HPLC-ESI-qTOF-MS/MS Characterization, Antioxidant Activities and Inhibitory Ability of Digestive Enzymes with Molecular Docking Analysis of Various Parts of Raspberry (Rubus ideaus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Materials
2.3. Extraction of Phenolic Compounds
2.4. Determination of Total Phenolic Content (TPC) and Flavonoid Content (TFC)
2.5. Phenolic Compositions Analysis by HPLC-ESI-HR-qTOF-MS/MS
2.6. Antioxidant Activities Assays
2.6.1. DPPH Radical Scavenging Activity Assay
2.6.2. ABTS Cation Radical Scavenging Activity Assay
2.6.3. Hydroxyl (OH−) Radical Scavenging Activity Assay
2.6.4. Ferric Reducing/Antioxidant Power (FRAP) Assay
2.7. Type II Diabetes Related Enzyme Inhibition Properties
2.7.1. α-Glucosidase Inhibition Activity Assay
2.7.2. α-Amylase Inhibition Activity Assay
2.8. Molecular Docking Analysis
2.9. Data Analysis
3. Results and Discussion
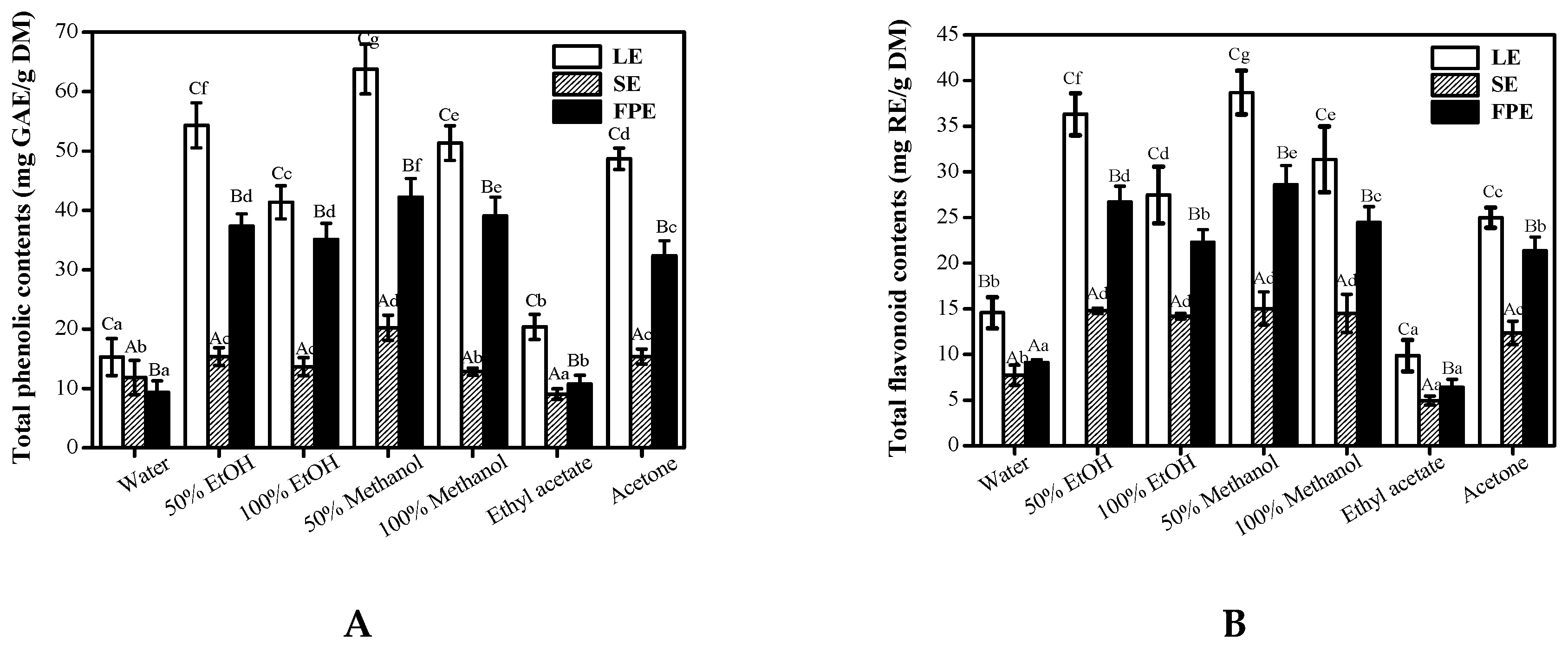
3.1. Total Phenolic Content and Total Flavonoid Content
3.2. HPLC-ESI-HR-qTOF-MS/MS Characterization and Quantification of Phenolic Compositions
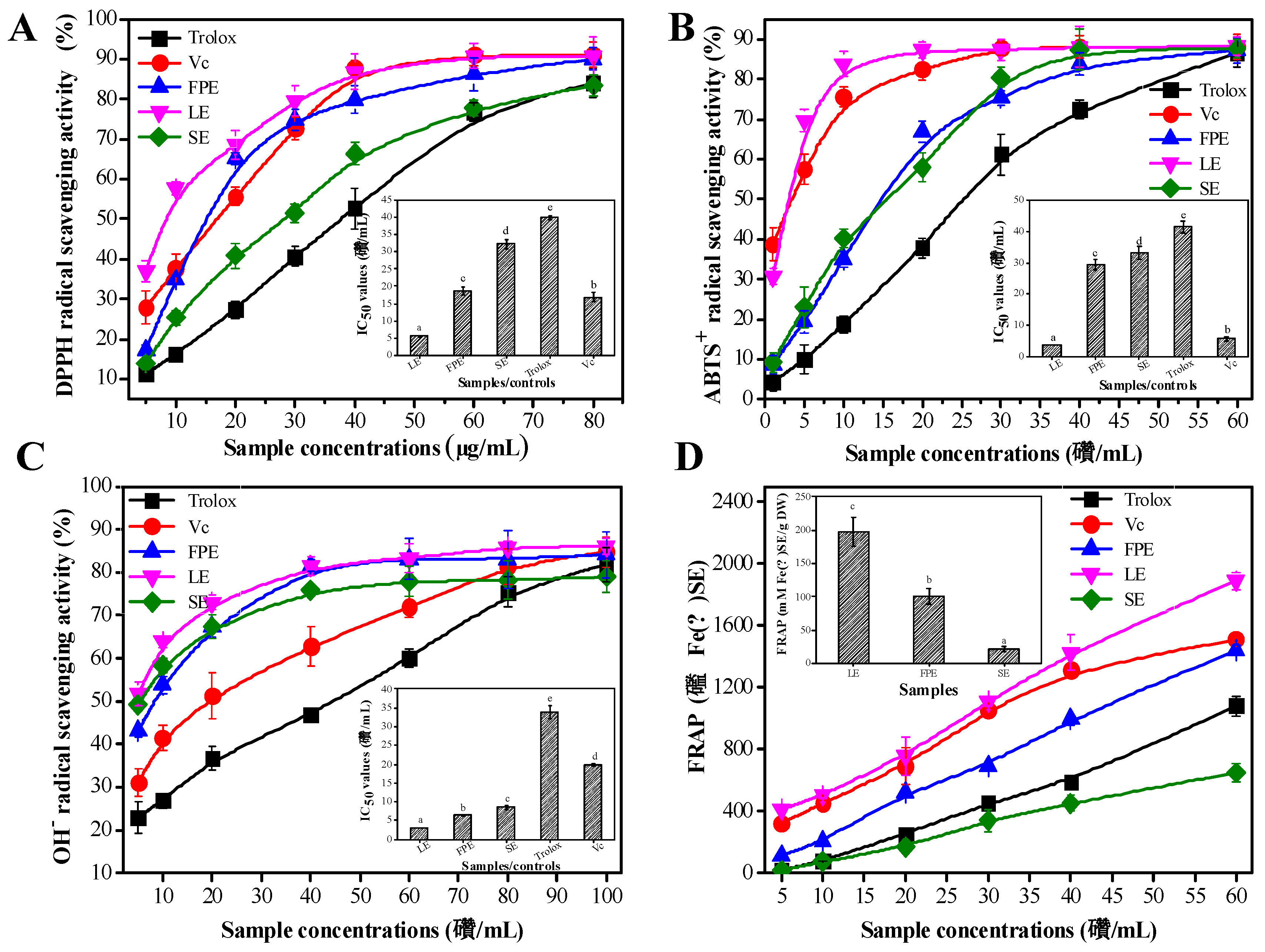
3.3. Antioxidant Activities
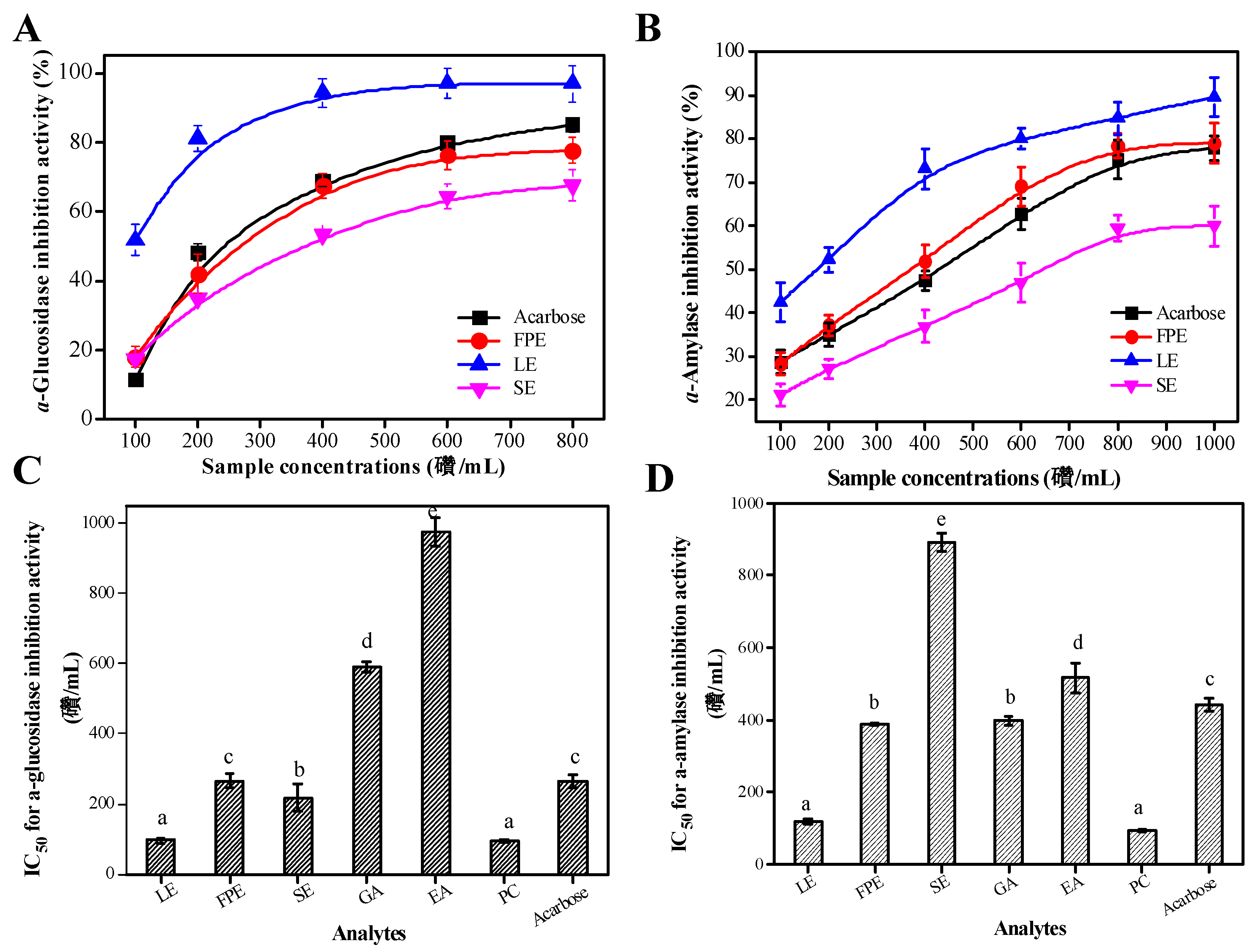
3.4. Type II Diabetes Related Enzymes Inhibitory Activities
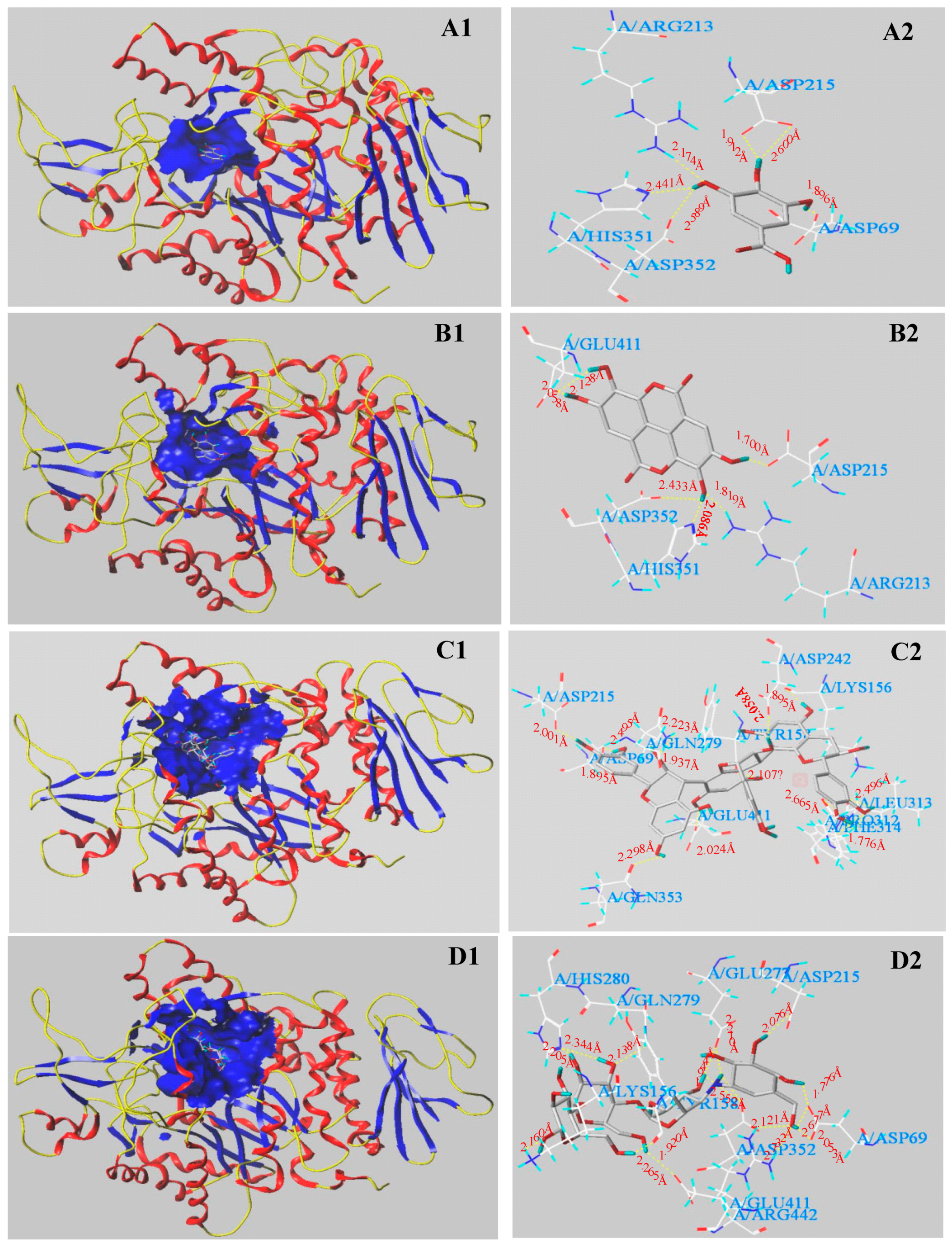
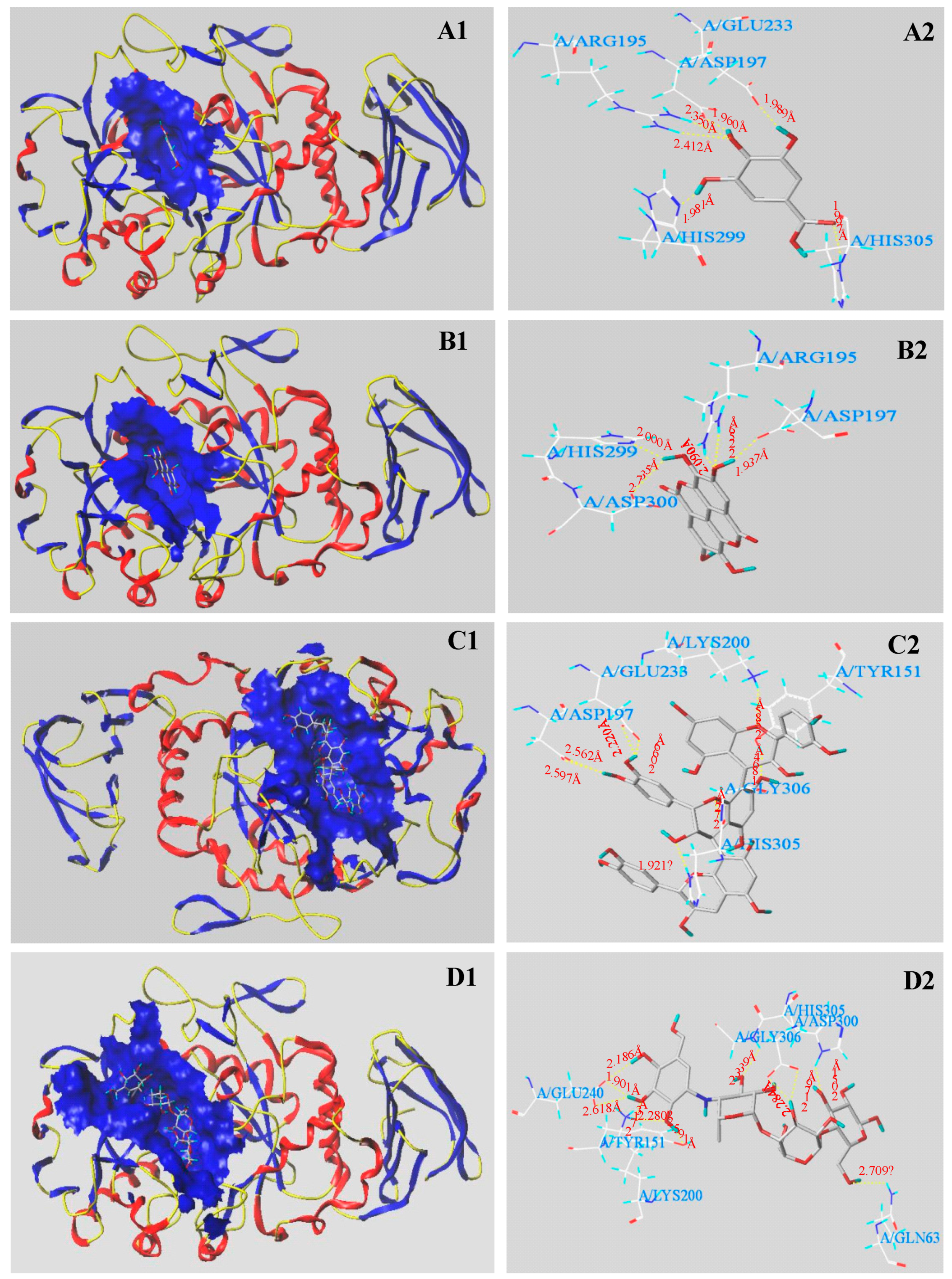
3.5. Molecular Docking Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LE | leaves extracts |
| SE | seed extracts |
| FPE | fruit pulp extracts |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl radical |
| ABTS | 2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| TPTZ | 2,4,6-tris(2-pyridyl)-s-triazine |
| FRAP | ferric reducing anti-oxidant power |
| p-NPG | p-Nitrophenyl-α-D-glucopyranoside |
| TPC | total phenolic content |
| TFC | flavonoid content |
| GA | gallic acid |
| EA | ellagic acid |
| PC | procyanidin C3 |
| Vc | ascorbic acid |
| DW | dried weight |
| HPLC-ESI-HR-qTOF-MS/MS | High performance liquid chromatography-electrospray ionization-high resolution-quadrupole time of flight-tandem mass spectrometry |
| PBS | phosphate buffer solution |
| GAE | gallic acid equivalents |
| RE | rutin equivalents |
References
- Rodriguezmonforte, M.; Sanchez, E.; Barrio, F.; Costa, B.; Floresmateo, G. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Younger, K.M. Antioxidant, anti-inflammatory, anti-carcinogenic and anti-diabetic. Nutr. Res. Rev. 2010, 23, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.D.; Hsu, H.F.; Chen, Z.H.; Wang, Y.T.; Huang, S.H.; Chen, H.J. Antioxidant, anti-inflammatory, and anti-proliferative activities of extracts from different parts of farmed and wild glossogyne tenuifolia. Ind. Crop. Prod. 2014, 57, 98–105. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, Y.J.; Cai, L.B.; Xu, F.R.; He, Q.Q. Fruit and vegetable consumption and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Crit. Rev. Food. Sci. 2015, 57, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zafrilla, P.; Ferreres, F.; Tomás-Barberán, F.A. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J. Agri. Food. Chem. 2001, 49, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.E.; Câmara, M.B.; Direito, R.; Rocha, J.; Serra, A.T.; Duarte, C.M.; Sepodes, B. Chemical characterization of a red raspberry fruit extract and evaluation of its pharmacological effects in experimental models of acute inflammation and collagen-induced arthritis. Food Funct. 2014, 5, 3241–3251. [Google Scholar]
- Han, N.; Gu, Y.; Ye, C.; Cao, Y.; Liu, Z.; Yin, J. Antithrombotic activity of fractions and components obtained from raspberry leaves (Rubus chingii). Food Chem. 2012, 132, 181–185. [Google Scholar] [CrossRef]
- Buricova, L.; Andjelkovic, M.; Cermakova, A.; Reblova, Z.; Jurcek, O.; Kolehmainen, E.; Kvasnicka, F. Antioxidant capacity and antioxidants of strawberry, blackberry, and raspberry leaves. Czech J. Food Sci. 2018, 29, 181–189. [Google Scholar] [CrossRef]
- Dvaranauskaite, A.; Venskutonis, P.R.; Labokas, J. Comparison of quercetin derivatives in ethanolic extracts of red raspberry (Rubus idaeus L.) leaves. Acta Aliment. 2008, 37, 449–461. [Google Scholar] [CrossRef]
- Luther, M.; Parry, J.; Moore, J.; Meng, J.; Zhang, Y.; Cheng, Z.; Yu, L. Inhibitory effect of Chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties. Food Chem. 2007, 104, 1065–1073. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Li, Y.; Wu, Z.Q. Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities. J. Funct. Foods. 2018, 46, 57–65. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crop. Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.; Liu, Y.; Wu, Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem. 2018, 264, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sheth, F.; De, S. Evaluation of comparative antioxidant potential of four cultivars of Hibiscus rosasinensis L. by HPLC-DPPH method. Free Rad. Antioxid. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Rezaei-Sadabady, R.; Eidi, A.; Zarghami, N.; Barzegar, A. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetinencapsulated liposomes. Artif. Cell. Nanomed. B. 2016, 44, 128–134. [Google Scholar] [CrossRef]
- Wang, L.; Bei, Q.; Wu, Y.; Liao, W.; Wu, Z.Q. Characterization of soluble and insoluble-bound polyphenols from Psidium guajava L. leaves co-fermented with Monascus anka and Bacillus sp. and their bio-activities. J. Funct. Foods. 2017, 32, 149–159. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, X.; Zhuang, X.; Han, W.; Guo, W.; Xiong, J.; Zhang, X. Rice bran polysaccharides and oligosaccharides modified by Grifola frondosa fermentation: Antioxidant activities and effects on the production of NO. Food Chem. 2017, 223, 49–53. [Google Scholar] [CrossRef]
- Wong, C.C.; Li, H.B.; Cheng, K.W.; Chen, F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Wang, Y.M.; Yang, Y.L.; Zeng, Y.; Mei, L.J.; Shi, Y.P. Antioxidants and α-glucosidase inhibitors from “liucha” (young leaves and shoots of Sibiraea laevigata). Food Chem. 2017, 230, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zaharudin, N.; Armando, A.S.; Dragsted, L.O. Inhibitory effects of edible seaweeds, polyphenolics and alginates on the activities of porcine pancreatic α-amylase. Food Chem. 2017, 245, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, Y.; Ma, Y.; Cheng, G.; Cai, S. Phenolic composition, antioxidant properties, and inhibition toward digestive enzymes with molecular docking analysis of different fractions from Prinsepia utilis royle fruits. Molecules 2018, 23, 3373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Gao, F.; Zhao, Y.; Cai, S.; Pang, M. The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. J. Funct. Foods. 2018, 40, 729–735. [Google Scholar] [CrossRef]
- Ye, F.; Qiang, L.; Hang, L.; Zhao, G. Solvent effects on phenolic content, composition, and antioxidant activity of extracts from florets of sunflower (Helianthus annuus L.). Ind. Crop. Prod. 2015, 76, 574–581. [Google Scholar] [CrossRef]
- A Comparative Study on Phytochemical Screening, Quantification of Phenolic Contents and Antioxidant Properties of Different Solvent Extracts from Various Parts of Pistacia lentiscus L. Available online: https://www.sciencedirect.com/science/article/pii/S1018364718303355 (accessed on 11 May 2018).
- Prabakaran, S.; Ramu, L.; Veerappan, S.; Pemiah, B.; Kannappan, N. Effect of different solvents on volatile and non-volatile constituents of red bell pepper (Capsicum annuum L.) and their in vitro antioxidant activity. J. Food. Meas. Charact. 2017, 11, 1531–1541. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food. Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, R.; Paweł, P.; Joanna, R.; Aneta, K.; Audrius, M.; Monika, N.; Boguslaw, B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. [Google Scholar]
- Rafińska, K.; Pomastowski, P.; Rudnicka, J.; Krakowska, A.; Maruśka, A.; Narkute, M.; Buszewski, B. Bioactive compounds and antioxidant activity of wheat bran and barley husk in the extracts with different polarity. Int. J. Food Prop. 2019, 22, 646–658. [Google Scholar]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef]
- Djordjevic, T.M.; ller-Marinkovic, S.S.; Dimitrijevic-Brankovic, S.I. Antioxidant activity and total phenolic content in some cereals and legumes. Int. J. Food Prop. 2011, 14, 175–184. [Google Scholar] [CrossRef]
- Hong, H.K.; Kwak, J.H.; Kang, S.C.; Lee, J.W.; Park, J.H.; Ahn, J.W. Antioxidative constituents from the whole plants of euphorbia supina. Korean J. Pharmacogn. 2008, 39, 260–264. [Google Scholar]
- Characterization and Antioxidant Activity of Gallic Acid Derivative. Available online: https://aip.scitation.org/doi/abs/10.1063/1.5011887 (accessed on 27 November 2017).
- Bialonska, D.; Kasimsetty, S.G.; Khan, S.I.; Ferreira, D. Urolithins, intestinal microbial metabolites of pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agr. Food. Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef] [PubMed]
- Khaled, H.; Kais, M.; Zahra, A.; Ahmed, A.; Abdelfattah, E. Inhibition of key digestive enzymes related to diabetes and hyperlipidemia and protection of liver-kidney functions by trigonelline in diabetic rats. Sci. Pharmaceut. 2013, 81, 233–246. [Google Scholar]
- Hua, F.; Zhou, P.; Wu, H.; Chu, G.; Xie, Z.; Bao, G. Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar]
- Wang, L.; Liu, Y.; Luo, Y.; Huang, K.; Wu, Z. Quickly screening for potential α-glucosidase inhibitors from guava leaves tea by bio-affinity ultrafiltration coupled with HPLC-ESI-TOF/MS method. J. Agri. Food. Chem. 2018, 66, 1576–1582. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hogan, S.; Chung, H.; Welbaum, G.E.; Zhou, K. Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition. Food Chem. 2010, 119, 592–599. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseinighazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar]
- Sun, L.J.; Warren, F.J.; Gidley, M.J.; Guo, Y.L.; Miao, M. Mechanism of binding interactions between young apple polyphenols andporcine pancreatic α-amylase. Food Chem. 2019, 283, 468–474. [Google Scholar] [CrossRef]
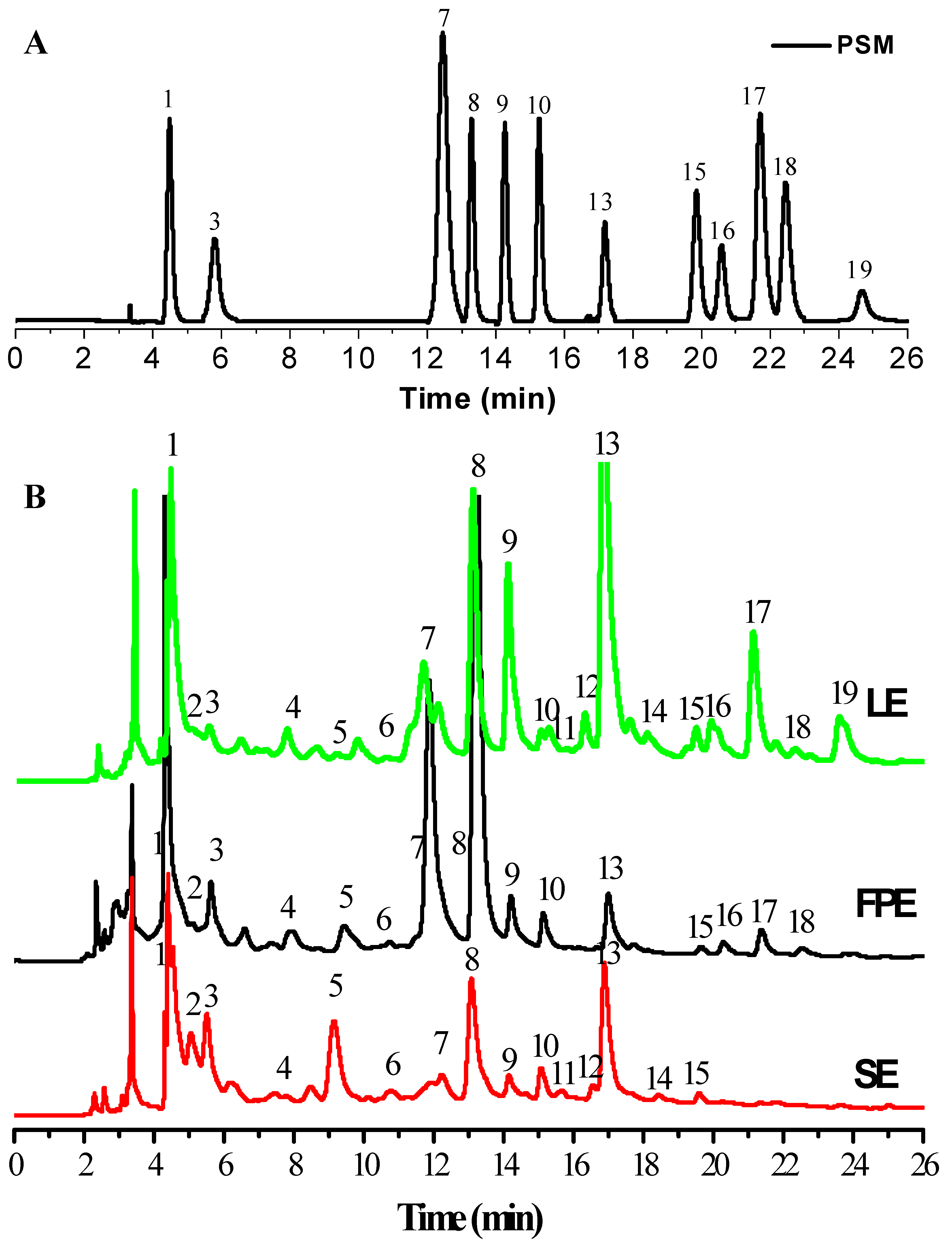
| Peak No. | Retention Time (min) | λmax (nm) | Molecular ion (m/z) | MS (m/z) | Mw | Formula | Error (ppm) | Compounds (Abbreviation) | Reference | LE | FPE | SE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.31 | 215, 271 | 169.2101[M-H]− | 169.1221 | 170 | C7H6O5 | −0.7 | Gallic acid (GA) | Standard | √ | √ | √ |
| 2 | 5.12 | 210, 268 | 353.2410[M+H]− | 191.0121, 98.9212 | 354 | C16H17O9 | 1.2 | Chlorogenic acid | MS/MS | √ | ||
| 3 | 5.86 | 208, 279 | 291.0869 [M+H]+ | 291.0869, 209.1545, 138.1423 | 290 | C15H14O6 | −0.4 | Epicatechin | Standard | √ | √ | √ |
| 4 | 7.87 | 215, 280 | 579.1503[M+H]+ | 579.1503, 291.0708 | 578 | C30H26O12 | 2.7 | Procyanidin dimer 1 | MS/MS | √ | √ | |
| 5 | 9.17 | 214, 280 | 579.1496[M+H]+ | 579.1496, 291.0503 | 578 | C30H26O12 | 0.5 | Procyanidin dimer 2 | MS/MS | √ | √ | |
| 6 | 10.78 | 218, 284 | 867.2129 [M+H]+ | 867.2131, 563.1548, 291.0294 | 866 | C45H38O18 | 2.2 | Procyanidin trimer 1 | MS/MS | √ | ||
| 7 | 11.98 | 220, 285 | 579.1514 [M+H]+ | 579.1514, 447.0508, 303.0134, 291.032 | 578 | C30H26O12 | 0.3 | Procyanidin B2 | Standard | √ | √ | |
| 8 | 13.19 | 225, 280 | 867.2140 [M+H]+ | 867.2131, 579.1502, 291.0155,185.0085 | 866 | C45H38O18 | 0.2 | Procyanidin C3 (PC) | Standard | √ | √ | √ |
| 9 | 14.27 | 253, 358 | 435.0564 [M+H]+ | 435.0564, 303.0142, 185.0085 | 434 | C19H14O12 | 0.9 | Ellagic acid pentoside | Standard | √ | √ | √ |
| 10 | 15.13 | 253, 354 | 611.1607 [M+H]+ | 611.1607, 449.0557, 303.0563 | 610 | C27H30O16 | −2.5 | Rutin | Standard | √ | √ | √ |
| 11 | 15.87 | 254, 351 | 611.1607 [M+H]+ | 611.1254, 449.1338, 435.0557, 287.0716 | 610 | C27H30O16 | −2.5 | kaempferol-galactoside-glucoside | MS/MS | √ | ||
| 12 | 16.81 | 257, 353 | 867.2147 [M+H]+ | 611.1607, 479.0557, 303.0515, 209.1547 | 610 | C27H30O16 | 1.7 | Quercetin-3-O-glucuronide-arabinoside | MS/MS | √ | √ | |
| 13 | 16.95 | 256, 351 | 303.0501 [M+H]+ | 303.0501, 193.1221 | 302 | C14H6O8 | −0.3 | Ellagic acid (EA) | Standard | √ | √ | √ |
| 14 | 18.21 | 254, 359 | 465.1032 [M+H]+ | 465.1032, 303.0498 | 464 | C21H20O12 | −0.5 | Quercetin 3-O-galactoside | MS/MS | √ | √ | |
| 15 | 19.67 | 254, 356 | 465.1037 [M+H]+ | 465.1037, 303.0506 | 464 | C21H20O12 | −0.7 | Quercetin 3-O-glucoside | Standard | √ | √ | √ |
| 16 | 21.57 | 262,391 | 435.0924 [M+H]+ | 435.0924, 303.0500, 219.1754 | 434 | C20H18O11 | −0.2 | Avicularin | Standard | √ | √ | |
| 17 | 20.42 | 253, 357 | 463.0807 [M+H]+ | 463.0807, 287.0546, 133.1526 | 462 | C21H18O12 | −0.6 | Kaempferol-7-O-glucuronide | Standard | √ | √ | |
| 18 | 22.72 | 254, 351 | 479.0827 [M+H]+ | 479.0829, 303.0506 | 478 | C21H18O13 | 0.2 | Quercetin-7-O-glucuronide | Standard | √ | √ | |
| 19 | 23.51 | 257, 363 | 463.0878 [M+H]+ | 463.0878, 317.0123, 287.0557 | 462 | C21H18O12 | −1.4 | Kaempferol-3-O-glucuronide | Standard | √ |
| Analytes | Contents (μg/g DW) | ||
|---|---|---|---|
| LE | FPE | SE | |
| Gallic acid | 539.42 ± 2.09c | 339.45 ± 2.17b | 127.15 ± 3.21a |
| Epicatechin | 3.47 ± 0.02b | 0.41 ± 0.07a | 0.32 ± 0.12a |
| * Procyanidin dimer 1 | 6.71 ± 0.07c | 4.39 ± 0.05b | 2.13 ± 0.09a |
| * Procyanidin dimer 2 | 1.79 ± 0.05a | 2.07 ± 0.03b | 13.35 ± 1.12c |
| Procyanidin B2 | 6.82 ± 0.12b | 21.72 ± 0.07c | 0.17 ± 0.02a |
| # Procyanidin trimer 1 | N.D. | 2.34 ± 0.01a | 3.25 ± 0.03a |
| Procyanidin C3 | 149.17 ± 0.01b | 252.37 ± 0.05c | 29.12 ± 0.11a |
| Ellagic acid pentoside | 67.88 ± 0.12c | 12.82 ± 0.09b | 5.87 ± 0.11a |
| Rutin | 2.53 ± 0.16a | 5.07 ± 0.07b | 4.89 ± 0.15b |
| Ellagic acid | 527.26 ± 3.27c | 95.42 ± 0.53b | 48.32 ± 0.23a |
| Quercetin 3-glucoside | 7.39 ± 0.03c | 4.35 ± 0.02b | 1.57 ± 0.23a |
| Avicularin | 35.87 ± 0.12b | 16.73 ± 0.09a | N.D. |
| Kaempferol-7-O-glucuronide | 21.31 ± 0.01b | 14.31 ± 0.02a | N.D. |
| Quercetin-7-O-glucuronide | 2.32 ± 0.05a | 2.14 ± 0.07a | N.D. |
| Kaempferol-3-O-glucuronide | 5.47 ± 0.02 | N.D. | N.D. |
| Digestive Enzymes | Main Phenolics | C-Score | T-Score | PMF-Score | CHEM-Score | G-Score | D-Score |
|---|---|---|---|---|---|---|---|
| α-Glucosidase | GA | 4 | 3.86 | −117.262 | −15.181 | −201.051 | −108.348 |
| EA | 5 | 1.66 | −143.165 | −25.320 | −169.858 | −134.180 | |
| PC | 4 | 5.58 | −169.841 | −20.602 | −259.179 | −186.583 | |
| Acarbose | 4 | 11.50 | −267.829 | −18.801 | −399.410 | −268.642 | |
| α-Amylase | GA | 5 | 4.61 | −100.463 | −14.772 | −137.871 | −77.144 |
| EA | 5 | 3.56 | −108.516 | −19.079 | −156.583 | −100.861 | |
| PC | 5 | 6.74 | −157.306 | −26.662 | −326.221 | −178.056 | |
| Acarbose | 5 | 7.07 | −174.749 | −7.459 | −311.510 | −211.197 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-qTOF-MS/MS Characterization, Antioxidant Activities and Inhibitory Ability of Digestive Enzymes with Molecular Docking Analysis of Various Parts of Raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. https://doi.org/10.3390/antiox8080274
Wu L, Liu Y, Qin Y, Wang L, Wu Z. HPLC-ESI-qTOF-MS/MS Characterization, Antioxidant Activities and Inhibitory Ability of Digestive Enzymes with Molecular Docking Analysis of Various Parts of Raspberry (Rubus ideaus L.). Antioxidants. 2019; 8(8):274. https://doi.org/10.3390/antiox8080274
Chicago/Turabian StyleWu, Lingfeng, Yufeng Liu, Yin Qin, Lu Wang, and Zhenqiang Wu. 2019. "HPLC-ESI-qTOF-MS/MS Characterization, Antioxidant Activities and Inhibitory Ability of Digestive Enzymes with Molecular Docking Analysis of Various Parts of Raspberry (Rubus ideaus L.)" Antioxidants 8, no. 8: 274. https://doi.org/10.3390/antiox8080274
APA StyleWu, L., Liu, Y., Qin, Y., Wang, L., & Wu, Z. (2019). HPLC-ESI-qTOF-MS/MS Characterization, Antioxidant Activities and Inhibitory Ability of Digestive Enzymes with Molecular Docking Analysis of Various Parts of Raspberry (Rubus ideaus L.). Antioxidants, 8(8), 274. https://doi.org/10.3390/antiox8080274