Bioactive Compounds and Antioxidant Activity of Mango Peel Liqueurs (Mangifera indica L.) Produced by Different Methods of Maceration
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Raw Material
2.3. Production of the Liqueurs
2.4. Physicochemical Analysis and Color Measurement by CIEL*a*b* System
2.5. Determination of the Bioactive Compounds Profile by RP-HPLC/DAD/FD
2.6. Total Bioactive Content and In Vitro Antioxidant Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Quality Parameters
3.2. Total Phenolic Content and Bioactive Profile in Mango Peel Liqueurs
3.2.1. Flavanols
3.2.2. Flavonols and Trans-Resveratrol
3.2.3. Phenolic Acids
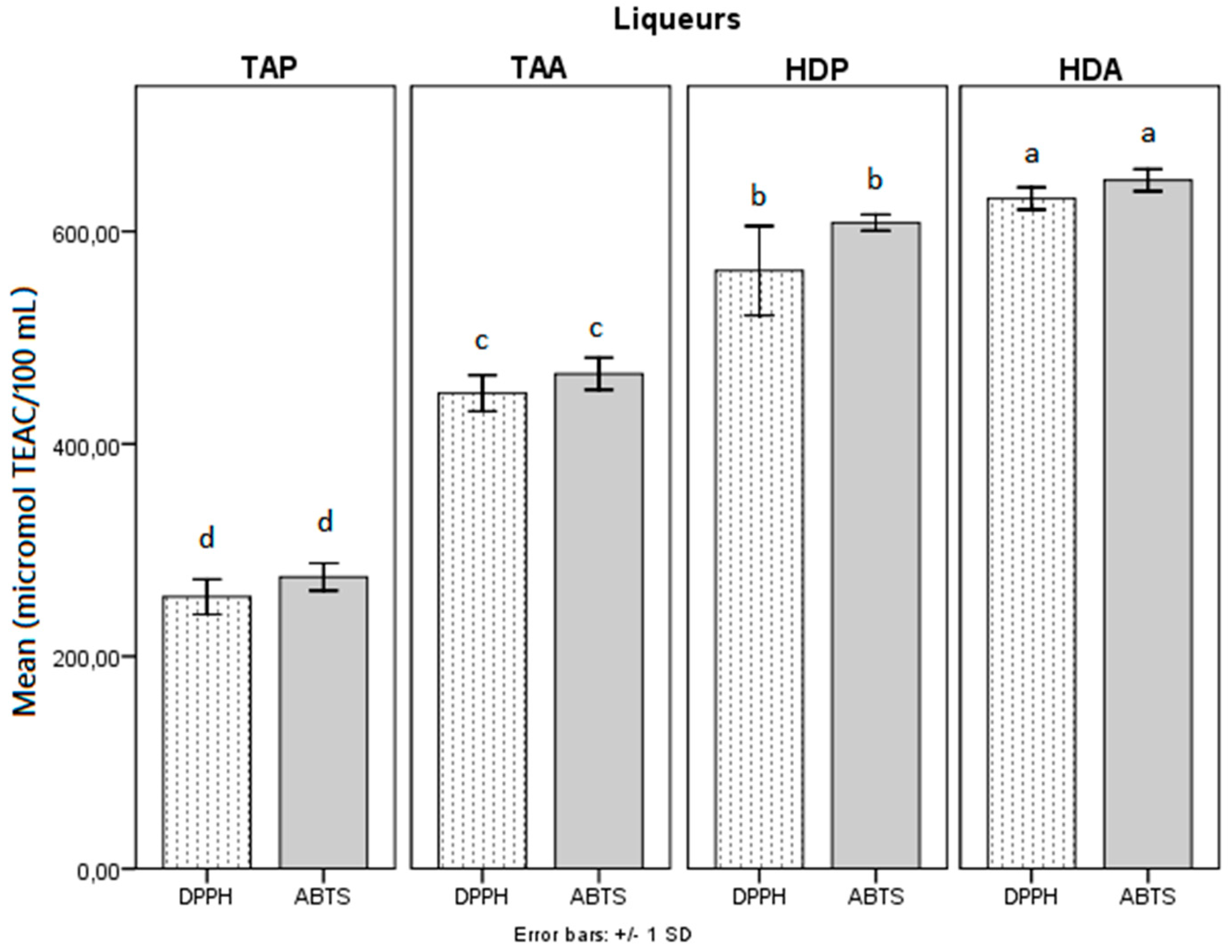
3.3. In Vitro Antioxidant Activity of Mango Peel Liqueurs
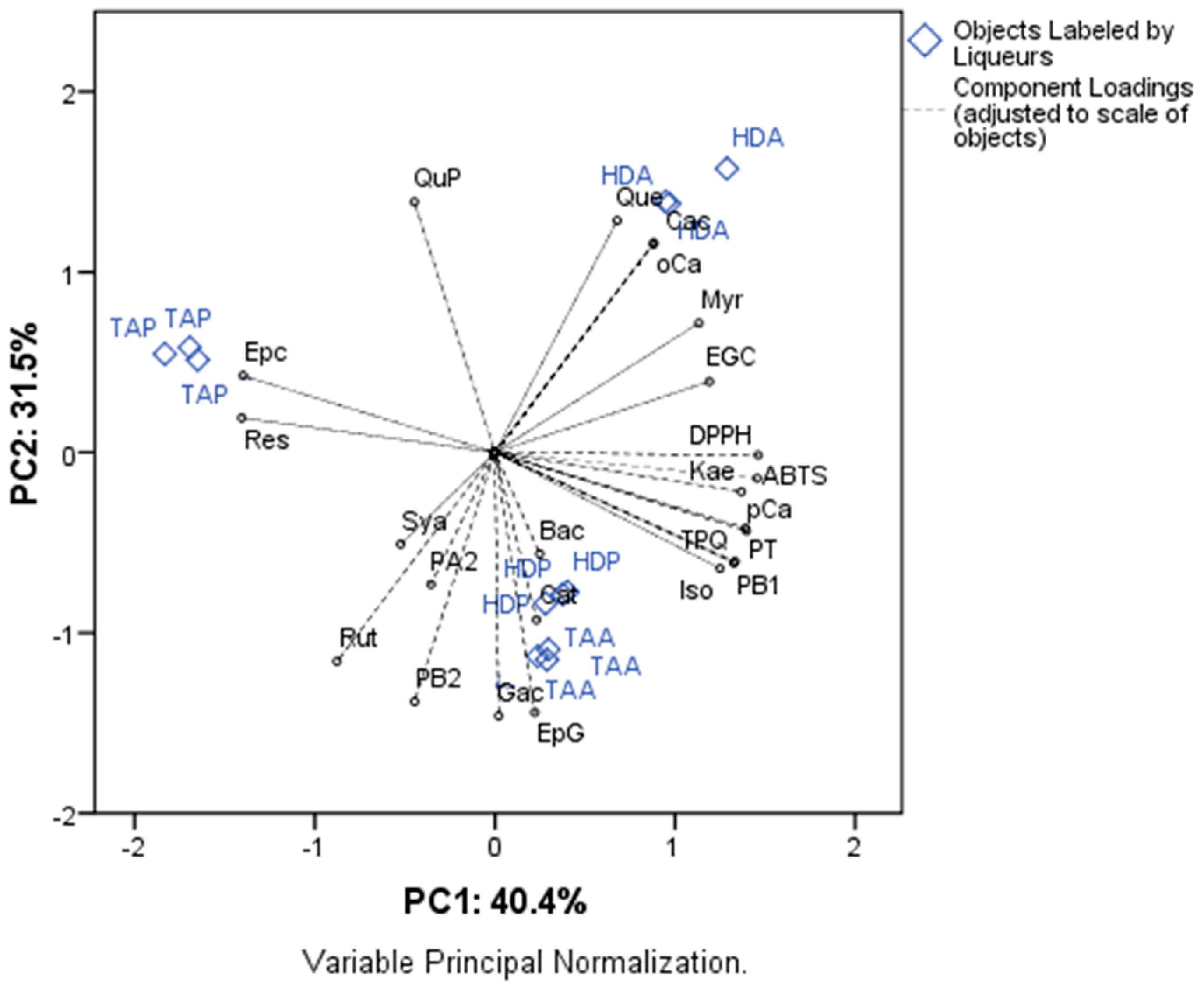
3.4. Principal Components Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Use of HPLC- and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.M.R.; Barbosa, L.C.A.; Queiroz, J.H.; Knödlerd, M.; Schieber, A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 2008, 110, 620–626. [Google Scholar] [CrossRef]
- Arbos, K.A.; Stevani, P.C.; de Fátima Castanha, R. Atividade antimicrobiana, antioxidante e teor de compostos fenólicos em casca e amêndoa de frutos de manga. Rev. Ceres 2013, 60, 161–165. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Denoya, G.I.; Sivakumar, D.; Polenta, G.A.; Soundy, P. Comparison of the contents of bioactive compounds and quality parameters in selected mango cultivars. J. Food Qual. 2013, 36, 394–402. [Google Scholar] [CrossRef]
- Liu, F.; Fu, S.; Bi, X.; Chen, F.; Liao, X.; Hu, X.; Wu, J. Physico-Chemical and antioxidant properties of four mango (Mangifera indica) cultivars in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef]
- Masibo, M.; He, A.Q. Mango Bioactive Compounds and Related Nutraceutical Properties—A Review. Food Rev. Int. 2009, 25, 346–370. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.M. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Sokoł-Łetowska, A.; Kucharska, A.Z.; Wińskab, K.; Szumny, A.; Nawirska-Olszańskaa, A.; Mizgier, P.; Wyspiańskaa, D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef]
- Summo, C.; Trani, A.; Faccia, M.; Caponio, F.; Gambacorta, G. Volatiles and acceptability of liqueurs from kumquat and grapefruit. Ital. J. Food Sci. 2016, 28, 258–270. [Google Scholar]
- Rodríguez-Solana, R.; Vázquez-Araújo, L.; Salgado, J.M.; Domíngueza, J.M.; Cortés-Diégueza, S. Optimization of the process of aromatic and medicinal plant maceration in grape marc distillates to obtain herbal liqueurs and spirits. J. Sci. Food Agric. 2016, 96, 4760–4771. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.N.A.; Santos, D.C.; Gomes, J.P.; Rocha, A.P.T.; Albuquerque, E.M.B. Estabilidade física e química de licores de graviola durante o armazenamento em condições ambientais. Rev. Bras. Eng. Agric. Ambient.-Agriambi 2015, 19, 245–251. [Google Scholar] [CrossRef]
- Magro, L.D.; Goetze, D.; Ribeiro, C.T.; Paludo, N.; Rodrigues, E.; Hertz, P.F.; Klein, M.P.; Rodrigues, R.C. Identification of Bioactive Compounds from Vitis labrusca L. Variety Concord Grape Juice Treated with Commercial Enzymes: Improved Yield and Quality Parameters. Food Bioprocess Technol. 2016, 9, 365–377. [Google Scholar] [CrossRef]
- Lima, M.S.; Dutra, M.C.P.; Toaldo, I.M.; Corrêa, L.C.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic Compounds, Organic Acids and Antioxidant Activity of Grape Juices Produced in Industrial Scale by Different Processes of Maceration. Food Chem. 2015, 188, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.M.; Azevedo, L.C.; Correa, L.C.; Bordignon-Luiz, M.T.; Lima, M.d.S. Phenolic profile, organic acids and antioxidant activity of frozen pulp and juice of the jambolan (Syzygium cumini). J. Food Biochem. 2016, 40, 211–219. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1997. [Google Scholar]
- Natividade, M.M.P.; Correa, L.C.; Souza, S.V.C.; Pereira, G.E.; Lima, L.C.D.O. Simultaneous analysis of 25 phenolic compounds in grape juice for HPLC: Method validation and characterization of São Francisco Valley samples. Microchem. J. 2013, 110, 665–674. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Kim, Y.K.; Guo, Q.; Packer, L. Free radical scavenging activity of red ginseng aqueous extracts. Toxicology 2002, 172, 149–156. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites 2018, 8, 74. [Google Scholar] [CrossRef]
- Sultana, B.; Hussain, Z.; Asif, M.; Minir, A. Investigation on the Antioxidant Activity of Leaves, Peels, Stems Bark, and Kernel of Mango (Mangifera indica L.). J. Food Sci. 2012, 77, C840–C852. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of Mango Byproducts: Study of the Effect of Extraction Solvent and Temperature on Their Antioxidant Properties. J. Food Sci. 2012, 71, C80–C88. [Google Scholar] [CrossRef]
- Meneses, M.A.; Caputo, G.; Scognamiglio, M.; Reverchon, E.; Adami, R. Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. J. Food Eng. 2015, 163, 43–53. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Yahia, E.M.; Gonzalez-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC-DAD-MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Leeuw, R.V.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant capacity and phenolic composition of red wines from various grape varieties: Specificity of Pinot Noir. J. Food Compos. Anal. 2014, 36, 40–50. [Google Scholar] [CrossRef]
- Silva, G.G.; Dutra, M.d.C.P.; de Oliveira, J.B.; Rybka, A.C.P.; Pereira, G.E.; dos Santos Lima, M. Processing methods with heat increases bioactive phenolic compounds and antioxidant activity in grape juices. J. Food Biochem. 2019, 43, e12732. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Ionica, M.; Tutulescu, F. Phenolics Content, Antioxidant Activity and Color of Green Walnut Extracts for Preparing Walnut Liquor. Not. Bot. Hort. Agrobot. Cluj-Napoca 2014, 42, 551–555. [Google Scholar] [CrossRef]
| Liqueurs | Tommy Atkins | Haden | ||
|---|---|---|---|---|
| Maceration Treatments | Alcoholic | Pectinase | Alcoholic | Pectinase |
| pH | 4.85 ± 0.08 b | 3.61 ± 0.02 d | 5.01 ± 0.06 a | 3.94 ± 0.05 c |
| Titratable acidity (g L−1) | 1.0 ± 0.0 b | 4.4 ± 0.0 a | 1.1 ± 0.0 b | 4.6 ± 0.0 a |
| Total sugars % | 14.9 ± 0.1 a | 15.0 ± 0.1 a | 15.0 ± 0.1 a | 15.0 ± 0.2 a |
| Alcoholic strength % (v/v) | 18.2 ± 0.3 a | 18.0 ± 0.2 a | 17.9 ± 0.3 a | 18.0 ± 0.2 a |
| Colour | ||||
| L* | 59 ± 1 c | 62 ± 1 b | 55 ± 1 d | 70 ± 2 a |
| a* | 8.2 ± 0.2 a | 6.1 ± 0.2 b | 8.6 ± 0.1 a | 1.2 ± 0.1 c |
| b* | 80.7 ± 0.9 a | 71.5 ± 0.7 b | 63.3 ± 0.2 c | 37.3 ± 0.8 d |
| Liqueurs | Tommy Atkins | Haden | ||
|---|---|---|---|---|
| Maceration Treatments | Pectinase | Alcoholic | Pectinase | Alcoholic |
| FLAVANOLS | ||||
| (+)-Catechin | 12.0 ± 0.1 c | 141.3 ± 2.3 a | 16.0 ± 0.0 b | 8.0 ± 0.1 d |
| (−)-Epicatechin | 22.0 ± 0.3 a | 14.0 ± 0.2 c | 13.3 ± 1.1 c | 20.0 ± 0.3 b |
| (−)-Epicatechin gallate | 406.7 ± 0.0 b | 776.7 ± 12.2 a | 266.2 ± 17.4 c | 718.7 ± 15.1 a |
| (−)-Epigallocatechin gallate | 64 ± 0 c | 141.7 ±17.5 a,b | 174 ± 14 a | 134 ± 2 b |
| Procyanidin A2 | 36.7 ± 1.1 b | 78 ± 2 a | 22.0 ± 0.0 c | 14.0 ± 0.8 d |
| Procyanidin B1 | 40 ± 0 c | 88 ± 1 a | 29.3 ± 1.1 d | 80 ± 1 b |
| Procyanidin B2 | ND | 8.7 ± 1.1 a | 4.0 ± 0.1 b | 10.0 ± 0.1 a |
| Total Flavanols quantification | 581 ± 9 | 1254 ± 26 | 541 ± 30 | 950 ± 67 |
| FLAVONOLS | ||||
| Kaempferol 3-glucoside | 23.3 ± 1.1 c | 30.7 ± 2.3 b,c | 41.3 ± 9.2 a,b | 46.0 ± 0.1 a |
| Myricetin | 6.7 ± 1.1 c | 13.3 ± 1.1 b | 17.3 ± 2.3 a | 8.0 ± 0.1 c |
| Isorhamnetin | 11.3 ± 1.1 b | 24.0 ± 4.0 a | 20.7 ± 6.4 a,b | 30.7 ± 1.1 a |
| Rutin | 42.0 ± 0.2 b | 39.3 ± 1.1 b | 14 ± 2 c | 58.0 ± 0.1 a |
| Quercetin 3-glucoside | 14.0 ± 0.0 b | 6.0 ± 0.1 c | 22.0 ± 0.1 a | 6.0 ± 0.1 c |
| Quercetin-3-O-glucopyranoside | 343.3 ± 1.1 a | 14.0 ± 3.5 c | 348.7 ± 18.6 a | 42.7 ± 1.1 b |
| Total Flavonols quantification | 4401 ± 2 | 127 ± 5 | 464 ± 14 | 191 ± 1 |
| STILBENE | ||||
| trans-Resveratrol | 4.0 ± 0.3 a | ND | 2.0 ± 0.2 b | 2.0 ± 0.1 b |
| PHENOLIC ACIDS | ||||
| Gallic acid | 18.0 ± 8.3 d | 2271 ± 12 b | 1225.3 ± 12.8 c | 7512 ± 28 a |
| Cinnamic acid | 8.0 ± 0.1 c | 45.3 ± 9.4 b | 79.3 ± 2.3 a | 4.7 ± 1.1 c |
| p-Coumaric acid | 2.0 ± 0.1 b | 72 ± 4 a | 4.0 ± 0.3 b | 4.0 ± 0.1 b |
| o-Coumaric acid | 20.0 ± 0.2 c | 114 ± 6 b | 306.0 ± 14.4 a | 21.3 ± 1.1 c |
| Benzoic acid | ND | ND | ND | 1777.3 ± 7.6 a |
| Syringic acid | 96.0 ± 3.5 b | 391.3 ± 21.4 a | ND | 90 ± 2 b |
| Total phenolics acids quantification | 145 ± 9 | 2530 ± 45 | 1615 ± 29 | 9269 ± 2 |
| Total phenolics quantification by HPLC | 1167 ± 6 | 4303 ± 31 | 2622 ± 25 | 10523 ± 129 |
| Total Phenolics § | 38,758 ± 133 b | 64,787 ± 170 a | 63,479 ± 116 a | 70,564 ± 186 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, E.M.; de Souza, M.E.A.O.; Corrêa, L.C.; Viana, A.C.; de Azevêdo, L.C.; dos Santos Lima, M. Bioactive Compounds and Antioxidant Activity of Mango Peel Liqueurs (Mangifera indica L.) Produced by Different Methods of Maceration. Antioxidants 2019, 8, 102. https://doi.org/10.3390/antiox8040102
Coelho EM, de Souza MEAO, Corrêa LC, Viana AC, de Azevêdo LC, dos Santos Lima M. Bioactive Compounds and Antioxidant Activity of Mango Peel Liqueurs (Mangifera indica L.) Produced by Different Methods of Maceration. Antioxidants. 2019; 8(4):102. https://doi.org/10.3390/antiox8040102
Chicago/Turabian StyleCoelho, Emanuela Monteiro, Marcelo Eduardo Alves Olinda de Souza, Luiz Claudio Corrêa, Arão Cardoso Viana, Luciana Cavalcanti de Azevêdo, and Marcos dos Santos Lima. 2019. "Bioactive Compounds and Antioxidant Activity of Mango Peel Liqueurs (Mangifera indica L.) Produced by Different Methods of Maceration" Antioxidants 8, no. 4: 102. https://doi.org/10.3390/antiox8040102
APA StyleCoelho, E. M., de Souza, M. E. A. O., Corrêa, L. C., Viana, A. C., de Azevêdo, L. C., & dos Santos Lima, M. (2019). Bioactive Compounds and Antioxidant Activity of Mango Peel Liqueurs (Mangifera indica L.) Produced by Different Methods of Maceration. Antioxidants, 8(4), 102. https://doi.org/10.3390/antiox8040102





