion’s formulation of purple sweet potato (Ipomoea batatas L.) ethanol extract as antioxidant by using various concentration of span 80. Int. J. Pharm Tech Res. 2016, 9, 234–239. [Google Scholar]
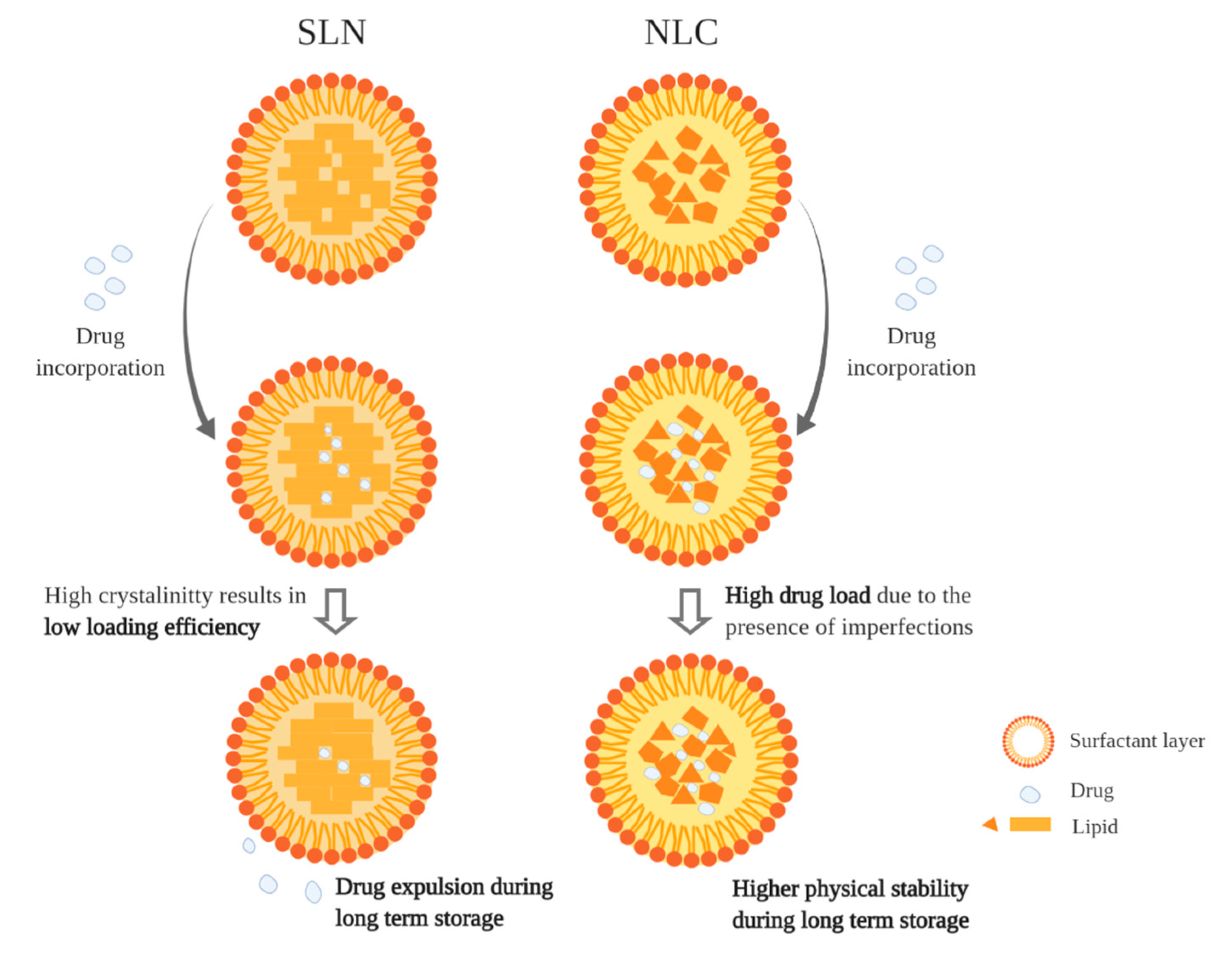
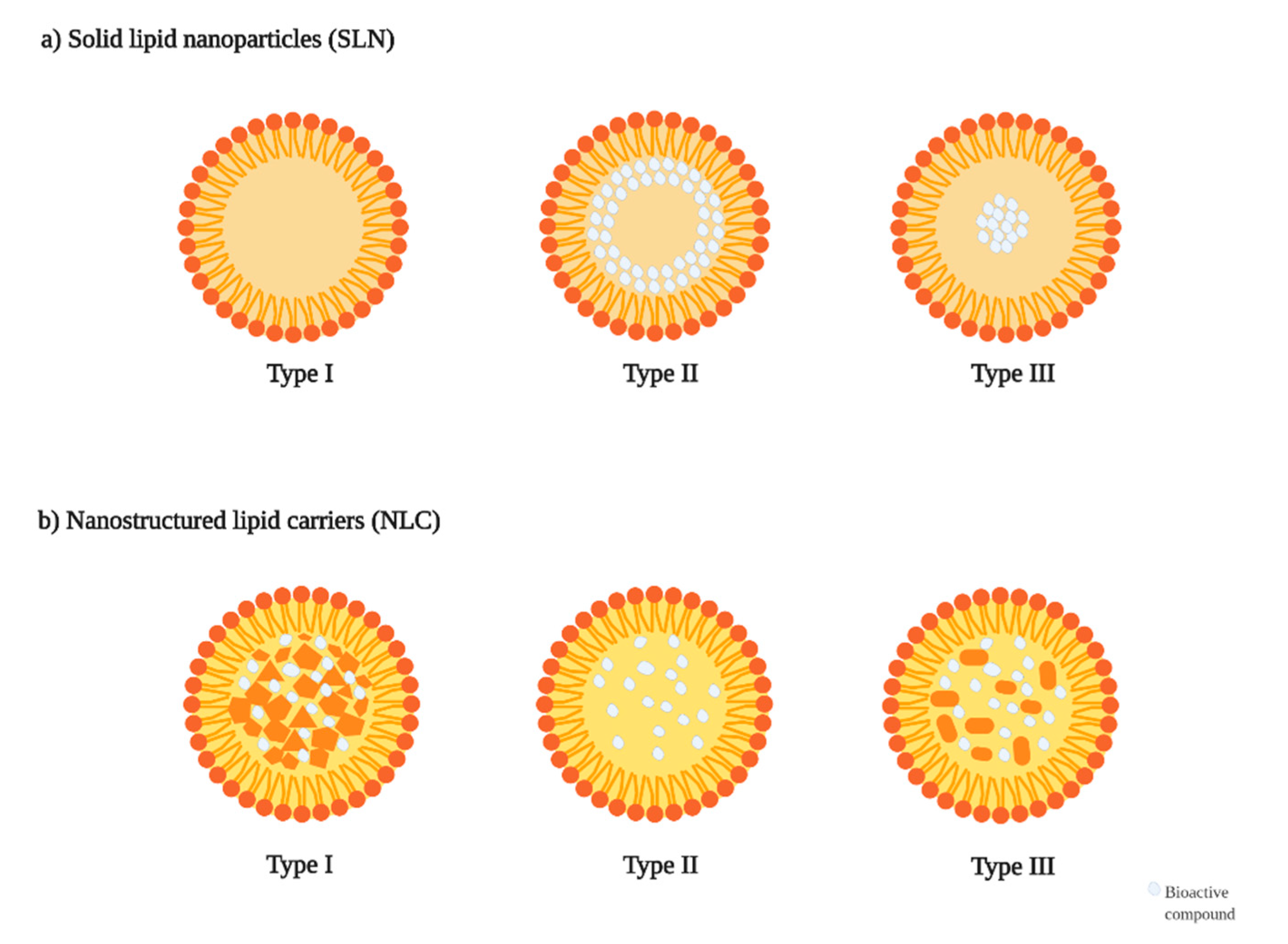
| Main Class | Subclass | Phenolic Compound | Food Sources | Beneficial Effects | Possible Applications |
|---|---|---|---|---|---|
| Flavonoids | Flavan-3-ol | Epigallocatechin gallate | Green tea | Anti-oxidant effects [12] | Protection against chemical carcinogens [13] |
| Flavones | Apigenin | Oranges | Inhibition of rat brain monoamine oxidases (MAOs) [14] | Development of antidepressant and antianxiety agents [15] | |
| Flavonols | Quercetin | Onions | Anti-oxidant effects and anti-inflammatory effects [16] | Neuropathology prevention [17] | |
| Anthocyanins | Delphinidin-3-glucoside | Grapes | Anti-oxidant and anti-carcinogenic effects [18] | Inhibition of breast cancer cell proliferation [19] | |
| Flavanones | Hesperetin | Citrus fruits | Activity against apoptotic neurodegeneration [20] | Development of therapeutic agents for Alzheimer’s disease [20] | |
| Isoflavones | Glycitein | Soybean | Estrogenic effects [21] | Several health effects and disease prevention [21] | |
| Phenolic Acids | Hydroxybenzoic acids | Gallic acid | Green tea | Anti-inflammatory activity [22] | Development of novel and organic anti-inflammatory agents [22] |
| Hydroxycinnamic acids | Caffeic acid | Wheat, rice, and oats | Anti-bacterial activity [23] | Development of alternatives to overcome antibiotic resistance [23] | |
| Stilbenes | Resveratrol | Grapes | Anti-oxidant effects [24] | Skin anti-aging products | |
| Lignins | Lignin | Plants (secondary cell walls) | Development of nanoparticles for drug delivery [25] | Cancer therapy |
| Lipid Nanoparticle | Incorporated Molecule | Lipids | Surfactants | Application |
|---|---|---|---|---|
| SLN | Vitamin E | Precirol ATO 5 | Tween 80 | Skin applications [46] |
| SLN | Quercetin | Glyceryl monostearate (GMS) | Tween 80 and polyethylene glycol (PEG) 400 | Food applications [47] |
| SLN | Baicalen | Tripalmitin, Gelucire 48/9, and Gelucire 62/5 | Poloxamer 188 | Treatment of ischemic stroke [48] |
| SLN | Apomorphine hydrochloride | Tripalmitin, hydrogenated soybean phosphatidylcholine (HSPC), glyceryl monostearate (GMS), and polyethylene glycol monostearate (PMS) | Pluronic F68 L-ascorbic acid | Treatment of Parkinson’s disease [49] |
| SLN | Tetandrine | Compritol 888 ATO | Myrj 52 | Treatment of ocular diseases [50] |
| SLN | Ofloxacin | Palmitic acid | Polyvinyl alcohol (PVA) | Improvement of pharmacological activity [51] |
| NLC | Curcumin | Precirol ATO 5 and Miglyol 812 | Lutrol F68 and Tween 80 | Intraperitoneal administration [52] |
| NLC | Calcipotriol, methotrexate | Precirol ATO 5, squalene mixture, Myverol 18-04K | Pluronic F68 | Treatment of psoriasis [53] |
| NLC | Paclitaxel | Glyceryl monostearate (GMS), soya lecithin, soybean oil | Hexadecyl trimethyl ammonium bromide (CTAB) | Cancer targeting [54] |
| NLC | mTHPC (commercial formulation Foscan) | Soybean oil and Suppocire NB | Lipoid S75, Myrj S40 | Photodynamic therapy [55] |
| NLC | Mediterranean essential oils | Labrafil, Softisan 100 | Kolliphor RH40, Tween 80 | Treatment of Candida skin infections [56] |
| Phenolic Compound | Preparation Method | Size (nm) | Polidispersity Index (PdI) | Zeta Potential (mV) | Encapsulation Efficiency (EE) or Loading Capacity (LC) (%) |
|---|---|---|---|---|---|
| Epigallocatechin gallate | Hot homogenization | 107–122 | 0.102–0.149 | −56.7 to −52.9 | EE 50–70 |
| Curcumin | Solvent emulsification/ evaporation | 130–180 | 0.240–0.260 | −31 to −24 mV | EE 82 |
| Ferulic acid | Micro-emulsification | 96.16 ± 2.96 | 0.196 ± 0.038 | Water: −36.40 ± 1.38 NaCl 0.9%: −1.97 ± 0.25 PBS: −11.7 ± 2.24 | LC 20 |
| Resveratrol | Solvent diffusion–solvent evaporation | 134 ± 7.6 | 0.182 ± 0.017 | −34.3 ± 2.5 | EE 88.9 ± 3.1 |
| Quercetin | Ultrasonication | 416.9 ± 11.4 to 341.2 ± 1.0 | n.d. | −50.4 ± 0.0 to 29.4 ± 0.4 | EE 15.2 ± 1.1 to 46.2 ± 2.2 |
| Polyphenol-enriched Hibiscus sabdariffa | Double emulsion | 66.5 ± 0.3 to 294 ± 2 | 0.17 ± 0.02 to 0.29 ± 0.01 | −29.1 ± 0.6 to −21.0 ± 1.0 mV | EE 52.9 ± 0.9 and 93 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. https://doi.org/10.3390/antiox9100998
Borges A, de Freitas V, Mateus N, Fernandes I, Oliveira J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants. 2020; 9(10):998. https://doi.org/10.3390/antiox9100998
Chicago/Turabian StyleBorges, Alexandra, Victor de Freitas, Nuno Mateus, Iva Fernandes, and Joana Oliveira. 2020. "Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds" Antioxidants 9, no. 10: 998. https://doi.org/10.3390/antiox9100998
APA StyleBorges, A., de Freitas, V., Mateus, N., Fernandes, I., & Oliveira, J. (2020). Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants, 9(10), 998. https://doi.org/10.3390/antiox9100998








