Synechococcus sp. PCC 7002 Performs Anoxygenic Photosynthesis and Deploys Divergent Strategies to Cope with H2Sn and H2O2
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Verification of Anoxygenic Photosynthesis
2.3. Induction Experiments with H2Sn and H2O2
2.4. RNA Extraction and Transcriptome Sequencing
2.5. RNA-Sequencing Analysis
2.6. Tolerance of Synechococcus sp. PCC7002 to H2Sn and H2O2
2.7. Data Availability
3. Results
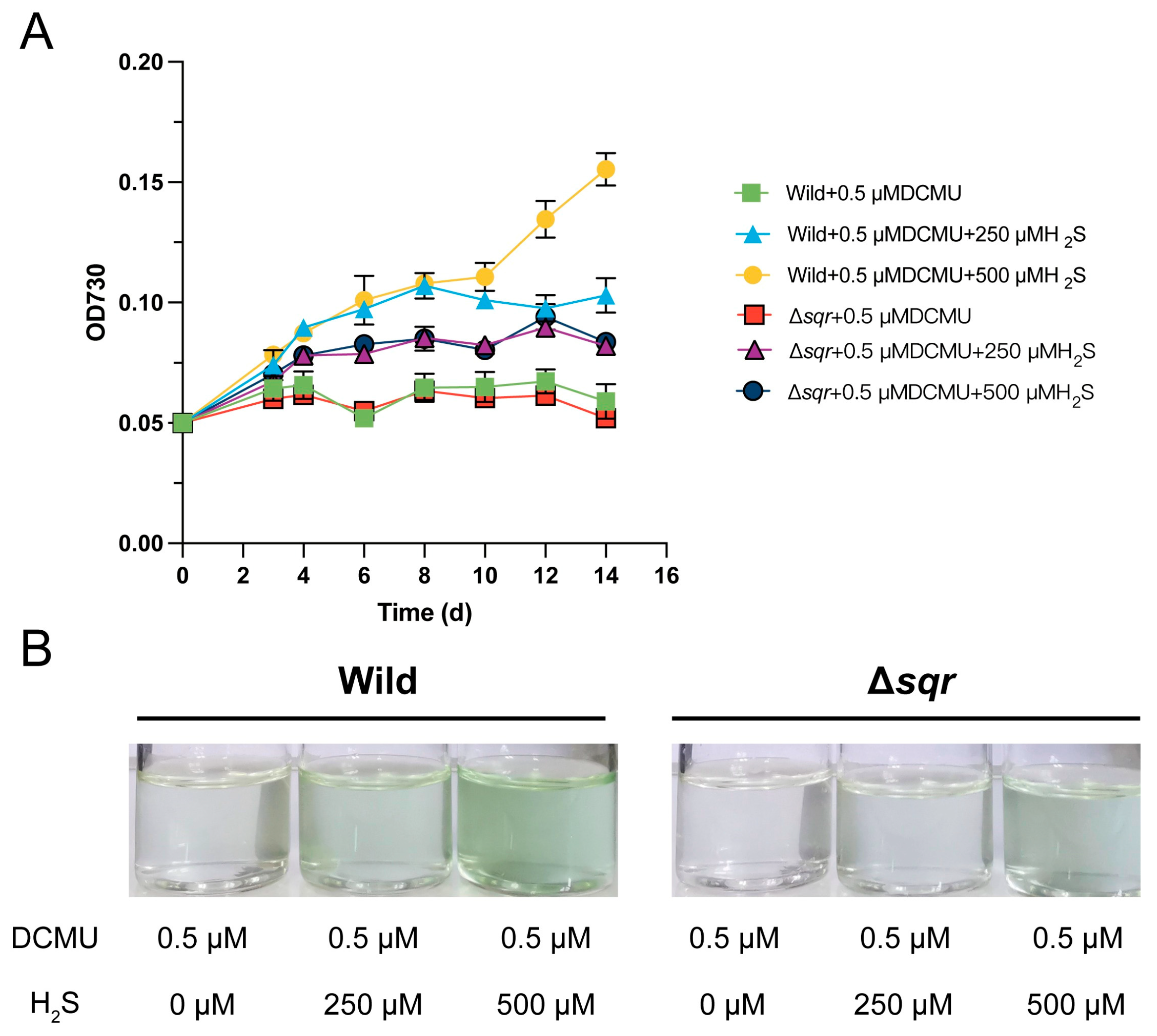
3.1. Synechococcus sp. PCC7002 Performed Anoxygenic Photosynthesis
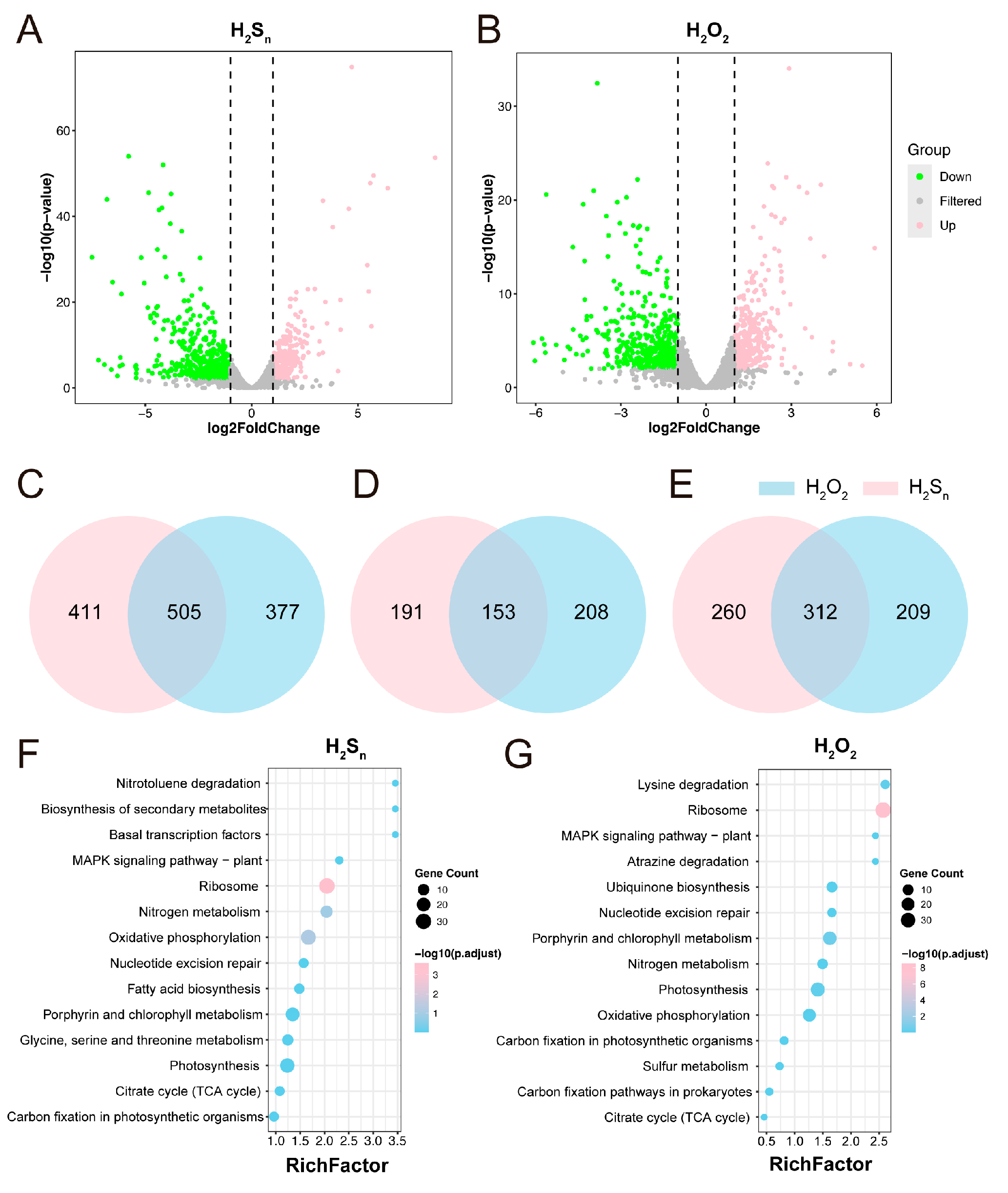
3.2. The Transcriptional Response of Synechococcus sp. 7002 to H2Sn and H2O2
3.3. The Effect of H2Sn and H2O2 on Photosynthesis of Synechococcus sp. PCC7002
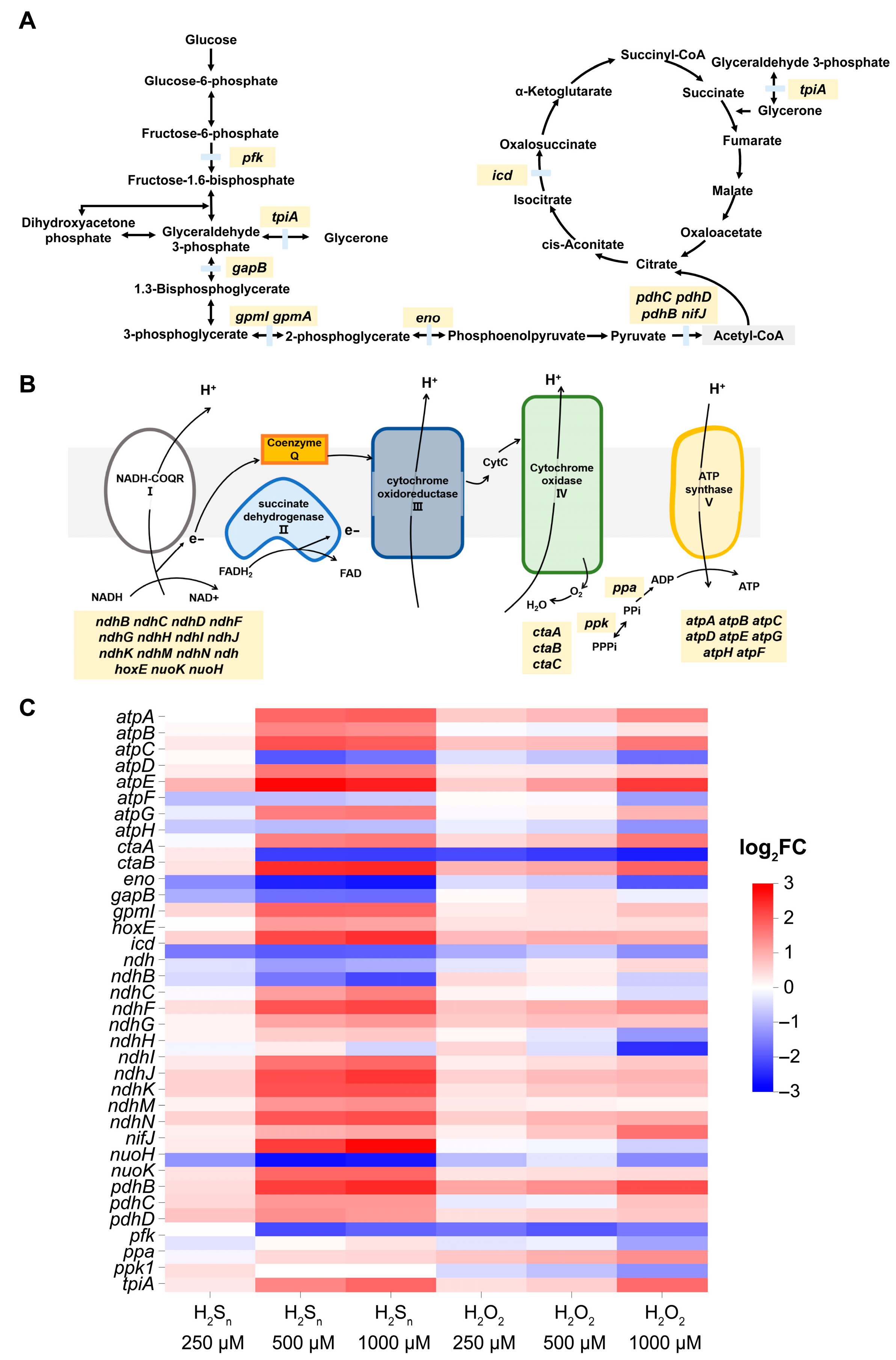
3.4. The Effect of H2Sn and H2O2 on TCA Cycle, Glycolysis, and Oxidative Phosphorylation of Synechococcus sp. PCC7002
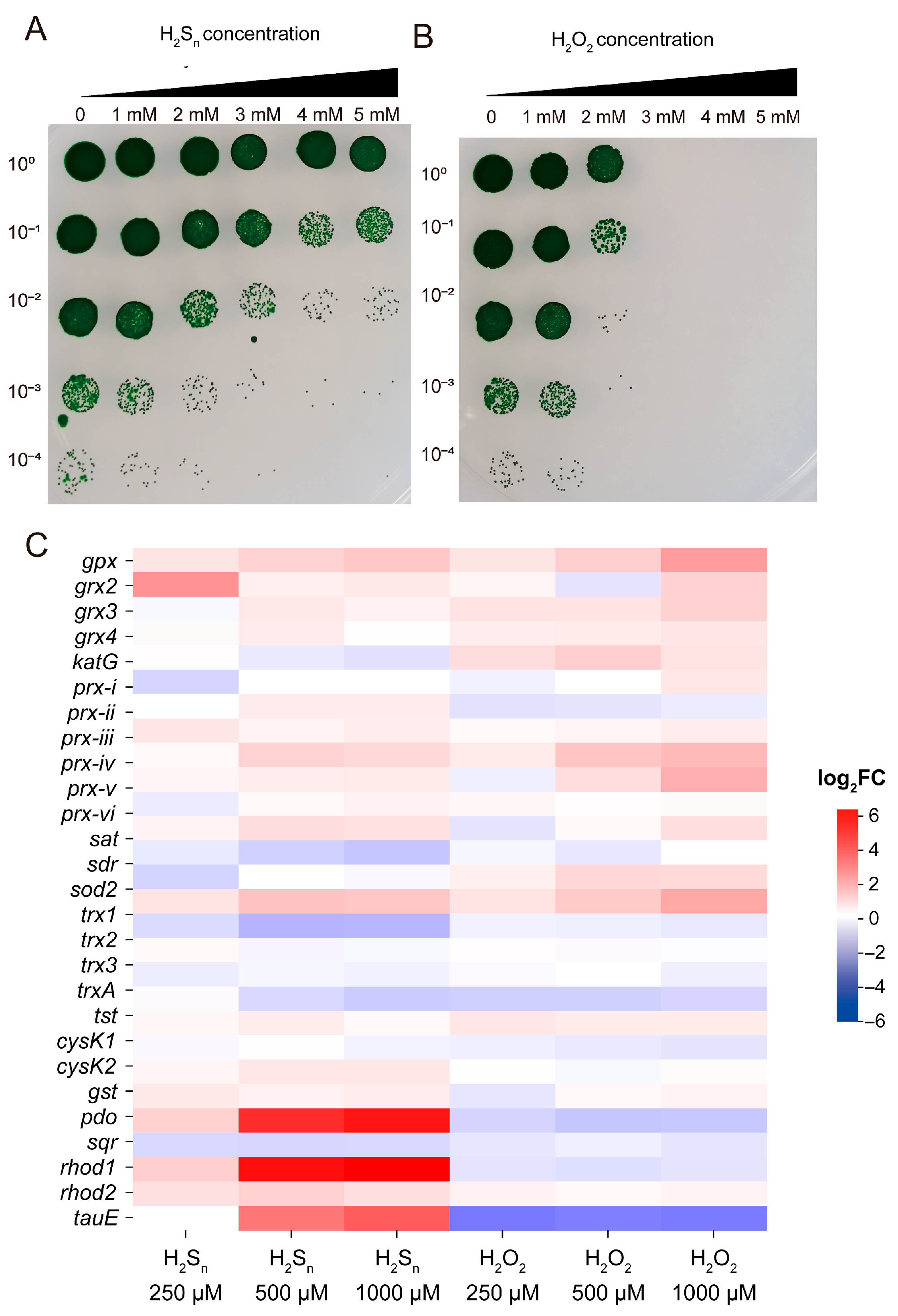
3.5. Effects of High Concentrations of H2Sn and H2O2 on Synechococcus sp. PCC7002 Growth and Their Tolerance Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Baracaldo, P.; Bianchini, G.; Wilson, J.D.; Knoll, A.H. Cyanobacteria and biogeochemical cycles through Earth history. Trends Microbiol. 2022, 30, 143–157. [Google Scholar] [CrossRef]
- Grim, S.L.; Voorhies, A.A.; Biddanda, B.A.; Jain, S.; Nold, S.C.; Green, R.; Dick, G.J. Omics-Inferred Partitioning and Expression of Diverse Biogeochemical Functions in a Low-O2 Cyanobacterial Mat Community. mSystems 2021, 6, e01042-21. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Liu, L.; Jiao, J.Y.; Li, M.M.; Hu, C.J.; Lv, A.P.; Qi, Y.L.; Li, Y.X.; Rao, Y.Z.; Qu, Y.N.; et al. Exploring the Origins and Evolution of Oxygenic and Anoxygenic Photosynthesis in Deeply Branched Cyanobacteriota. Mol. Biol. Evol. 2024, 41, msae151. [Google Scholar] [CrossRef] [PubMed]
- Soo, R.M.; Hemp, J.; Hugenholtz, P. Evolution of photosynthesis and aerobic respiration in the cyanobacteria. Free Radic. Biol. Med. 2019, 140, 200–205. [Google Scholar] [CrossRef]
- Kushkevych, I.; Bosáková, V.; Vítězová, M.; Rittmann, S.K.M.R. Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide. Antioxidants 2021, 10, 829. [Google Scholar] [CrossRef]
- Klatt Judith, M.; Al-Najjar Mohammad, A.A.; Yilmaz, P.; Lavik, G.; de Beer, D.; Polerecky, L. Anoxygenic Photosynthesis Controls Oxygenic Photosynthesis in a Cyanobacterium from a Sulfidic Spring. Appl. Environ. Microbiol. 2015, 81, 2025–2031. [Google Scholar] [CrossRef]
- Callieri, C. Synechococcus plasticity under environmental changes. FEMS Microbiol. Lett. 2017, 364, fnx229. [Google Scholar] [CrossRef]
- Shabana, E.F.; Ali, G.H. Phytoplankton activities in hypersaline, anoxic conditions. II-Photosynthetic activity of some sulphide adapted cyanobacterial strains isolated from Solar Lake, Taba, Egypt. Water Sci. Technol. 1999, 40, 127–132. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Lau, N.; Pluth, M.D. Reactive sulfur species (RSS): Persulfides, polysulfides, potential, and problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Giedroc, D.P.; Antelo, G.T.; Fakhoury, J.N.; Capdevila, D.A. Sensing and regulation of reactive sulfur species (RSS) in bacteria. Curr. Opin. Chem. Biol. 2023, 76, 102358. [Google Scholar] [CrossRef]
- Wu, G.; Niu, X.; Chen, J.; Wu, C.; Li, Y.; Li, Y.; Cui, D.; He, X.; Wang, F.; Li, S. Hydrogen Sulfide Alleviates Oxidative Damage Under Chilling Stress Through Mitogen-Activated Protein Kinase in Tomato. Antioxidants 2024, 13, 323. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants Under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Landry, A.P.; Ballou, D.P.; Banerjee, R. Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase. Chembiochem 2021, 22, 949–960. [Google Scholar] [CrossRef]
- Liu, H.; Xin, Y.; Xun, L. Distribution, diversity, and activities of sulfur dioxygenases in heterotrophic bacteria. Appl. Environ. Microbiol. 2014, 80, 1799–1806. [Google Scholar] [CrossRef]
- Lü, C.; Xia, Y.; Liu, D.; Zhao, R.; Gao, R.; Liu, H.; Xun, L. Cupriavidus necator H16 Uses Flavocytochrome c Sulfide Dehydrogenase to Oxidize Self-Produced and Added Sulfide. Appl. Environ. Microbiol. 2017, 83, e01610-17. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Zhang, X.; Xin, Y.; Xia, Y.; Xun, L.; Liu, H. Rhodanese Rdl2 produces reactive sulfur species to protect mitochondria from reactive oxygen species. Free Radic. Biol. Med. 2021, 177, 287–298. [Google Scholar] [CrossRef]
- Xia, Y.; Lü, C.; Hou, N.; Xin, Y.; Liu, J.; Liu, H.; Xun, L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, J.; Lü, C.; Xia, Y.; Liu, H.; Jiao, N.; Xun, L.; Liu, J. Synechococcus sp. Strain PCC7002 Uses Sulfide:Quinone Oxidoreductase to Detoxify Exogenous Sulfide and to Convert Endogenous Sulfide to Cellular Sulfane Sulfur. mBio 2020, 11, e03420-19. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, R.; Cui, F.; Lü, C.; Liu, H.; Liu, H.; Xia, Y.; Xun, L. The Heterotrophic Bacterium Cupriavidus pinatubonensis JMP134 Oxidizes Sulfide to Sulfate with Thiosulfate as a Key Intermediate. Appl. Environ. Microbiol. 2020, 86, e01835-20. [Google Scholar] [CrossRef]
- Hou, N.; Yan, Z.; Fan, K.; Li, H.; Zhao, R.; Xia, Y.; Xun, L.; Liu, H. OxyR senses sulfane sulfur and activates the genes for its removal in Escherichia coli. Redox Biol. 2019, 26, 101293. [Google Scholar] [CrossRef]
- Liu, D.; Song, H.; Li, Y.; Huang, R.; Liu, H.; Tang, K.; Jiao, N.; Liu, J. The Transcriptional Repressor PerR Senses Sulfane Sulfur by Cysteine Persulfidation at the Structural Zn2+ Site in Synechococcus sp. PCC7002. Antioxidants 2023, 12, 423. [Google Scholar] [CrossRef]
- Stevens, S.E.; Porter, R.D. Transformation in Agmenellum quadruplicatum. Proc. Natl. Acad. Sci. USA 1980, 77, 6052–6056. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kalvari, I.; Nawrocki, E.P.; Ontiveros-Palacios, N.; Argasinska, J.; Lamkiewicz, K.; Marz, M.; Griffiths-Jones, S.; Toffano-Nioche, C.; Gautheret, D.; Weinberg, Z.; et al. Rfam 14: Expanded coverage of metagenomic, viral and microRNA families. Nucleic Acids Res. 2021, 49, D192–D200. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple hypothesis testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- The Gene Ontology, C.; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- McClure, R.; Balasubramanian, D.; Sun, Y.; Bobrovskyy, M.; Sumby, P.; Genco, C.A.; Vanderpool, C.K.; Tjaden, B. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res. 2013, 41, e140. [Google Scholar] [CrossRef]
- Bryant, D.A.; Gisriel, C.J. The structural basis for light harvesting in organisms producing phycobiliproteins. Plant Cell 2024, 36, 4036–4064. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zheng, Z.; Li, X.; Wang, G.; Zhang, K.; Wei, P.; Zhao, J.; Gao, N. Structural insight into the mechanism of energy transfer in cyanobacterial phycobilisomes. Nat. Commun. 2021, 12, 5497. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Klatt, J.M.; de Beer, D.; Macalady, J.L. Cyanobacterial photosynthesis under sulfidic conditions: Insights from the isolate Leptolyngbya sp. strain hensonii. ISME J. 2018, 12, 568–584. [Google Scholar] [CrossRef]
- de Beer, D.; Weber, M.; Chennu, A.; Hamilton, T.; Lott, C.; Macalady, J.; Klatt, J.M. Oxygenic and anoxygenic photosynthesis in a microbial mat from an anoxic and sulfidic spring. Environ. Microbiol. 2017, 19, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.T.; Wolfe-Simon, F.; Pearson, A.; Knoll, A.H. Anoxygenic photosynthesis modulated Proterozoic oxygen and sustained Earth’s middle age. Proc. Natl. Acad. Sci. USA 2009, 106, 16925–16929. [Google Scholar] [CrossRef]
- Grim, S.L.; Dick, G.J. Photosynthetic Versatility in the Genome of Geitlerinema sp. PCC 9228 (Formerly Oscillatoria limnetica ‘Solar Lake’), a Model Anoxygenic Photosynthetic Cyanobacterium. Front. Microbiol. 2016, 7, 1546. [Google Scholar] [CrossRef] [PubMed]
- Soo, R.M.; Hemp, J.; Parks, D.H.; Fischer, W.W.; Hugenholtz, P. On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science 2017, 355, 1436–1440. [Google Scholar] [CrossRef]
- Mulo, P.; Sicora, C.; Aro, E.-M. Cyanobacterial psbA gene family: Optimization of oxygenic photosynthesis. Cell. Mol. Life Sci. 2009, 66, 3697–3710. [Google Scholar] [CrossRef] [PubMed]
- Mulo, P.; Sakurai, I.; Aro, E.M. Strategies for psbA gene expression in cyanobacteria, green algae and higher plants: From transcription to PSII repair. Biochim. Et Biophys. Acta 2012, 1817, 247–257. [Google Scholar] [CrossRef]
- Schneider, D.; Berry, S.; Volkmer, T.; Seidler, A.; Rögner, M. PetC1 is the major Rieske iron-sulfur protein in the cytochrome b6f complex of Synechocystis sp. PCC 6803. J. Biol. Chem. 2004, 279, 39383–39388. [Google Scholar] [CrossRef]
- Wang, H.; Moussa, M.G.; Huang, W.; Han, D.; Dang, B.; Hao, H.; Zhang, L.; Xu, Z.; Jia, W. Exogenous hydrogen sulfide increased Nicotiana tabacum L. resistance against drought by the improved photosynthesis and antioxidant system. Sci. Rep. 2024, 14, 25534. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.; Yue, Z.; Wang, J.; Chen, T.; Li, J.; Dai, H.; Dou, T.; Yu, J.; Liu, Z. Effect of hydrogen sulfide on cabbage photosynthesis under black rot stress. Plant Physiol. Biochem. 2024, 208, 108453. [Google Scholar] [CrossRef]
- Klatt, J.M.; Haas, S.; Yilmaz, P.; de Beer, D.; Polerecky, L. Hydrogen sulfide can inhibit and enhance oxygenic photosynthesis in a cyanobacterium from sulfidic springs. Environ. Microbiol. 2015, 17, 3301–3313. [Google Scholar] [CrossRef]
- Qian, H.; Yu, S.; Sun, Z.; Xie, X.; Liu, W.; Fu, Z. Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat. Toxicol. 2010, 99, 405–412. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Z.; Kong, F.; Zhang, M.; Yu, Y.; Shi, X. Growth, physiochemical and antioxidant responses of overwintering benthic cyanobacteria to hydrogen peroxide. Environ. Pollut. 2016, 219, 649–655. [Google Scholar] [CrossRef]
- Huang, D.; Jing, G.; Zhu, S. Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants 2023, 12, 1644. [Google Scholar] [CrossRef]
- Zini, R.; Berdeaux, A.; Morin, D. The differential effects of superoxide anion, hydrogen peroxide and hydroxyl radical on cardiac mitochondrial oxidative phosphorylation. Free Radic. Res. 2007, 41, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Hirst, J. Mitochondrial Complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Módis, K.; Ju, Y.; Ahmad, A.; Untereiner, A.A.; Altaany, Z.; Wu, L.; Szabo, C.; Wang, R. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016, 113, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Liu, J.; Cao, B.; Li, B.; Tian, S. Hydrogen peroxide acts on sensitive mitochondrial proteins to induce death of a fungal pathogen revealed by proteomic analysis. PLoS ONE 2011, 6, e21945. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Gao, Y.; Arif, F.; Arora, K.; Patel, S.; DeLeon, E.R.; Sutton, T.R.; Feelisch, M.; Cortese-Krott, M.M.; Straub, K.D. Metabolism of hydrogen sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by superoxide dismutase. Redox Biol. 2018, 15, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Arif, M.; Arif, F.; Arora, N.; Straub, K.D. Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biol. 2017, 12, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Takata, T.; Clear, K.J.; Gao, Y.; Ma, Z.; Pfaff, E.; Mouli, K.; Kent, T.A.; Jones, P., Jr.; Fukuto, J.; et al. The SOD1 Inhibitor, LCS-1, Oxidizes H2S to Reactive Sulfur Species, Directly and Indirectly, Through Conversion of SOD1 to an Oxidase. Antioxidants 2024, 13, 991. [Google Scholar] [CrossRef]
- Olson, K.R. Reactive oxygen species or reactive sulfur species: Why we should consider the latter. J. Exp. Biol. 2020, 223, jeb196352. [Google Scholar] [CrossRef]
- Olson, K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid. Redox Signal. 2020, 33, 1125–1142. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Meng, Y.; Ren, H.; Huang, R.; Liu, J.; Liu, D. Synechococcus sp. PCC 7002 Performs Anoxygenic Photosynthesis and Deploys Divergent Strategies to Cope with H2Sn and H2O2. Antioxidants 2025, 14, 1122. https://doi.org/10.3390/antiox14091122
Wang Y, Meng Y, Ren H, Huang R, Liu J, Liu D. Synechococcus sp. PCC 7002 Performs Anoxygenic Photosynthesis and Deploys Divergent Strategies to Cope with H2Sn and H2O2. Antioxidants. 2025; 14(9):1122. https://doi.org/10.3390/antiox14091122
Chicago/Turabian StyleWang, Yafei, Yue Meng, Hongwei Ren, Ranran Huang, Jihua Liu, and Daixi Liu. 2025. "Synechococcus sp. PCC 7002 Performs Anoxygenic Photosynthesis and Deploys Divergent Strategies to Cope with H2Sn and H2O2" Antioxidants 14, no. 9: 1122. https://doi.org/10.3390/antiox14091122
APA StyleWang, Y., Meng, Y., Ren, H., Huang, R., Liu, J., & Liu, D. (2025). Synechococcus sp. PCC 7002 Performs Anoxygenic Photosynthesis and Deploys Divergent Strategies to Cope with H2Sn and H2O2. Antioxidants, 14(9), 1122. https://doi.org/10.3390/antiox14091122





