Antioxidant, Anti-Inflammatory, and Multidrug Resistance Modulation Activity of Silychristin Derivatives
Abstract
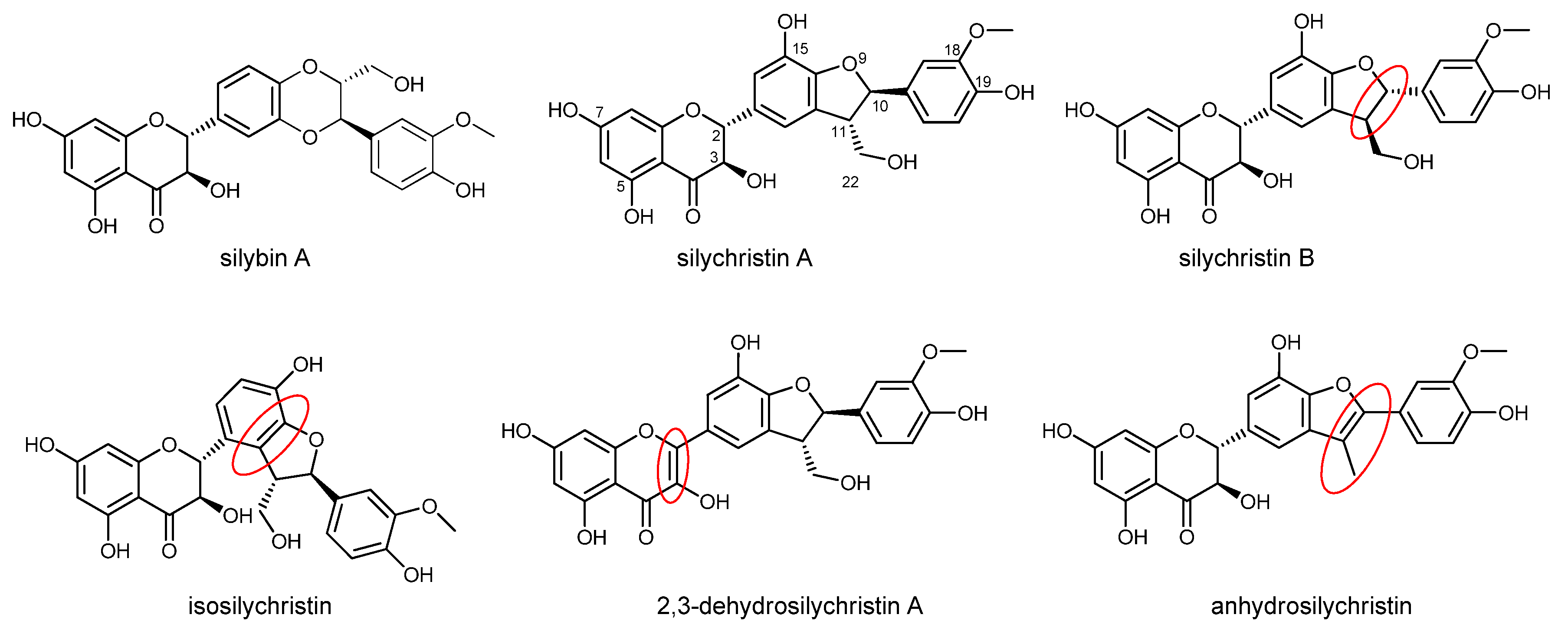
:1. Introduction
2. Materials and Methods
2.1. Analytical Standards and Chemicals
2.2. Antioxidant Capacity
2.3. Anti-Inflammatory Properties
2.4. Inhibition of P-Glycoprotein
2.5. Sensitization of MDR Cell Line
2.6. Inhibition of Expression of Transporters Responsible for MDR Phenotype
2.7. Data Processing and Statistical Analysis
3. Results
3.1. Antioxidant Capacity
3.2. Anti-Inflammatory Properties
3.3. Inhibition of P-Glycoprotein
3.4. Sensitization of the Multidrug-Resistant Cell Line
3.5. Inhibition of ABC Transporters Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-azo-bis-(2-methylpropionamidine) dihydrochloride |
| ABC | ATP binding cassette |
| ADR | Adriamycin, trade name of doxorubicin |
| BCRP | breast cancer resistance protein |
| BHA | butylated hydroxyanisole |
| BHT | butylhydroxytoluen |
| CAA | cellular antioxidant activity |
| COX-2 | cyclooxygenase-2 |
| DCFH-DA | 2′,7′-dichlorofluorescin diacetate |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DMEM | Dulbecco’s Modified Eagle’s Medium - high glucose |
| EMEM | Eagle´s Minimum Essential Medium |
| FBS | fetal bovine serum |
| FCR | Folin–Ciocalteu reagent |
| FRAP | ferric reducing ability of plasma |
| HepG2 | human hepatocellular adenocarcinoma |
| HOC | human ovarian adenocarcinoma |
| HOC/ADR | human ovarian adenocarcinoma resistant to doxorubicin |
| IL-1 | Interleukin-1 |
| iNOS | inducible isoform of nitric oxide synthases |
| LPS | lipopolysaccharides |
| LOX | lipoxygenase |
| MDR | multidrug resistance |
| MEM | Essential Medium Eagle no phenol red |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| ORAC | oxygen radical absorption capacity |
| P-gp | P-glycoprotein |
| PBS | Phosphate-buffered saline |
| RAW 264.7 | mouse macrophages |
| RLU | relative luminescence units |
| THTT | thyroid hormone transmembrane transporters |
| TNF-α | tumor necrosis factor alpha |
Appendix A
| Gene Symbol | Assay ID | GenBank Accession No. | Gene Name | Amplicon Length (bp) | Expression Difference | P | Resistant versus Sensitive |
|---|---|---|---|---|---|---|---|
| Reference | |||||||
| PPIA | Hs99999904_m1 | NM_021130.3 | Peptidylprolyl isomerase A | 98 | |||
| Target | |||||||
| ABCA1 | Hs00194045_m1 | NM_005502.3 | ABC, sub-family A (ABC1), member 1 | 125 | 3.140 | 0.000 | overexpression |
| ABCA2 | Hs00242232_m1 | NM_212533.2 | ABC, sub-family A (ABC1), member 2 | 58 | 1.402 | 0.000 | overexpression |
| ABCA3 | Hs00184543_m1 | NM_001089.2 | ABC, sub-family A (ABC1), member 3 | 77 | 0.487 | 0.161 | |
| ABCA4 | Hs00184367_m1 | NM_000350.2 | ABC, sub-family A (ABC1), member 4 | 71 | neither in HOC nor in HOC/ADR | ||
| ABCA5 | Hs00363322_m1 | NM_172232.2 | ABC, sub-family A (ABC1), member 5 | 100 | 4.595 | 0.000 | overexpression |
| ABCA6 | Hs00365329_m1 | NM_080284.2 | ABC, sub-family A (ABC1), member 6 | 83 | neither in HOC nor in HOC/ADR | ||
| ABCA7 | Hs00185303_m1 | NM_019112.3 | ABC, sub-family A (ABC1), member 7 | 80 | 2.632 | 0.000 | overexpression |
| ABCA8 | Hs00992371_m1 | NM_007168.2 | ABC, sub-family A (ABC1), member 8 | 85 | neither in HOC nor in HOC/ADR | ||
| ABCA9 | Hs00329320_m1 | NM_080283.3 | ABC, sub-family A (ABC1), member 9 | 145 | neither in HOC nor in HOC/ADR | ||
| ABCA10 | Hs00365268_m1 | NM_080282.3 | ABC, sub-family A (ABC1), member 10 | 127 | neither in HOC nor in HOC/ADR | ||
| ABCA12 | Hs00292421_m1 | NR_103740.1 | ABC, sub-family A (ABC1), member 12 | 77 | neither in HOC nor in HOC/ADR | ||
| ABCA13 | Hs01110169_m1 | NM_152701.3 | ABC, sub-family A (ABC1), member 13 | 80 | neither in HOC nor in HOC/ADR | ||
| ABCB1 | Hs00184491_m1 | NM_000927.4 | ABC, sub-family B (MDR/TAP), member 1 | 110 | no detectable level of expression in HOC | ||
| ABCB2 | Hs00388677_m1 | NM_000593.5 | Transporter 1, ABC, sub-family B (MDR/TAP) | 60 | 0.263 | 0.320 | not affected |
| ABCB3 | Hs00241060_m1 | NM_018833.2 | Transporter 2, ABC, sub-family B (MDR/TAP) | 66 | 0.290 | 0.000 | attenuated |
| ABCB4 | Hs00240956_m1 | NM_018850.2 | ABC, sub-family B (MDR/TAP), member 4 | 73 | neither in HOC nor in HOC/ADR | ||
| ABCB6 | Hs00180568_m1 | NM_005689.2 | ABC, sub-family B (MDR/TAP), member 6 | 60 | 4.339 | 0.000 | overexpression |
| ABCB7 | Hs00188776_m1 | NM_004299.3 | ABC, sub-family B (MDR/TAP), member 7 | 92 | 1.485 | 0.320 | not affected |
| ABCB8 | Hs00185159_m1 | NM_007188.3 | ABC, sub-family B (MDR/TAP), member 8 | 74 | 1.611 | 0.000 | overexpression |
| ABCB9 | Hs00608640_m1 | NM_203444.2 | ABC, sub-family B (MDR/TAP), member 9 | 75 | 1.185 | 0.000 | overexpression |
| ABCB10 | Hs00429240_m1 | NM_012089.2 | ABC, sub-family B (MDR/TAP), member 10 | 133 | 1.270 | 0.000 | overexpression |
| ABCB11 | Hs00184824_m1 | NM_003742.2 | ABC, sub-family B (MDR/TAP), member 11 | 63 | neither in HOC nor in HOC/ADR | ||
| ABCC1 | Hs00219905_m1 | NM_004996.3 | ABC, sub-family C (CFTR/MRP), member 1 | 74 | 1.241 | 0.000 | overexpression |
| ABCC2 | Hs00166123_m1 | NM_000392.3 | ABC, sub-family C (CFTR/MRP), member 2 | 75 | 399.968 | 0.000 | overexpression |
| ABCC3 | Hs00358656_m1 | NM_003786.3 | ABC, sub-family C (CFTR/MRP), member 3 | 98 | neither in HOC nor in HOC/ADR | ||
| ABCC4 | Hs00195260_m1 | NM_005845.3 | ABC, sub-family C (CFTR/MRP), member 4 | 86 | 1.326 | 0.000 | overexpression |
| ABCC5 | Hs00981089_m1 | NM_005688.2 | ABC, sub-family C (CFTR/MRP), member 5 | 68 | 1.619 | 0.000 | overexpression |
| ABCC6 | Hs00184566_m1 | NM_001171.5 | ABC, sub-family C (CFTR/MRP), member 6 | 56 | 0.941 | 0.000 | attenuated |
| ABCC9 | Hs00245832_m1 | NM_020297.2 | ABC, sub-family C (CFTR/MRP), member 9 | 70 | neither in HOC nor in HOC/ADR | ||
| ABCC10 | Hs00675716_m1 | NM_033450.2 | ABC, sub-family C (CFTR/MRP), member 10 | 142 | 2.591 | 0.000 | overexpression |
| ABCD1 | Hs00163610_m1 | NM_000033.3 | ABC, sub-family D (ALD), member 1 | 101 | 4.417 | 0.000 | overexpression |
| ABCD2 | Hs00193054_m1 | NM_005164.3 | ABC, sub-family D (ALD), member 2 | 109 | 0.830 | 0.502 | not affected |
| ABCD3 | Hs00161065_m1 | NM_002858.3 | ABC, sub-family D (ALD), member 3 | 91 | 1.697 | 0.159 | not affected |
| ABCD4 | Hs00245534_m1 | NM_005050.3 | ABC, sub-family D (ALD), member 4 | 117 | 0.964 | 0.842 | not affected |
| ABCE1 | Hs01009190_m1 | NM_001040876.1 | ABC, sub-family E (OABP), member 1 | 91 | 1.207 | 0.000 | overexpression |
| ABCF1 | Hs00153703_m1 | NM_001090.2 | ABC, sub-family F (GCN20), member 1 | 69 | 1.706 | 0.000 | overexpression |
| ABCF2 | Hs00606493_m1 | NM_005692.4 | ABC, sub-family F (GCN20), member 2 | 113 | 1.330 | 0.000 | overexpression |
| ABCF3 | Hs00217977_m1 | NM_018358.2 | ABC, sub-family F (GCN20), member 3 | 61 | 1.792 | 0.000 | overexpression |
| ABCG1 | Hs00245154_m1 | NM_207629.1 | ABC, sub-family G (WHITE), member 1 | 58 | 2.532 | 0.000 | overexpression |
| ABCG2 | Hs00184979_m1 | NM_004827.2 | ABC, sub-family G (WHITE), member 2 | 92 | 0.467 | 0.000 | attenuated |
| ABCG4 | Hs00223446_m1 | NM_001142505.1 | ABC, sub-family G (WHITE), member 4 | 93 | 24.936 | 0.000 | overexpression |
| ABCG8 | Hs00223690_m1 | NM_022437.2 | ABC, sub-family G (WHITE), member 8 | 63 | neither in HOC nor in HOC/ADR | ||
| Silychristin A | 2,3-Dehydrosilychristin A | Isosilychristin | Anhydrosilychristin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Symbol | Expression Difference | P | Treated versus Untreated | Expression Difference | P | Treated versus Untreated | Expression Difference | P | Treated versus Untreated | Expression Difference | P | Treated versus Untreated |
| PPIA | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||
| ABCA1 | 1.217 | 0.321 | 0.801 | 0.000 | DOWN | 0.856 | 0.665 | 0.948 | 0.672 | |||
| ABCA2 | 0.755 | 0.000 | DOWN | 0.756 | 0.000 | DOWN | 0.743 | 0.000 | DOWN | 0.675 | 0.000 | DOWN |
| ABCA3 | 0.971 | 0.829 | 0.834 | 0.000 | DOWN | 0.755 | 0.336 | 0.686 | 0.000 | DOWN | ||
| ABCA5 | 0.971 | 0.829 | 0.965 | 0.675 | 0.864 | 0.499 | 0.822 | 0.341 | ||||
| ABCA7 | 1.220 | 0.172 | 1.241 | 0.000 | UP | 1.142 | 0.166 | 1.054 | 0.846 | |||
| ABCB1 | 0.932 | 0.321 | 0.863 | 0.654 | 0.796 | 0.000 | DOWN | 0.722 | 0.000 | DOWN | ||
| ABCB2 | 0.980 | 0.657 | 0.825 | 0.347 | 0.753 | 0.166 | 0.546 | 0.000 | DOWN | |||
| ABCB3 | 1.007 | 0.828 | 0.902 | 0.832 | 0.962 | 0.669 | 0.693 | 0.000 | DOWN | |||
| ABCB6 | 0.799 | 0.000 | DOWN | 0.707 | 0.000 | DOWN | 0.758 | 0.166 | 0.557 | 0.000 | DOWN | |
| ABCB7 | 0.855 | 0.321 | 0.735 | 0.157 | 0.769 | 0.166 | 0.820 | 0.174 | ||||
| ABCB8 | 0.833 | 0.000 | DOWN | 0.811 | 0.328 | 0.953 | 0.665 | 0.554 | 0.000 | DOWN | ||
| ABCB9 | 0.924 | 0.657 | 0.743 | 0.000 | DOWN | 0.848 | 0.000 | DOWN | 0.590 | 0.000 | DOWN | |
| ABCB10 | 0.883 | 0.657 | 0.774 | 0.157 | 0.626 | 0.166 | 0.568 | 0.000 | DOWN | |||
| ABCC1 | 0.917 | 0.321 | 0.774 | 0.000 | DOWN | 0.711 | 0.166 | 0.654 | 0.000 | DOWN | ||
| ABCC2 | 1.025 | 0.828 | 0.869 | 0.675 | 0.761 | 0.166 | 0.596 | 0.000 | DOWN | |||
| ABCC3 | 6.375 | 0.172 | 5.600 | 0.000 | UP | 4.569 | 0.170 | 3.812 | 0.174 | |||
| ABCC5 | 0.895 | 0.321 | 0.773 | 0.000 | DOWN | 0.697 | 0.166 | 0.615 | 0.000 | DOWN | ||
| ABCC6 | 1.094 | 0.321 | 0.861 | 0.675 | 0.841 | 0.499 | 1.159 | 0.000 | UP | |||
| ABCC10 | 1.006 | 0.828 | 0.752 | 0.000 | DOWN | 0.752 | 0.000 | DOWN | 0.628 | 0.000 | DOWN | |
| ABCD1 | 1.775 | 0.000 | UP | 1.664 | 0.000 | UP | 1.700 | 0.000 | UP | 1.552 | 0.000 | UP |
| ABCD2 | 2.925 | 0.000 | UP | 4.374 | 0.000 | UP | 0.827 | 0.497 | 4.267 | 0.329 | ||
| ABCD3 | 0.957 | 0.657 | 0.793 | 0.504 | 0.293 | 0.166 | 0.964 | 0.331 | ||||
| ABCD4 | 2.146 | 0.172 | 0.752 | 0.000 | DOWN | 1.822 | 0.000 | UP | 1.686 | 0.174 | ||
| ABCE1 | 1.854 | 0.000 | UP | 1.811 | 0.157 | 2.080 | 0.000 | UP | 2.234 | 0.329 | ||
| ABCF1 | 0.161 | 0.000 | DOWN | 0.182 | 0.000 | DOWN | 0.114 | 0.000 | DOWN | 0.208 | 0.000 | DOWN |
| ABCF2 | 2.053 | 0.000 | UP | 2.028 | 0.000 | UP | 1.753 | 0.000 | UP | 1.672 | 0.000 | UP |
| ABCF3 | 1.116 | 0.172 | 0.464 | 0.000 | DOWN | 0.993 | 0.669 | 0.799 | 0.000 | DOWN | ||
| ABCG1 | 1.448 | 0.172 | 0.844 | 0.000 | DOWN | 0.900 | 0.665 | 1.760 | 0.000 | UP | ||
| ABCG2 | 0.891 | 0.657 | 0.804 | 0.000 | DOWN | 0.922 | 0.830 | 0.716 | 0.000 | DOWN | ||
| ABCG4 | 1.854 | 0.000 | UP | 1.533 | 0.000 | UP | 1.513 | 0.166 | 1.583 | 0.000 | UP | |
References
- Biedermann, D.; Buchta, M.; Holečková, V.; Sedlák, D.; Valentová, K.; Cvačka, J.; Bednářova, L.; Křenková, A.; Kuzma, M.; Skuta, C.; et al. Silychristin: Skeletal alterations and biological activities. J. Nat. Prod. 2016, 79, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Křenek, K.; Marhol, P.; Peikerová, Ž.; Křen, V.; Biedermann, D. Preparatory separation of the silymarin flavonolignans by Sephadex LH-20 gel. Food Res. Int. 2014, 65, 115–120. [Google Scholar] [CrossRef]
- Zanarotti, A. Stereochemistry of silychristin mild dehydrogenation of flavanonols. Heterocycles 1982, 19, 1585–1586. [Google Scholar] [CrossRef]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojovic, M.; Popovic-Bijelic, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Vostalová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Svobodová, A.R. Skin protective activity of silymarin and its flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Gorlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017, 13, 94–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Vrba, J.; Papoušková, B.; Roubalová, L.; Zatloukalová, M.; Biedermann, D.; Křen, V.; Valentová, K.; Ulrichová, J.; Vacek, J. Metabolism of flavonolignans in human hepatocytes. J. Pharm. Biomed. 2018, 152, 94–101. [Google Scholar] [CrossRef]
- Valentová, K.; Purchartová, K.; Rýdlová, L.; Roubalová, L.; Biedermann, D.; Petrásková, L.; Křenková, A.; Pelantová, H.; Holečkova-Moravcová, V.; Tesařová, E.; et al. Sulfated metabolites of flavonolignans and 2,3-dehydroflavonolignans: Preparation and properties. Int. J. Mol. Sci. 2018, 19, 2349. [Google Scholar] [CrossRef]
- Qin, N.B.; Hu, X.; Li, S.G.; Wang, J.; Li, Z.L.; Li, D.H.; Xu, F.X.; Gao, M.; Hua, H.M. Hypoglycemic effect of silychristin a from Silybum marianum fruit via protecting pancreatic islet cells from oxidative damage and inhibiting α-glucosidase activity in vitro and in rats with type 1 diabetes. J. Funct. Food 2017, 38, 168–179. [Google Scholar] [CrossRef]
- Hrčková, G.; Kubásková, T.M.; Benada, O.; Kofroňová, O.; Tumová, L.; Biedermann, D. Differential effects of the flavonolignans silybin, silychristin and 2,3-dehydrosilybin on Mesocestoides vogae larvae (cestoda) under hypoxic and aerobic in vitro conditions. Molecules 2018, 23, 2999. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Dziedzic, A.; Synowiec, E.; Sliwinski, T.; Saluk-Bijak, J. Flavonolignans inhibit IL1-β-induced cross-talk between blood platelets and leukocytes. Nutrients 2017, 9, 1022. [Google Scholar] [CrossRef] [PubMed]
- Kosina, P.; Paloncyová, M.; Svobodová, A.R.; Zálešák, B.; Biedermann, D.; Ulrichová, J.; Vostalová, J. Dermal delivery of selected polyphenols from Silybum marianum. Theoretical and experimental study. Molecules 2019, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Synowiec, E.; Sitarek, P.; Sliwinski, T.; Saluk-Bijak, J. Evaluation of the cytotoxicity and genotoxicity of flavonolignans in different cellular models. Nutrients 2017, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Tvrdý, V.; Catapano, M.C.; Rawlik, T.; Karlíčková, J.; Biedermann, D.; Křen, V.; Mladěnka, P.; Valentová, K. Interaction of isolated silymarin flavonolignans with iron and copper. J. Inorg. Biochem. 2018, 189, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Szelenberger, R.; Dziedzic, A.; Saluk-Bijak, J. Inhibitory effect of flavonolignans on the P2Y12 pathway in blood platelets. Molecules 2018, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Dziedzic, A.; Saluk-Bijak, J. Flavonolignans reduce the response of blood platelet to collagen. Int. J. Biol. Macromol. 2018, 106, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Inhibition properties of some flavonoids on carbonic anhydrase I and II isoenzymes purified from human erythrocytes. J. Biochem. Mol. Toxicol. 2017, 31, e21930. [Google Scholar] [CrossRef]
- Gažák, R.; Marhol, P.; Purchartová, K.; Monti, D.; Biedermann, D.; Riva, S.; Cvak, L.; Křen, V. Large-scale separation of silybin diastereoisomers using lipases. Process Biochem. 2010, 45, 1657–1663. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967–984. [Google Scholar] [CrossRef]
- Souček, P.; Anzenbacher, P.; Skoumalová, I.; Dvořák, M. Expression of cytochrome P450 genes in CD34+ hematopoietic stem and progenitor cells. Stem Cells 2005, 23, 1417–1422. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The miqe guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Taleb, A.; Ahmad, K.A.; Ihsan, A.U.; Qu, J.; Lin, N.; Hezam, K.; Koju, N.; Hui, L.; Ding, Q.L. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. Pharmacother. 2018, 102, 689–698. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Becker, K.; Schroecksnadel, S.; Gostner, J.; Zaknun, C.; Schennach, H.; Überall, F.; Fuchs, D. Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds. Phytomedicine 2014, 21, 164–171. [Google Scholar] [CrossRef]
- Sersen, F.; Vencel, T.; Annus, J. Silymarin and its components scavenge phenylglyoxylic ketyl radicals. Fitoterapia 2006, 77, 525–529. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Mirvaghefi, A.R.; Rafei, G.R. Effects of long-term silymarin oral supplementation on the blood biochemical profile of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2011, 37, 885–896. [Google Scholar] [CrossRef]
- Naso, L.G.; Ferrer, E.G.; Butenko, N.; Cavaco, I.; Lezama, L.; Rojo, T.; Etcheverry, S.B.; Williams, P.A.M. Antioxidant, DNA cleavage, and cellular effects of silibinin and a new oxovanadium(iv)/silibinin complex. J. Biol. Inorg. Chem. 2011, 16, 653–668. [Google Scholar] [CrossRef]
- Gabrielová, E.; Křen, V.; Jabůrek, M.; Modrianský, M. Silymarin component 2,3-dehydrosilybin attenuates cardiomyocyte damage following hypoxia/reoxygenation by limiting oxidative stress. Physiol. Res. 2015, 64, 79–91. [Google Scholar]
- Rong, Y.Z.; Wang, Z.W.; Wu, J.H.; Zhao, B. A theoretical study on cellular antioxidant activity of selected flavonoids. Spectrochim. Acta A 2012, 93, 235–239. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Borges, F.F.V.; Silva, C.R.E.; Goes, W.M.; Godoy, F.R.; Franco, F.C.; Veras, J.H.; Bailao, E.; Silva, D.D.E.; Cardoso, C.G.; da Cruz, A.D.; et al. Protective effects of silymarin and silibinin against DNA damage in human blood cells. BioMed Res. Int. 2018. [Google Scholar] [CrossRef]
- Weidmann, A.E. Dihydroquercetin: More than just an impurity? Eur. J. Pharmacol. 2012, 684, 19–26. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Svobodová, A.; Vostálová, J.; Gažák, R.; Hrbáč, J.; Sedmera, P.; Křen, V.; Lazzaroni, R.; Duroux, J.-L.; et al. Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin flavonolignans: A joint experimental and theoretical study. J. Phys. Chem. A 2008, 112, 1054–1063. [Google Scholar] [CrossRef]
- Valentová, K.; Biedermann, D.; Křen, V. 2,3-Dehydroderivatives of silymarin flavonolignans: Prospective natural compounds for the prevention of chronic diseases. Proceedings 2019, 11, 21. [Google Scholar] [CrossRef]
- Biedermann, D.; Moravcová, V.; Valentová, K.; Kuzma, M.; Petrásková, L.; Císařová, I.; Křen, V. Oxidation of flavonolignan silydianin to unexpected lactone-acid derivative. Phytochem. Lett. 2019, 30, 14–20. [Google Scholar] [CrossRef]
- Esmaeil, N.; Anaraki, S.B.; Gharagozloo, M.; Moayedi, B. Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 2017, 50, 194–201. [Google Scholar] [CrossRef]
- Gupta, O.P.; Sing, S.; Bani, S.; Sharma, N.; Malhotra, S.; Gupta, B.D.; Banerjee, S.K.; Handa, S.S. Anti-inflammatory and anti-arthritic activities of silymarin acting through inhibition of 5-lipoxygenase. Phytomedicine 2000, 7, 21–24. [Google Scholar] [CrossRef]
- Agarwal, R.; Agarwal, C.; Ichikawa, H.; Singh, R.P.; Aggarwal, B.B. Anticancer potential of silymarin: From bench to bed side. Anticancer Res. 2006, 26, 4457–4498. [Google Scholar]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition and why does it matter? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Juráňová, J.; Aury-Landas, J.; Boumediene, K.; Bauge, C.; Biedermann, D.; Ulrichová, J.; Franková, J. Modulation of skin inflammatory response by active components of silymarin. Molecules 2019, 24, 123. [Google Scholar] [CrossRef]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid hormones and cancer: A comprehensive review of preclinical and clinical studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef]
- Johannes, J.; Jayarama-Naidu, R.; Meyer, F.; Wirth, E.K.; Schweizer, U.; Schomburg, L.; Kohrle, J.; Renko, K. Silychristin, a flavonolignan derived from the milk thistle, is a potent inhibitor of the thyroid hormone transporter MCT8. Endocrinology 2016, 157, 1694–1701. [Google Scholar] [CrossRef]
- Groeneweg, S.; van den Berge, A.; Meima, M.E.; Peeters, R.P.; Visser, T.J.; Visser, W.E. Effects of chemical chaperones on thyroid hormone transport by MCT8 mutants in patient-derived fibroblasts. Endocrinology 2018, 159, 1290–1302. [Google Scholar] [CrossRef]
- Alevizopoulos, K.; Calogeropoulou, T.; Lang, F.; Stournaras, C. Na+/K+ ATPase inhibitors in cancer. Curr. Drug Targets 2014, 15, 988–1000. [Google Scholar] [CrossRef]
- Kubala, M.; Čechová, P.; Geletičová, J.; Biler, M.; Štenclová, T.; Trouillas, P.; Biedermann, D. Flavonolignans as a novel class of sodium pump inhibitors. Front. Physiol. 2016, 7, 115. [Google Scholar] [CrossRef]
- Chambers, C.S.; Viktorová, J.; Řehořová, K.; Biedermann, D.; Turková, L.; Macek, T.; Křen, V.; Valentová, K. Defying multidrug resistance! Modulation of related transporters by flavonoids and flavonolignans. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Zhang, S.; Morris, M.E. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J. Pharmacol. Exp. Ther. 2003, 304, 1258–1267. [Google Scholar] [CrossRef]
- Wu, J.W.; Lin, L.C.; Tsai, T.H. Drug-drug interactions of silymarin on the perspective of pharmacokinetics. J. Ethnopharmacol. 2009, 121, 185–193. [Google Scholar] [CrossRef]
- Chung, S.Y.; Sung, M.K.; Kim, N.H.; Jang, J.O.; Go, E.J.; Lee, H.J. Inhibition of P-glycoprotein by natural products in human breast cancer cells. Arch. Pharm. Res. 2005, 28, 823–828. [Google Scholar] [CrossRef]
- Džubák, P.; Hajdúch, M.; Gažák, R.; Svobodová, A.; Psotová, J.; Walterová, D.; Sedmera, P.; Křen, V. New derivatives of silybin and 2,3-dehydrosilybin and their cytotoxic and P-glycoprotein modulatory activity. Bioorg. Med. Chem. 2006, 14, 3793–3810. [Google Scholar] [CrossRef]
- Sadava, D.; Kane, S.E. Silibinin reverses drug resistance in human small-cell lung carcinoma cells. Cancer Lett. 2013, 339, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Večeřa, R.; Zacharová, A.; Orolin, J.; Škottová, N.; Anzenbacher, P. The effect of silymarin on expression of selected ABC transporters in the rat. Vet. Med. 2011, 56, 59–62. [Google Scholar] [CrossRef]
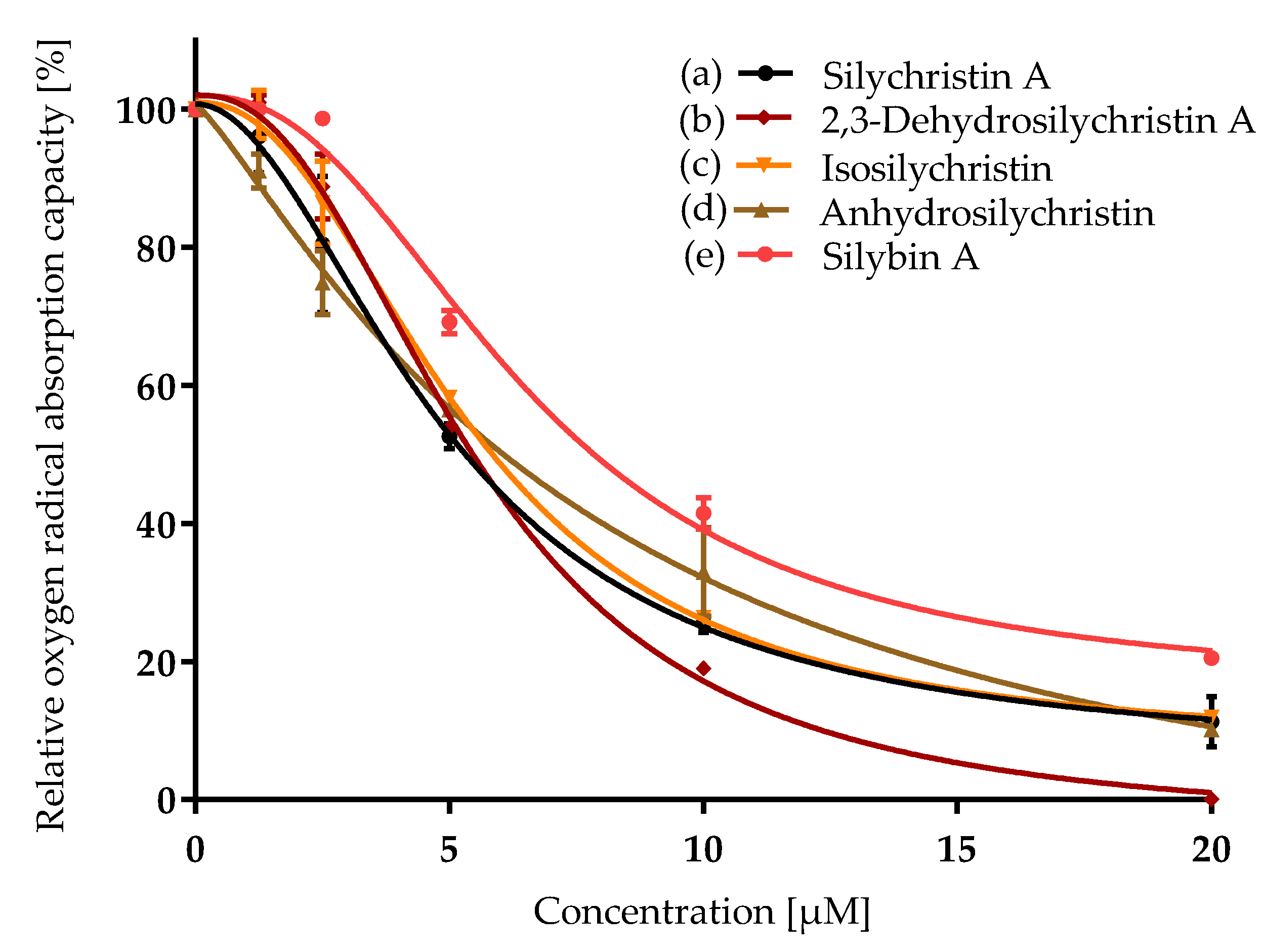
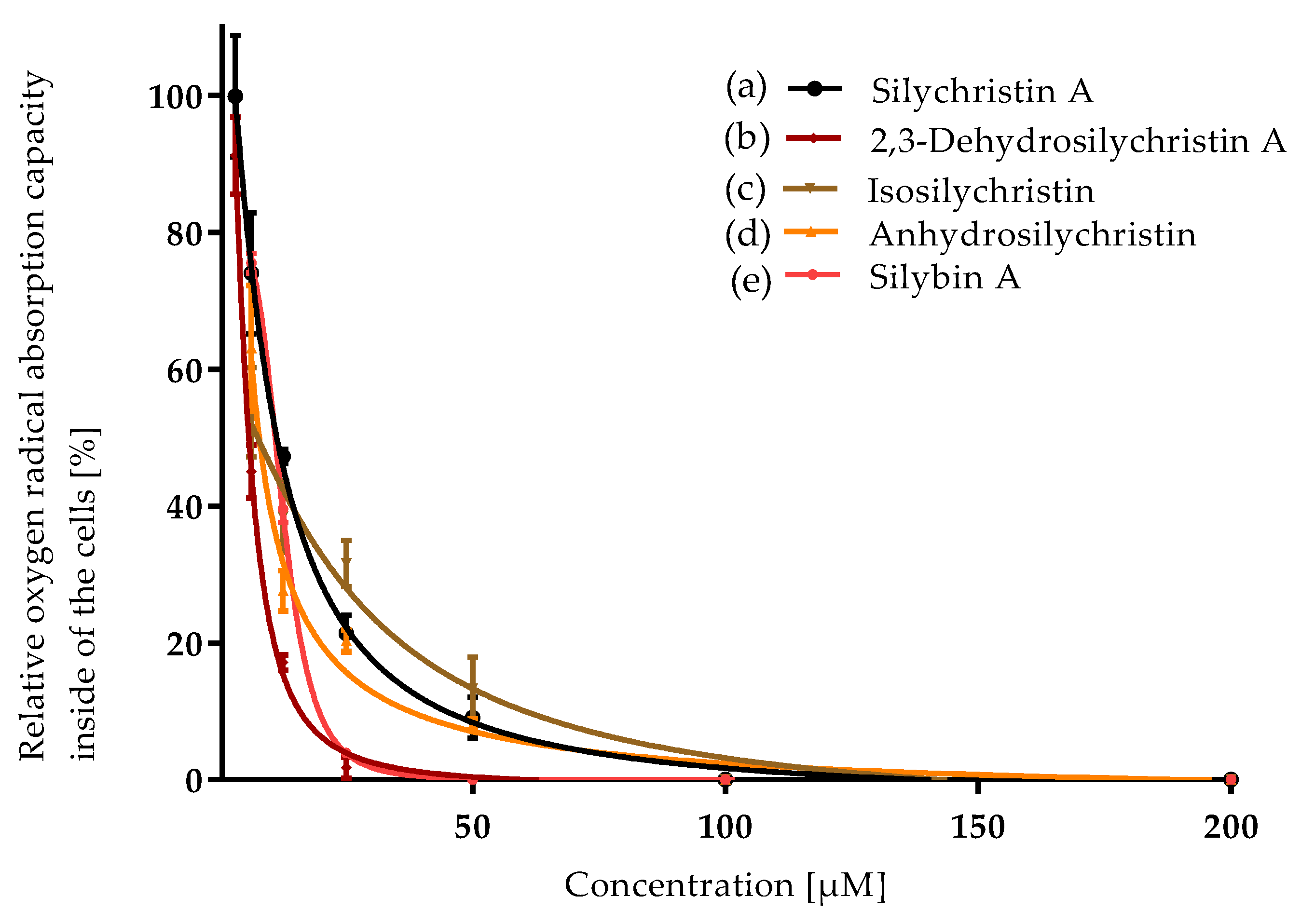
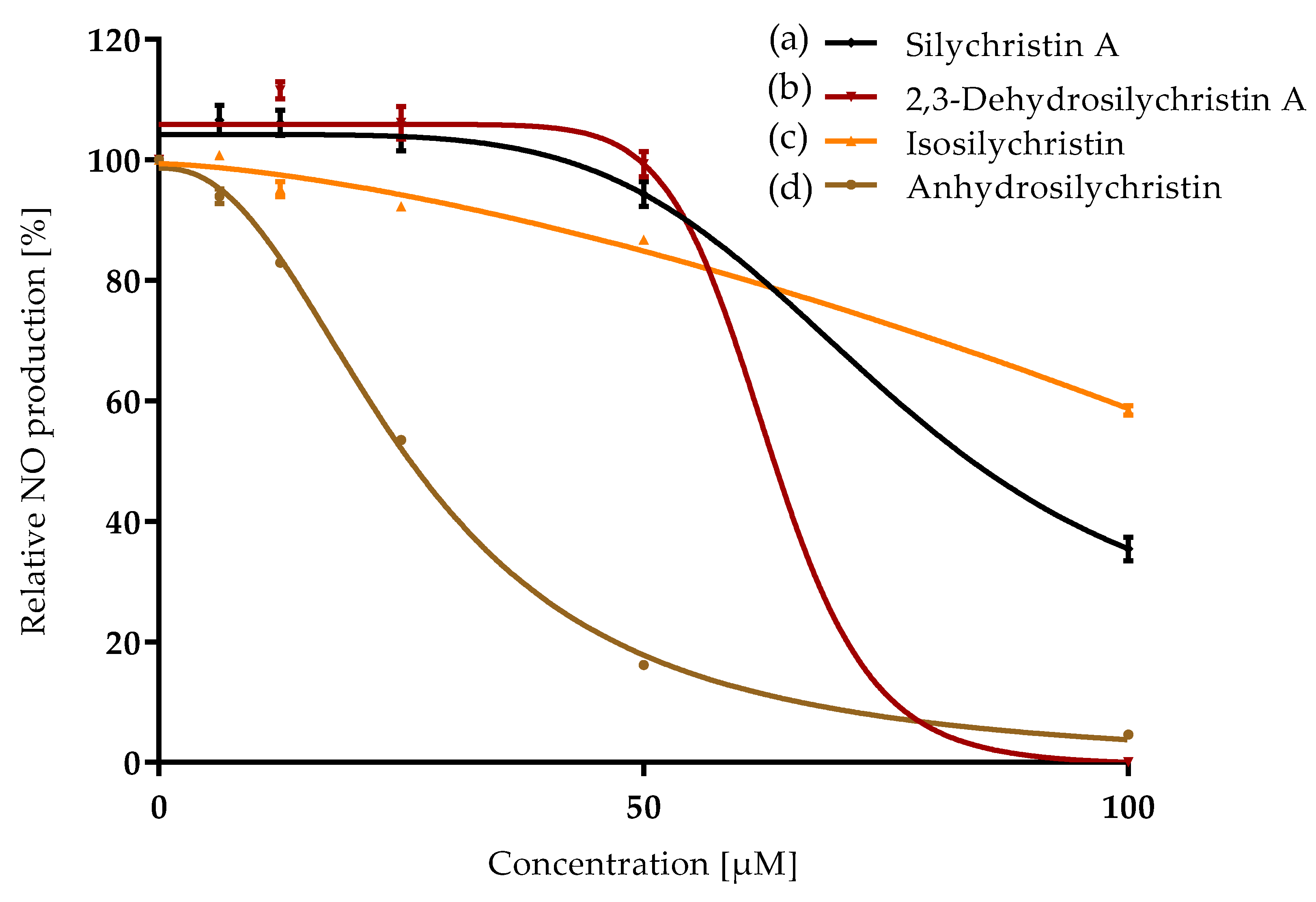
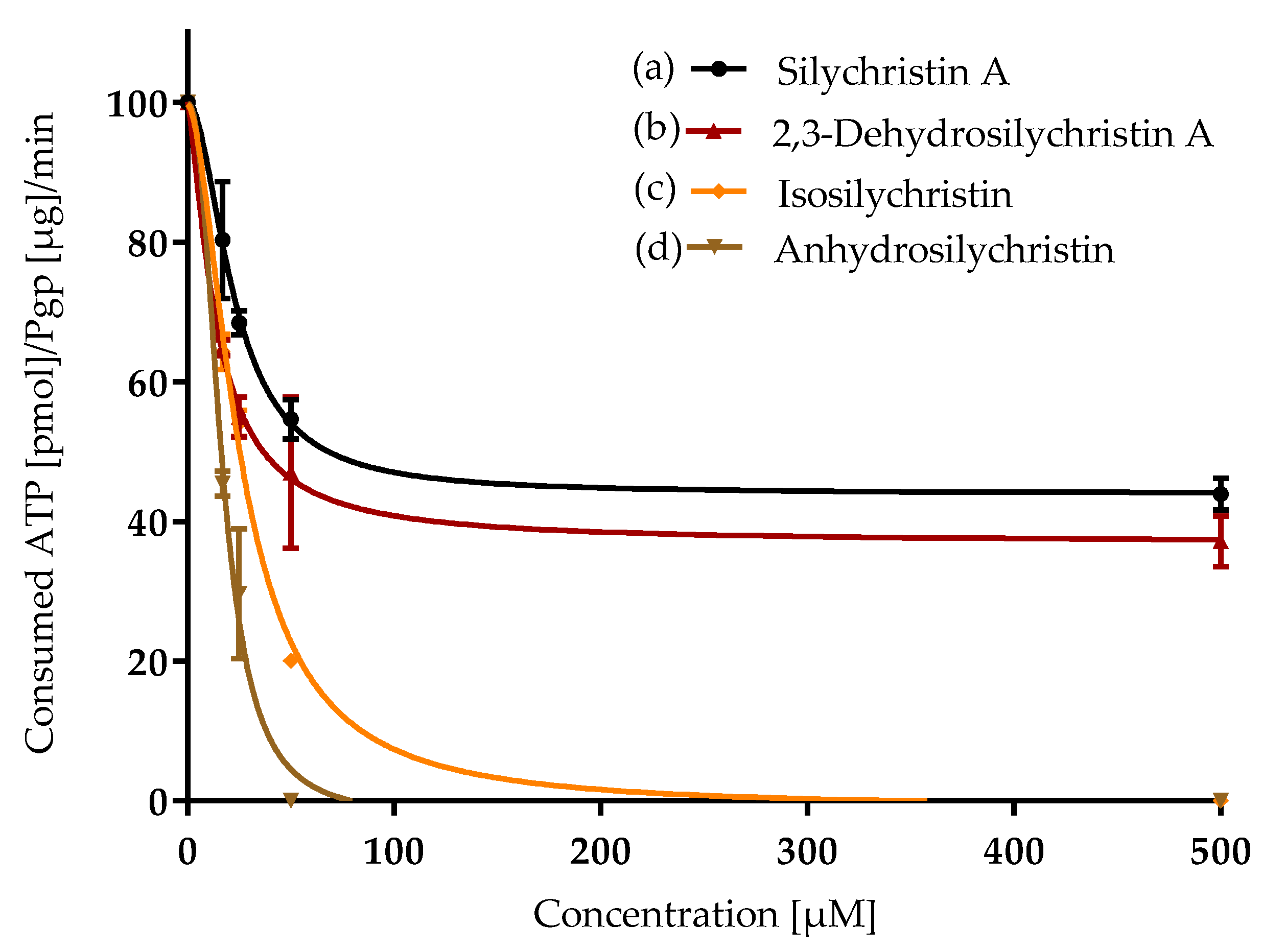
| Compound | ORAC IC50 [µM] | CAA IC50 [µM] | NO Production IC50 [µM] | P-gp IC50 [µM] |
|---|---|---|---|---|
| Silychristin A | 5.4 ± 0.2 a | 11.4 ± 0.5 b,c | 65 ± 3 d | 21 ± 1 b |
| 2,3-Dehydrosilychristin A | 5.4 ± 0.1 a | 6.0 ± 0.2 a | 36.0 ± 0.2 b | 15.6 ± 0.5 a |
| Isosilychristin | 6.0 ± 0.1 a | 13 ± 2 c | 59.8 ± 0.8 c | 25.9 ± 0.4 c |
| Anhydrosilychristin | 5.77 ± 0.05 a | 8.0 ± 0.7 a, b | 22.5 ± 0.3 a | 16.8 ± 0.2 a |
| IC50 [µM] | HOC | HOC/ADR |
|---|---|---|
| Silychristin A | 144 ± 2 | 184 ± 5 |
| 2,3-Dehydrosilychristin A | 50.8 ± 0.9 | 68 ± 9 |
| Isosilychristin | 209 ± 3 | 115 ± 4 |
| Anhydrosilychristin | 45 ± 4 | 66 ± 3 |
| Doxorubicin | 0.022 ± 0.001 | 6.2 ± 0.3 |
| Compound | 10 µM | 20 µM |
|---|---|---|
| Silychristin A | (1.7 ± 0.2) × | (4.0 ± 0.2) × |
| 2,3-Dehydrosilychristin A | (1.3 ± 0.1) × | (4.8 ± 0.3) × |
| Isosilychristin | (1.0 ± 0.1) × | (1.5 ± 0.1) × |
| Anhydrosilychristin | (1.5 ± 0.1) × | (1.0 ± 0.0) × |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viktorová, J.; Dobiasová, S.; Řehořová, K.; Biedermann, D.; Káňová, K.; Šeborová, K.; Václavíková, R.; Valentová, K.; Ruml, T.; Křen, V.; et al. Antioxidant, Anti-Inflammatory, and Multidrug Resistance Modulation Activity of Silychristin Derivatives. Antioxidants 2019, 8, 303. https://doi.org/10.3390/antiox8080303
Viktorová J, Dobiasová S, Řehořová K, Biedermann D, Káňová K, Šeborová K, Václavíková R, Valentová K, Ruml T, Křen V, et al. Antioxidant, Anti-Inflammatory, and Multidrug Resistance Modulation Activity of Silychristin Derivatives. Antioxidants. 2019; 8(8):303. https://doi.org/10.3390/antiox8080303
Chicago/Turabian StyleViktorová, Jitka, Simona Dobiasová, Kateřina Řehořová, David Biedermann, Kristýna Káňová, Karolína Šeborová, Radka Václavíková, Kateřina Valentová, Tomáš Ruml, Vladimír Křen, and et al. 2019. "Antioxidant, Anti-Inflammatory, and Multidrug Resistance Modulation Activity of Silychristin Derivatives" Antioxidants 8, no. 8: 303. https://doi.org/10.3390/antiox8080303
APA StyleViktorová, J., Dobiasová, S., Řehořová, K., Biedermann, D., Káňová, K., Šeborová, K., Václavíková, R., Valentová, K., Ruml, T., Křen, V., & Macek, T. (2019). Antioxidant, Anti-Inflammatory, and Multidrug Resistance Modulation Activity of Silychristin Derivatives. Antioxidants, 8(8), 303. https://doi.org/10.3390/antiox8080303