1. Introduction
In modern nutrition, snacks have gained widespread popularity as a food choice, especially in the context of a fast-paced lifestyle. Although the rise in snack consumption is more gradual in developing countries than in Western nations, the market continues to expand steadily [
1]; in fact, as Michelle Lieszkovszky said in a 2020 interview, “snacking is becoming a lifestyle” [
2]. Simultaneously, the current food industry is challenged to meet the demands of health-conscious consumers seeking foods with functional health benefits, and to address sustainability and innovation challenges by—for instance—developing functional foods.
Within this context, the development of “healthy snacks” has emerged as a rapidly expanding global trend, offering a convenient and efficient way to deliver nutrients and bioactive compounds with proven health-promoting properties [
1].
With the growing demand for sustainable alternative protein sources and the need to address the low consumption of legumes, pulses are increasingly being incorporated into functional food products—particularly “healthy snacks”—either as a primary ingredient or as a functional component designed to provide additional health benefits [
2].
Regular consumption of legumes has been associated with reduced low-grade inflammation and improved immune function, supporting overall health [
3,
4,
5]. Nutritionally, legumes are low in total fats and calories, yet rich in monounsaturated and polyunsaturated fatty acids (MUFAs and PUFAs), fibre, plant sterols, vitamins, minerals, and have a low glycaemic index (GI) [
6,
7].
Their high polyphenol content contributes to their antioxidant potential, offering protection against conditions such as cardiovascular disease, osteoporosis, gastrointestinal disorders, and neurodegeneration [
8,
9,
10].
Generally, polyphenols are believed to exert these effects primarily through their antioxidant activity, acting as radical scavengers, modulating immune responses and cellular inflammation [
11]. For these reasons, plant-based diets are increasingly incentivised as health-promoting and environmentally sustainable options [
12,
13]. Among legumes, chickpeas (
Cicer arietinum L.) stand out due to their high nutritional value [
14]. Their inclusion in food products is rising, supported by evidence of antioxidant, anti-inflammatory, and antifungal properties [
15,
16,
17,
18]. In fact, recent findings suggest that chickpeas may support gut health by reducing inflammation, supporting the microbiota, and enhancing epithelial barrier function [
10,
19,
20,
21].
Despite these recognised benefits, it is well known that legume consumption remains relatively low [
22]. Consequently, there is a persistent need to develop strategies that encourage greater legume consumption, which could lead to better health outcomes and a more sustainable food supply [
23].
The incorporation of chickpeas into snack formulations has attracted increasing interest, especially in response to the growing demand for convenient and health-oriented foods. However, their sensory acceptance remains limited due to their characteristic taste. To address this, recent approaches have focused on combining legumes with other functional ingredients to improve both nutritional value and palatability. In this context, dark chocolate represents a promising complementary ingredient, not only for its potential to enhance flavour but also for its well-documented health benefits [
24]. Notably, in recent years, there has been a marked increase in both the production of and research on functional chocolates, highlighting their relevance in the development of novel health-promoting snack products [
24].
Chocolate, derived from the processing of cocoa (Theobroma cacao L.), is a globally beloved product enjoyed by people of all ages due to its appealing sensory qualities.
Cocoa is known to be rich in polyphenols, which give it strong antioxidant properties [
25]. Many in vitro studies have focused on these antioxidant effects and the beneficial properties of cocoa [
26,
27]. Research has shown that the regular consumption of cocoa (up to 20 g every three days) can help to reduce inflammation and may be beneficial for heart health, cancer prevention. and the immune system. Specifically, it can affect the body’s inflammatory responses and improve immune function in the gut, showing promise as a food with immune-regulating abilities [
9,
25].
Given these findings, the development of functional chocolates—such as chocolates enriched with bioactive compounds from other functional foods (e.g., pulses)—is gaining significant attention in the scientific community. This approach not only enhances the nutritional value of chocolate but also improves the taste of other functional foods [
24].
The primary aim of this study was to explore the nutrigenomic and anti-inflammatory properties of bioactive molecules found in chickpea- and dark chocolate-based snacks after they undergo in vitro gastrointestinal digestion. To successfully achieve this goal, we evaluated the potential usefulness of Caco-2 cells (colon adenocarcinoma cells) as a model for the intestinal epithelium, using trans-well systems to simulate the small intestinal epithelium for transport studies. The barrier properties of Caco-2 were assessed by measuring transepithelial electrical resistance (TEER) and the transport of water-insoluble probes, such as Lucifer Yellow (LY), to assess the epithelial integrity. Despite several protocols describing the Caco-2 trans-well model [
21,
28,
29,
30,
31], the reproducibility of efficient intestinal epithelium appears to be challenging [
29,
32,
33,
34,
35,
36]. Thus, we tested several conditions to provide additional information on how to reproduce this model.
Furthermore, we used THP-1 cells, an established model for studying monocyte and macrophage functions [
5], to assess whether the in vitro digested chickpea- and dark chocolate-based snacks could stimulate the THP-1 immune response by inducing macrophage polarisation towards either a pro-inflammatory or anti-inflammatory phenotype.
2. Materials and Methods
2.1. Sample Preparation
Fertitecnica Colfiorito, a company based in Colfiorito, Italy, kindly provided us with chickpeas, 60% dark chocolate, and Cioccorito (a snack made from equal parts chickpeas and dark chocolate, 50%/50% w/w). The process of producing roasted chickpeas involved several carefully controlled steps. First, the raw material went through a magnetic check to ensure high quality. After that, raw materials were soaked in water to hydrate. Once they were ready, they were cooked for 70–90 min at a low temperature of 15–20 °C. The cooking process was followed by a drying phase, using hot air at around 120–130 °C for about 15–20 min. Once dried, the chickpeas were sifted and cooled with cold air. Finally, they were coated in chocolate using a basting technique. The final touch was polishing them with the carnauba wax to give them a nice, shiny finish.
2.2. In Vitro Digestion
The in vitro digestion process followed a method developed by Brodkorb and his team [
37]. To begin, 1 g each of puffed chickpea, dark chocolate, and the snack was mixed individually with a simulated oral fluid solution for 2 min at 37 °C. This oral fluid contained the enzyme salivary amylase (S-A1031-5KU, Sigma–Aldrich, St. Louis, MO, USA) at a final activity of 75 U/mL to mimic the human oral fluid.
After 2 min, the second step was the stomach phase. Then, 2 mL of a simulated gastric fluid (SGF)—which included an electrolyte solution, pepsin, and gastric lipase (S-L3126-25G, Sigma–Aldrich, St. Louis, MO, USA)—was mixed with the oral fluid of the previous step at a ratio of 1:1 (v/v). All the enzymes in the SGF achieved a final activity of 2000 U/mL and 60 U/ml.
The acidity was also adjusted by adding HCl to bring the pH to 3, emulating the environment within the human stomach. This mixture was then incubated for 2 h at 37 °C, gently shaken with a Julabo SW-20C shaking water bath to simulate the digestive process.
Once the stomach phase was performed, the gastric chyme was then diluted 1:1 (v/v) with the simulated intestinal fluid (SIF) solution. According to this, 4 mL of the stomach was mixed with 4 mL of SIF, which contained an electrolyte mix and an enzyme called pancreatin (with a final trypsin activity of 100 U/mL) (S-P1750-25G, Sigma–Aldrich, St. Louis, MO, USA), mimicking intestinal digestion. Then, 1M NaOH was used to adjust the pH to 7. The samples were incubated for another 2 h at 37 °C.
After digestion was completed, the samples were centrifuged at 4000×
g for at least 30 min at room temperature, and the supernatant was filtered using Millex-GP 0.22 μm polyethersulphone syringe filters (S-SLGS033SS, Sigma–Aldrich, St. Louis, MO, USA) and used for the experiments. The solid part was stored at −80 °C before its application in other analyses [
37].
2.3. Total Polyphenol Content (TPC)
Although the determination of polyphenols is hampered by their structural complexity, there are many methods for determining the total amount of polyphenolic compounds (TPC) in plants [
38]. Colourimetric or biochemical methods for the total quantification of polyphenols are very useful and reliable when quantitative information on the entire set of phenolic compounds is required. The conceptual basis of the method was to quantify the total concentration of phenolic hydroxyl groups in the extract being analysed, irrespective of the individual molecules in which they are found [
39]. In this study, the TPC was measured by means of a colourimetric assay using the reaction that leads to the reduction in the Folin–Ciocalteu Reagent to a blue pigment in an alkaline environment, as previously described [
40]. Briefly, the digested samples were tested at different concentrations presented as the amount of original digested food product (mg) solubilised in the digestion fluids (mL): 30.3 mg/mL, 15.1 mg/mL, 7.6 mg/mL, 3.8 mg/mL, and 1.9 mg/mL. An aliquot of the previously diluted samples was mixed with Folin reagent in a 96-well microplate and maintained in agitation for 5 min at room temperature. Then Na
2CO
3 and distilled water were added. Subsequently, the samples were incubated for 120 min at room temperature in the dark. Then, absorbance was read using a spectrophotometer (FLUOstar Omega, BMG LABTECH’s, Ortenberg, software version 1.30) at 760 nm against a blank. The TPC was then expressed as mg gallic acid equivalents (GAE) per mL of digested sample. All digested samples were analysed in quadruplicate.
2.4. DPPH Antioxidant Capacity Assay
The DPPH test was used to estimate the antioxidant activity of the digests based on spectrophotometric measurements of the capacity of antioxidants to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals [
41], according to this reaction: DPPH. +AH → DPPH-H +A. A reduction in absorbance corresponds to a decrease in DPPH concentration, which occurs due to the transfer of electrons from the antioxidant compounds [
42]. The diluted digested samples were tested at different concentrations presented as the amount of original digested food product (mg) solubilised in digestion fluids (mL): 30.3 mg/mL, 15.1 mg/mL, 7.6 mg/mL, 3.8 mg/mL, and 1.9 mg/mL. The digested samples were then mixed with the 3 mM DPPH working solution in a 96-well microplate and reacted for 20 min in the dark, after which the absorbance was determined at 515 nm with a FLUOstar Omega multi-mode microplate reader (BMG LABTECH’s, Ortenberg, Germany) against a blank. Antioxidant activity was calculated as the percentage of the inhibition of the DPPH radical compared to the control (DPPH without antioxidant). Different concentrations of Trolox, a known antioxidant, were used to prepare a standard curve for DPPH antioxidant activity determination. The Trolox calibration curve was used to express the results as Trolox equivalent (µM TE)/mL of the digested product. All digested samples were analysed in quadruplicate.
2.5. ABTS Antioxidant Capacity Assay
In this assay, the stable-coloured cation radical (Ry+) is generated by the oxidation of the diammonium salt of 2,2′-azino-bis (3-ethylbenzothiazoline-6- sulphonic acid) (ABTS) by means of a solution of potassium persulphate (K2S2O8). This cation radical (ABTSy+) has an absorption peak at 734 nm. Antioxidant compounds (AOH), which can transfer a hydrogen atom or electron to the cation radical, cause discolouration of the solution. The decrease in the peak at 734 nm of the cation radical (ABTSy+) after a predetermined incubation time (10–15 min maximum) was then analysed by UV-Vis spectrophotometry. The absorbance decrease (decolourisation) was proportional to the antioxidant charge in the sample. Briefly, for the ABTS assay, a fresh stock solution was prepared by mixing 7.4 mM ABTS and 2.6 mM potassium persulfate in equal amounts, which were kept for 12 h in the dark. Using the stock solution, a fresh ABTS working solution was prepared by diluting 1 mL of the stock solution with 40 mL of distilled water to obtain a 1.1 ± 0.02 units absorbance at 734 nm using a FLUOstar Omega multi-mode microplate reader. The diluted digested samples were tested at different concentrations presented as the amount of original digested food product (mg) solubilised in digestion fluids (mL): 30.3 mg/mL, 15.1 mg/mL, 7.6 mg/mL, 3.8 mg/mL, and 1.9 mg/mL. Then digested samples were mixed with the 7.4 mM ABTS working solution in a 96-well microplate and incubated in the dark for 20 min, followed by absorbance determination at 734 nm against a blank. Different concentrations of Trolox were used to prepare a standard curve for ABTS antioxidant activity determination. The calibration curve was used to express the antioxidant capacity as Trolox equivalent (µM TE)/mL of the digested product. All digested samples were analysed in quadruplicate.
2.6. Oxygen Radical Absorbance Capacity (ORAC)
The oxygen radical scavenging capacity (ORAC) test has been widely used to measure the antioxidant activity of nutraceuticals, pharmaceuticals, and foods. The ORAC test measures the classical ability of an antioxidant to quench free radicals via hydrogen donation.
Firstly, a 75 mM phosphate buffer solution, pH 7, was prepared. Subsequently, a fluorescein stock solution was prepared at a concentration of 4.2 mM in phosphate buffer. Starting with the 4.2 mM solution, a stock was prepared at a concentration of 0.08 µM in buffer. The diluted digested samples were tested at different concentrations presented as the amount of original digested food product (mg) solubilised in digestion fluids (mL): 30.3 mg/mL, 15.1 mg/mL, 7.6 mg/mL, 3.8 mg/mL, and 1.9 mg/mL. Then, the digested samples were mixed with 0.08 µM fluoresceine and 150 mM APPH (2,2,-azobis(2-methylpropionamidine) dihydrochloride). The plate was shaken for 10 s and read every 1.5 min for 90 min using a FLUOstar Omega microplate reader with excitation at 485 nm and emission at 530 nm. Different concentrations of Trolox (6.25–50 µM) were used to prepare a standard curve. Final ORAC values were calculated using the regression equation between Trolox and the net AUC and were expressed as µM TE/mL of digested product. All digested samples were analysed in quadruplicate.
2.7. Caco-2
The human epithelial cell line Caco-2 is extensively utilised as a model for the intestinal epithelial barrier [
43]. Originally derived from a colon carcinoma, the Caco-2 cell line’s most notable advantage is its ability to spontaneously differentiate into a monolayer of cells that exhibit many characteristics typical of absorptive enterocytes, including a brush border layer like that of the small intestine. Caco-2 cells were kindly provided by Prof. Massimo Nabissi (University of Camerino). Caco-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, ECB7501L, EuroClone S.p.A, Pero, Milan, Italy) supplemented with 10% heat-inactivated Foetal Bovine Serum (FBS, YOURSIAL-FBS-SA, Sigma–Aldrich, St. Louis, MO, USA), 1% L-glutamine (Stable L-Glutamine, 100X, 200 mM, ECB3004D, EuroClone S.p.A, Pero, Milan, Italy), 1% Non-Essential Amino Acids (NEAAs, ECB3054D, EuroClone S.p.A, Pero, Milan, Italy), and 1% penicillin/streptomycin (P/S 30-002-CI, Sigma–Aldrich, St. Louis, MO, USA). Cells were maintained at 37 °C in a humidified atmosphere with 5% CO
2. The medium was refreshed every 2 days, and cells were passaged when reaching 80% confluence. The passage number used for this study was 18.
2.8. Cell Viability Assay
The cytotoxic impact of chickpea, chocolate, and snack digested products was assessed through the 3-(4,5-Di-2-yl)-2,5-ditetrazolium bromide (MTT) assay (Thiazolyl blue tetrazolium bromide 98%, code 158990050, Acros Organic, NJ, USA). In brief, Caco-2 cells were plated in 96-well plates at a density of 1 × 104 cells/well in complete medium and exposed to various dilutions of digested product presented as the amount of the original digested food product (mg) solubilised in digestion fluids (mL) (30.3 mg/mL, 22.7 mg/mL, 15.1 mg/mL, 7.5 mg/mL, 3.8 mg/mL, and 1.5 mg/mL) for 2 h. Following the incubation period, the cells were treated with a 5 mg/mL MTT solution. After 4 h, the absorbance was measured at 550 nm using a FLUOstar Omega multidetector microplate reader (BMG LABTECH’s, Ortenberg). The results were expressed as a percentage (%) of control (cells grown in culture medium only) and were determined from dose–response curves. The experiment was performed in biological quadruplicates.
2.9. Intestinal Epithelium Model
To investigate the anti-inflammatory effects of the digested samples, Caco-2 cells were cultured in a trans-well-based system to evaluate how the digested products influence intestinal permeability and reduce inflammation. Firstly, to establish the optimal conditions for an intestinal epithelium model, different densities of seeding were used following the protocol outlined by Fengguang Pana et al. [
30] and by Kyeong Jin Kim et al. [
21] all with a few modifications.
Caco-2 cells were seeded on non-coated trans-well inserts (0.4 µm pore size, ThinCert®, Greiner Bio-one, Frickenhausen, Germany) at a density of 1 × 105 cells/cm2 (apical compartment), and 1 × 105 cells/insert (4.25 cm2 apical compartment) in a 6-well plate. The cell culture medium was added in both apical (AP) and basal (BL) compartments to facilitate the development of a differentiated intestinal epithelium. The medium was replaced every 2 days to prevent nutrient depletion. The 6-well plates were incubated in an atmosphere of 5% CO2 at 37 °C. Cells were cultured for 15 days and for 21 days in trans-wells until differentiation and complete epithelium formation. Pellets of all the densities were collected at time 15 days post-seeding and at time 21 days post-seeding to evaluate the development of the epithelium. Pellets were stored at −80 °C to prevent alteration of the genetic material to be analysed.
2.10. Caco-2 Treatments
Chickpea, chocolate, and snack samples were subjected to in vitro gastrointestinal digestion as described above, freshly prepared, and sterilised using a 0.22 μm filter before being added to the culture media (final concentration of 15.1 mg/mL and 3.8 mg/mL) for 2 h/day over 4 consecutive days prior to inducing inflammation following the method outlined by Frontela-Saseta and co-authors [
44] with some modifications. The pre-treatments started on the 14th day post-seeding until the 17th day post-seeding. Each day, following the 2 h incubation period with the digested products, the medium was replaced with fresh medium. The pre-treatments were conducted with a 2 h incubation period to mimic the human gastrointestinal residence time. After 4 consecutive days of pre-treatment with the digested products, a short-term inflammatory response was induced by exposing Caco-2 cells to both interleukin 1β (IL-1β) (SRP3083, Sigma–Aldrich, St. Louis, MO, USA) and lipopolysaccharide (LPS) (Sigma–Aldrich, St. Louis, MO, USA) following the method outlined by Lopes do Carmo and co-authors [
45]. IL-1β was added only on the basolateral side, and LPS was applied on both sides (apical and basolateral) [
45]. In the apical compartment, 2 mL of DMEM with 1% FBS (
v/
v) and LPS (10 µg/mL) was added, while in the basolateral compartment, 4 mL of DMEM with 1% FBS (
v/
v) and with both LPS (10 µg/mL) and IL-1β (10 ng/mL) was added. The monolayers were incubated for 3 h. A positive control, in which the Caco-2 monolayer was exposed to the inflammatory stimuli for 3 h, followed by the addition of DMEM (no treatment), was also performed. Monolayers only treated with DMEM were used as a negative control (no inflammation and no treatments). The experiment was performed in biological duplicates. The cell pellets were promptly frozen in liquid nitrogen and stored at −80 °C for subsequent analysis. The cell medium was centrifuged at 13,248×
g for 5 min, and the clear supernatant was aliquoted into new nuclease-free conical tubes and then stored at −80 °C for future use.
2.11. Permeability Assay
The permeability of the Caco-2 monolayer was determined by measuring the transepithelial electrical resistance (TEER), reflecting the integrity of the epithelium, because TEER quantifies the electrical resistance across the epithelial cell layer, which is directly related to the tightness of the tight junctions between cells. To assess the evolution of the intestinal epithelium formation, the barrier integrity was measured using Millicell
® ERS (Electrical Resistance System) Voltohmmeter (Millipore, Burlington, MA, USA) in the epithelium with the density of 1 × 10
5 cells/insert and 1 × 10
5 cells/cm
2 after 24 h, 4, 7, 10, 15, and 17 days post-seeding. Three values were measured for each well, which were then averaged and expressed as percentages. According to the literature, Caco-2 cells exhibit TEER values ranging from 150 to 400 Ω.cm
2 when forming an intact monolayer [
46,
47].
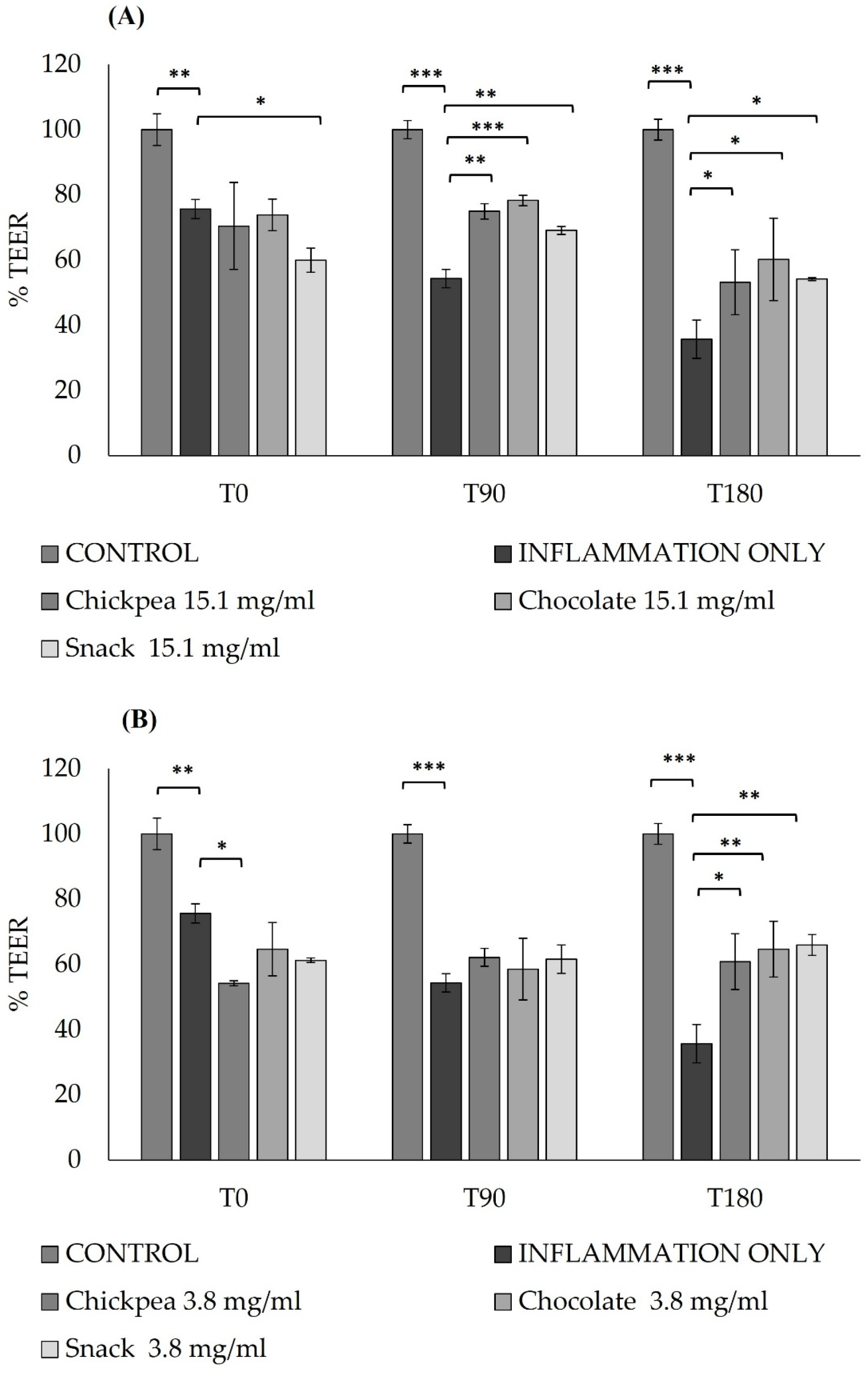
Additionally, TEER values were collected after pre-treatments with digestive products and a post-inflammation induction to evaluate any protective effect of the samples against inflammation.
The final values are expressed as Ohm × cm2 based on the following equation: TEER = (R − Rb) × A, where R is the resistance of the filter insert with cells, Rb is the resistance of the filter alone, and A is the growth area of the filter in cm2.
Also, the Lucifer Yellow (LY) (Sigma-Aldrich Life Science, St. Louis, MO, USA) assay was used to measure the permeability of untreated Caco-2 multilayers developed at different densities of seeding (1 × 105/cm2 and 1 × 105/well). Briefly, the medium from both the AP and BL compartments was collected after 15 days post-seeding and 21 days post-seeding for each density evaluated and stored at −80 °C for further analysis. Caco-2 cells on the AP side were gently washed twice with Phosphate-Buffered Saline (PBS) with Ca2+ and Mg2+ (Corning, Glendale, AZ, USA). LY at a concentration of 100 µM (in PBS with Ca2+ and Mg2+) was introduced into the AP compartment, while PBS (with Ca2+ and Mg2+) was added to the BL compartment. Subsequently, 150 µL of the solution from both compartments was promptly transferred to a 96-well plate, and fluorescence readings were taken using a fluorometer (FLUOstar Omega, BMG LABTECH’s, Ortenberg) at λEx/λEm = 485/520 nm. These measurements were repeated at 30, 60, and 120 min after the addition of the LY solution in the AP. The cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2 between each reading. All analyses were conducted in biological triplicates.
2.12. THP-1 Cells
Human monocytic THP-1 is a monocyte isolated from the peripheral blood of an acute monocytic leukaemia patient. This line is commonly employed in studies related to immune system disorders, immunology, and toxicology. THP-1 can be induced to differentiate into a macrophage-like phenotype through treatment with either phorbol-12-myristate-13- acetate (PMA), 1α, 25-dihydroxyvitamin D3 (vD3), or macrophage colony-stimulating factor (M-CSF) [
5].
THP-1 cells were purchased from Istituto zooprofilattico sperimentale della Lombardia e dell’Emilia Romagna “Bruno Ubertini”. Cells were cultured in Roswell Park Memorial Institute 1640 medium (RPMI-1640, ECB2000L, EuroClone S.p.A, Pero, Milan, Italy) supplemented with 2-mercaptoethanol to a final concentration of 0.05 mM, 20% heat-inactivated FBS (YOURSIAL-FBS-SA, Sigma–Aldrich, St. Louis, MO, USA), 1% L-glutamine (100X, 200 mM, ECB3004D, EuroClone S.p.A, Pero, Milan, Italy), 1% NEAA (ECB3054D, EuroClone S.p.A, Pero, Milan, Italy), 1% P/S (30-002-CI, Sigma–Aldrich, St. Louis, MO, USA), and 1% of sodium pyruvate (ECM0542D, EuroClone S.p.A, Pero, Milan, Italy). The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2.
Culture medium was replaced every 2 to 3 days, and cells were passaged when reaching 80% confluence before any experiment. The passage number used for this study was 13.
2.13. THP-1 Treatments
Firstly, to induce polarisation of THP-1 cells into the M0 phenotype macrophages, cells were treated with 100 ng/mL phorbol 12-myristate 13-acetate (PMA) for 24 h in a 6-well plate.
After an incubation period of 24 h to allow for complete adhesion of the cells, the daily treatments started.
THP-1 were exposed to chickpea, chocolate, and snack digested product at concentrations of 15.1 mg/mL; moreover, considering that the snack is made up of 50% chocolate and 50% chickpeas by weight, a concentration of 30.3 mg/mL was also tested to compare the effect of the snack with that observed in the individual components.
Chickpea, chocolate, and snack samples were subjected to in vitro gastrointestinal digestion as described above, freshly prepared, and sterilised using a 0.22 μm filter before being added to the culture media (final concentration of 15.1 mg/mL for chickpea chocolate and snack digested product and 30.3 mg/mL for only snack digested product) for 2 h/day over 4 consecutive days. Each day, following the 2 h incubation period with the digested products, the medium was replaced with fresh medium.
THP-1 monolayers, polarised and only treated with RMPI-1640, were used as a negative control. These cells were untreated, but the medium was replaced with only fresh medium each day.
The experiment was performed in biological duplicates. After 4 consecutive days of treatment with the digested products, cell pellets were promptly frozen in liquid nitrogen and stored at −80 °C for subsequent analysis. The cell medium was centrifuged at 13,248× g for 5 min, and the clear supernatant was aliquoted into new nuclease-free conical tubes and then stored at −80 °C.
2.14. Gene Expression Analysis
Total RNA was isolated from Caco-2 and THP-1 cells using the Total RNA Purification Plus Kit (Norgen Biotek, Thorold, ON, Canada) following the manufacturer’s protocol and quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Monza, Italy). Then, 1 µg of RNA was retrotranscribed to cDNA (complementary DNA) using the PrimeScript RT-PCR Kit (Takara Bio, Göteborg, Sweden). Gene expression analysis was conducted with quantitative real-time PCR (qPCR) on a Biorad CFX96 system, employing TB Green® Premix Ex Taq™ (Takara Bio, Göteborg, Sweden). The qPCR conditions included an initial denaturation for 30 s at 95 °C; after that, 5 s at 95 °C for denaturation and 30 s at 60 °C for annealing/extension were repeated for 40 cycles. Gene expression levels were normalised to β-actin and to Peptidylprolyl Isomerase A (PPIA) using the 2−∆∆CT method. Each assay was performed in technical duplicates, with an inter-run calibrator sample to standardise the results across different amplification plates. Biological duplicates were analysed for each treatment condition.
The genes of interest analysed from Caco-2 cells included the tight junction genes Zonulin 1 (ZO-1), Occludin (OCLN), and Claudin1 (CLDN1); the genes involved in Caco-2 differentiation ALPI Alkaline Phosphatase Intestinal (ALPI), Cytochrome P450 Family 3 Subfamily A Member 4 (CYP3A4), SLC15A1 Solute Carrier Family 15 Member 1 (SLC15A1), Solute Carrier Family 11 Member 2 (SLC11A2), and Sucrase-Isomaltase (SI); and the pro-inflammatory genes interleukin-6 (IL-6), IL-1β, Nuclear Factor Kappa B (NF-κB), Interleukin 8 (IL-8), Monocyte Chemoattractant Protein 1 (MCP1 or CCL2), Chemokine (C-C motif) Ligand 20 (CCL20), and Tumour Necrosis Factor Alpha (TNF-α). Primer sequences used in the study are provided in
Supplementary Materials Table S1.
For THP-1 cells, the analysis focused on genes related to inflammatory and anti-inflammatory pathways, including IL-1β, IL-6, IL-8, MCP1, NF-κB, C-type mannose receptor 1 (CD206), interleukin-10 (IL-10), Transforming Growth Factor Beta (TGFβ), Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ), and Cluster of Differentiation 163 (CD163). All genes evaluated were based on a study carried out by Matacchione G. et al. [
48]. The primer sequences used in the study are provided in
Supplementary Materials Table S1.
2.15. Statistical Analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 29.01.0, Armonk, NY, USA). The results were expressed as mean ± standard deviation. It was verified that the parameters followed a normal distribution. The existence of significant differences among the different parameters in the samples was evaluated using the non-parametric Kruskal–Wallis test. An ANOVA test with Bonferroni’s correction was used to test differences between means. The Bonferroni correction was applied to correct p values in multiple comparisons. Moreover, Spearman’s correlation was used to evaluate the presence of a positive linear correlation between the total polyphenol content and antioxidant value. A p value < 0.05 was considered significant throughout the study.
4. Discussion
Persistent inflammation and oxidative stress play a critical role in the development of chronic inflammatory conditions in the immune system.
Legumes, such as chickpeas, help to reduce this effect thanks to their high levels of phenolic content, which provide antioxidant and anti-inflammatory benefits [
49].
In this study, we showed that a puffed chickpea snack enriched with dark chocolate is enriched in polyphenol levels and antioxidant activity. After in vitro digestion, all digested products were non-cytotoxic at the tested concentrations. They maintained intestinal barrier integrity under inflammatory conditions and modulated the expression related to tight junction and inflammation genes in both Caco-2 and THP-1 cells.
Based on the antioxidant activity and capacity, the results showed that the TPC of all digests influenced the ABTS and ORAC values. The antioxidant capacity, as measured by ABTS and ORAC assays, was positively correlated with the TPC. Specifically, we found a correlation of
r = 0.727 for ABTS and
r = 0.572 for ORAC, indicating that higher TPC values led to stronger antioxidant responses. No significant correlation was observed between TPC and DPPH values (
r = 0.262), suggesting assay-specific responses. In particular, a positive correlation was found between the TPC and antioxidant activity measured by the ORAC method (
r = 0.572) and the ABTS assay (
r = 0.727). However, no significant correlation was observed between the TPC and the DPPH assay (
r = 0.262). The results obtained agree with those of the study by are consistent with Rajagukguk et al. [
50], who examined the total polyphenol content in a dark chocolate and chickpea snack bar. The TPC was found to significantly influence ORAC, DPPH, and ABTS antioxidant activity values, with reductions in the TPC leading to corresponding decreases in these measurements. It has been reported that the TPC significantly influenced antioxidant capacity in chickpea–dark chocolate snack bars across several assays [
50].
Moreover, other studies on chickpea-based products reported similar trends. Xiao Y. et al. [
51] observed positive correlations between the TPC and antioxidant activities (ABTS, DPPH, ORAC, and PCL). A similar highly significant correlation between the TPC and antioxidant assays (ABTS and ORAC) in chickpea samples was found by Sanchez-Magana, whose positive linear correlation (
r = 0.991 and
r = 0.972, respectively) can be considered extremely significant [
52]. This reduction may be attributed to the heat-related degradation or transformation of certain compounds.
Importantly, DPPH and ORAC measure different antioxidant mechanisms—electron transfer versus hydrogen atom transfer, respectively—thus reflecting the activity of distinct phenolic subgroups. Previous studies have also explored each ingredient individually. However, the lack of correlation with the DPPH assay in our results aligns with the findings of Xu et al. [
53], who observed a 10–35% reduction in DPPH activity after chickpeas soaking. This reduction in DPPH activity may be associated with heat-related degradation.
Importantly, ORAC and DPPH measure differently in their antioxidant mechanisms—electron transfer versus hydrogen atom transfer, respectively—thus reflecting the activity of distinct phenolic subgroups.
These results are consistent with previous studies that have analysed the antioxidant activity of individual ingredients. Chickpeas provide phenolic acids and flavonoids with known antioxidant effects, particularly after thermal processing [
53]. Similarly, dark chocolate, rich in polyphenols, has also demonstrated a strong antioxidant capacity in both in vitro and clinical studies [
54,
55].
Although the beneficial properties of these ingredients are well established, to our knowledge, no studies have yet investigated the synergistic effect of a chickpea- and dark chocolate-based snack after in vitro gastrointestinal digestion on total polyphenol content (TPC) and antioxidant activity.
Findings from related studies support the notion that in vitro digestion can enhance the bioaccessibility of phenolic compounds. For instance, recent research on a chickpea flour snack enriched with black carrot pomace (BCP) reported a significant post-digestion increase in the TPC, attributed to the enzymatic release of matrix-bound phenolic compounds during gastrointestinal digestion [
56]. Although the antioxidant activity (ABTS and DPPH) decreased after digestion in that study, a strong correlation between the TPC and ABTS was maintained throughout all phases—similar to what we observed in our samples.
These findings support the idea that in vitro digestion enhances the bioaccessibility of polyphenols, even though the measured antioxidant capacity may vary depending on the assay used and the digestion phase. The higher total polyphenol content and antioxidant activity observed in the digested snack samples can be attributed to the presence of dark chocolate. This aligns with findings by Jacimovic et al., who highlighted the health benefits of cocoa-rich dark chocolate, including its high content of bioactive compounds and its antioxidant and anti-inflammatory properties.
In our case, the higher TPC and antioxidant activity observed in the digested snack may largely be attributed to the dark chocolate component. Martini et al. [
57] showed that gastrointestinal digestion increases the bioaccessibility of phenolic compounds in dark chocolate, with a total release of 68.7% at the end of digestion and, even with limited systemic bioavailability, can reduce oxidative stress locally in the intestinal tract by neutralising reactive oxygen species.
Although systemic bioavailability may be limited, these compounds can act locally in the gut to neutralise reactive oxygen species and reduce oxidative stress [
57,
58,
59].
While our results confirm the beneficial antioxidant potential of the digested snack matrix, a methodological limitation must be recognised. Although this study did not quantify polyphenols in each individual phase of in vitro digestion, the focus of the analysis was to study the final bioaccessible fraction collected after the intestinal phase, which is generally considered the most physiologically relevant, as it contains compounds potentially available for absorption.
Moreover, it is well known that pH variations during the different stages of digestion can significantly affect the stability and release of polyphenols. Acidic gastric conditions can cause the degradation of some compounds, while neutral to slightly alkaline intestinal conditions can increase their solubility and availability [
60].
Given these promising antioxidant results and the known local effects of dietary polyphenols at the intestinal level, we next investigated whether the digested products could exert protective and anti-inflammatory properties using an in vitro intestinal model.
In recent years, in vitro models have gained attention in toxicological research due to ethical and scientific motivations. Among these models, differentiated Caco-2 cells are widely used stand out for their superior morphological and functional differentiation into enterocyte-like cells compared to other colon carcinoma cell lines. However, the literature reports significant discrepancies among different Caco-2 cell lines, which complicates the comparison of the results. This variability is due to differences in culture conditions, serum type, media supplements, passage number, clone origin, and other various parameters, complicating the comparison of the results [
29]. Therefore, it is essential to standardise the Caco-2 cell intestinal epithelium model and develop validated testing protocols to improve reproducibility [
35]. The literature shows a wide range of opinions on the ideal timing after seeding to achieve a reliably differentiated culture for experiments. Some researchers begin using Caco-2 cells as early as 6–9 days post-seeding [
61], while others wait 30 days to yield better results [
62].
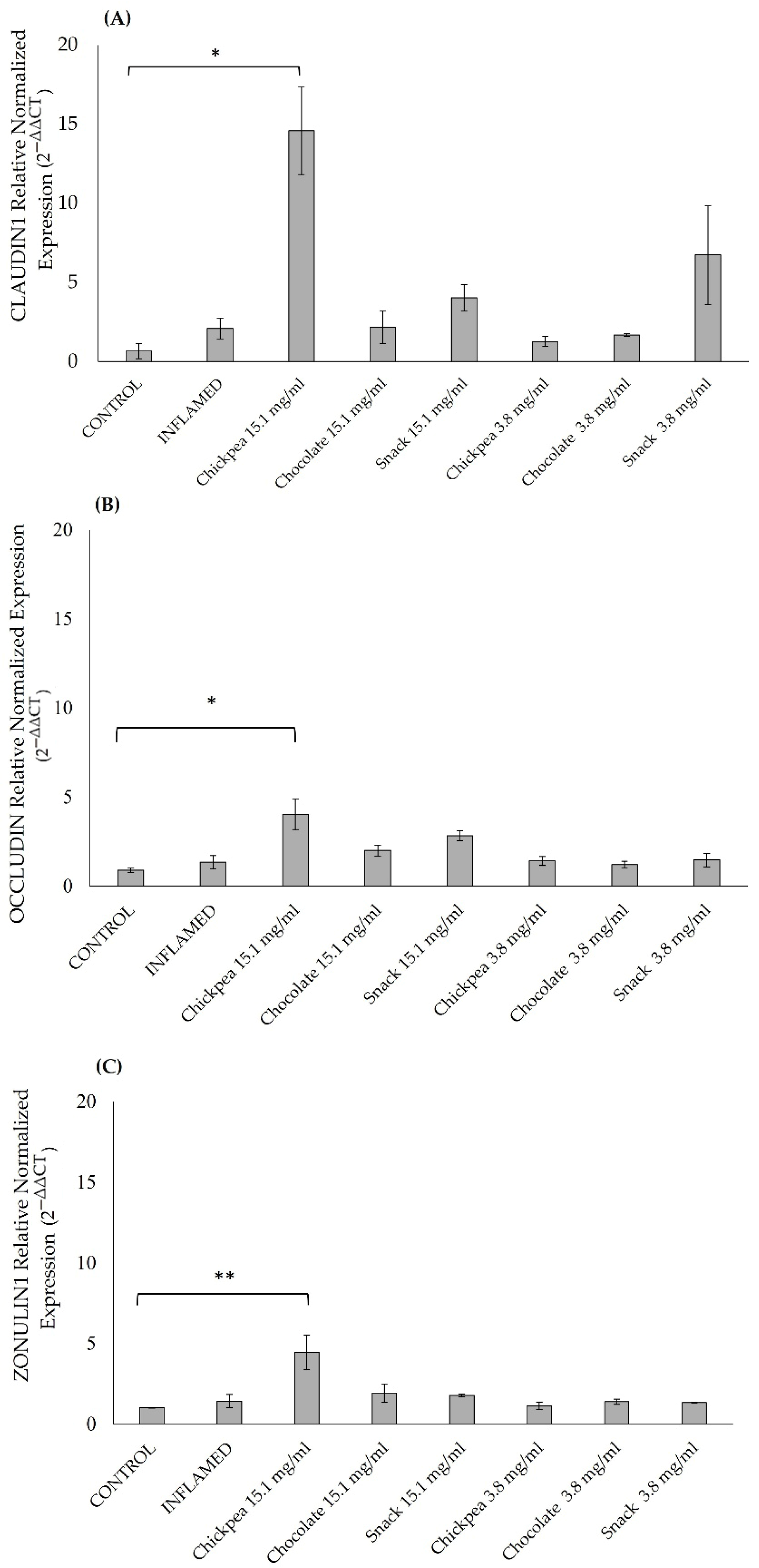
For all these reasons, with the aim of setting the best conditions to explore the anti-inflammatory effect of all digested products on an intestinal epithelium model, we tested two seeding densities (1 × 10
5/cm
2 and 1 × 10
5/well) and evaluated epithelial development after 15 and 21 days. Based on the results obtained, the gene expression of differentiation markers (ALPI, SLC11A2, and SLC15A1) and tight junction proteins (ZONULIN1, OCCLUDIN, and CLAUDIN1) indicated that 15 days post-seeding was the optimal time point, as expression levels declined by day 21. ALPI and SLC11A2 showed significant upregulation at 15 days, particularly at the higher seeding density, while CLAUDIN1 and ZONULIN1 were significantly elevated at the lower density. These findings are consistent with previous studies reporting peak epithelial differentiation around day 14–15 [
29]. To further assess epithelial integrity, TEER and Lucifer Yellow assays were performed. While Lucifer Yellow did not show significant differences in permeability between the seeding densities at 15 or 21 days, TEER measurements were more informative. The density of 1 × 10
5 cells/cm
2 showed an initial increase in resistance followed by a decline after day 10, indicating early epithelial degradation. In contrast, the 1 × 10
5 cells/well condition showed a steady increase in TEER, exceeding 150 Ω-cm
2 from day 7 and remaining stable for 17 days.
Although both TEER measurements and LY can be used to evaluate tight junction integrity, they assess different aspects. TEER evaluate ionic resistance across the epithelial monolayer, while LY assesses paracellular permeability based on non-electrolyte flux [
34]. In our study, LY did not differentiate between conditions, suggesting it may lack sensitivity for detecting subtle changes.
TEER is often considered more reliable for assessing the integrity of the epithelium [
46,
47]. This is because TEER directly measures electrical resistance across the cell monolayer, providing a quantitative estimate of the cohesion and integrity of cell junctions. These results confirmed that the 15-day culture using 1 × 10
5 cells/well was identified as the most suitable condition for establishing a well-formed and functionally differentiated intestinal epithelium.
The intestinal barrier plays an important role in the protection of the whole organism against toxic substances, maintaining gut health, and preventing disease [
35]. Moreover, the mucosal barrier acts as the first line of defence for gut contents and the body’s tissues. Damage to this barrier can enable pro-inflammatory molecules to pass through, leading to an overactive immune response, chronic inflammation, and tissue damage. Increased intestinal permeability is associated with several inflammatory conditions.
A diet rich in anti-inflammatory foods is key to protecting the intestinal barrier and reducing inflammation. Chickpeas, particularly, stand out for their nutritional benefits and positive impact on gut health and barrier integrity [
20].
This evidence suggests that chickpeas, possibly due to their richness in bioactive compounds, may play a crucial role in maintaining and protecting intestinal epithelial integrity. Their polyphenols have demonstrated significant antioxidant and anti-inflammatory properties, which are essential in mitigating intestinal inflammation and enhancing barrier function. Studies have shown that these compounds can also modulate inflammatory pathways and promote a healthy gut microbiome, thereby strengthening the epithelial barrier by improving the expression and function of tight junction proteins [
63].
In our study, digested chickpea, dark chocolate, and snack products demonstrated a dual protective effect on gut health, simultaneously preserving barrier integrity and modulating inflammatory responses. Pre-treatment of Caco-2 monolayers with the digested products significantly prevented the reduction in TEER values typically induced by inflammatory stimuli (LPS and IL-1β), indicating the preservation of epithelial tightness. This protective effect was further supported at the molecular level, as demonstrated by the upregulation of tight junction genes such as ZO-1, Claudin-1, and Occludin, particularly after treatment with digested chickpea products.
As shown by Ulluwishewa et al. [
64], polyphenols increase TEER across Caco-2 monolayers in a dose-dependent manner and promote tight junction assembly in Caco-2 cells [
65]. Amasheh et al. also found that polyphenols from chickpeas improved TEER of Caco-2 cell monolayers by increasing Claudin levels [
66] and protecting the integrity of HT-29/B6 cell monolayers from TNF-α-induced damage [
67]. Similarly, Suzukia et al. reported that polyphenols showed protective and promotive effects on intestinal tight junction barrier function by regulating tight junction protein expression [
68].
In parallel, the reduction in inflammatory markers supported the beneficial role of these food matrices. In Caco-2 cells, treatment with digested chickpea and chocolate significantly reduced IL-1β, a key pro-inflammatory cytokine involved in barrier disruption.
Additionally, a notable study examined how chocolate helps to preserve the integrity of the epithelial barrier, potentially through its ability to inhibit the apoptosis induced by oxysterols [
69]. It may also help reduce inflammation associated with obesity by lowering MCP-1 and IL-1β levels, which are involved in adipocyte development. Chocolate’s primary mechanism seems to be the suppression of inflammatory mediators, like IL-1β, which typically compromise the structural stability of cell membranes [
69]. This aligns with our findings, where chocolate not only preserved the intestinal barrier under inflammatory conditions but also significantly reduced IL-1β expression.
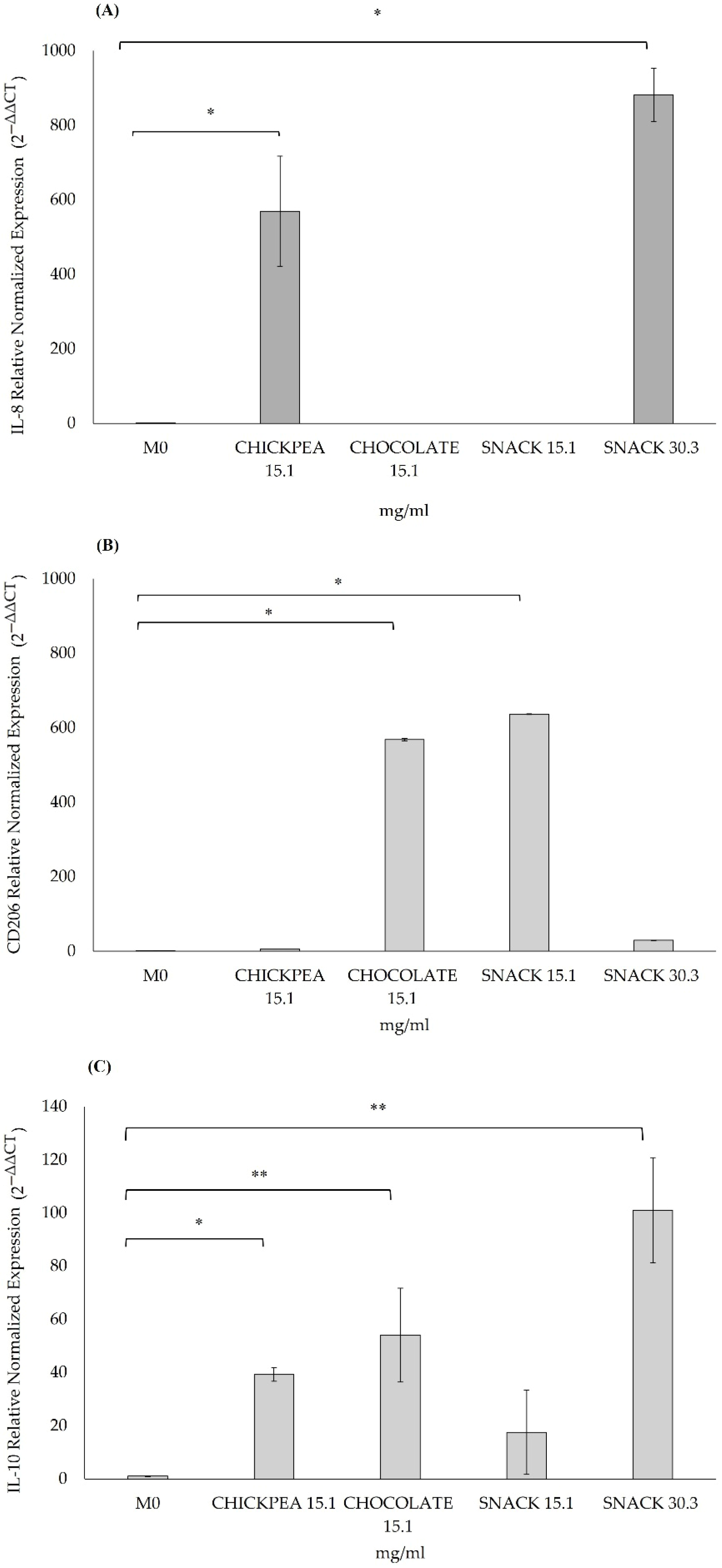
Furthermore, in THP-1 immune cells, exposure to the digested products increased the expression of anti-inflammatory markers such as IL-10 and CD206, indicating a shift to a M2-like macrophage phenotype associated with tissue repair and inflammation resolution.
Specifically, in our study, chocolate showed anti-inflammatory properties by increasing the expression of the CD206 and IL-10 genes. Similar effects were observed for the snack formulation at concentrations of 15.1 mg/mL and 30.3 mg/mL.
Based on previous studies, CD206 and IL-10 act as markers for M2 macrophages, known for their role in reducing inflammation and anti-inflammatory functions. Previous studies have established CD206 and IL-10 as reliable markers of M2 macrophages, which exert anti-inflammatory functions. Genine et al. reported that the THP-1/M2 phenotype expressed several key markers, including CD206, CD163, fibronectin, IL-10, CCL18, and CCL22, with a modest increase in CD206 and IL-10 observed after 24 h of incubation. In fact, after a 24 h incubation period, both CD206 and IL-10 showed a slight increase in THP-1/M2 cells [
70]. If CD206 gene expression increases following cocoa treatment in THP-1 cells, this could indicate a shift towards M2 macrophage polarisation, suggesting that chocolate, probably due to its polyphenols and bioactive components, promotes an anti-inflammatory response of M2 macrophages [
70]. Our results are consistent with those of Sarria et al., who reported that cocoa consumption reduced both modulated IL-1β and IL-10 levels, suggesting a decrease in inflammatory cytokines, also evaluated in a snack digested product for the contribution and the presence of chocolate [
71].
This dual downregulation was interpreted as a general reduction in inflammatory signalling, consistent with our observation of decreased levels of IL-1β and modulated IL-10 levels following treatment with the snack containing chocolate. Taken together, these data suggest that chocolate may exert a dual role: supporting anti-inflammatory polarisation through M2 macrophage markers while also contributing to a downregulation in inflammatory cytokines. This dual downregulation was consistent with our observation of decreased IL-1β expression and modulated IL-10 levels following treatment with the snack matrix, supporting the hypothesis that chocolate contributes to both anti-inflammatory macrophage polarisation and a reduction in inflammatory cytokines.
Interestingly, while chocolate exerted an anti-inflammatory effect, digested chickpeas showed a pro-inflammatory response induced by under these experimental conditions. Specific studies have shown that chickpeas, especially following digestion, may activate inflammatory pathways such as NF-κB, leading to the upregulation of cytokines including IL-8, IL-6, and TNF-α [
72,
73,
74].
Additionally, other studies demonstrated that the interaction with gut microbiota might influence immune responses that elevate cytokine levels, including IL-8, in intestinal cells [
63,
74].
These findings support our results, where chickpea and snack digested products increased IL-8 gene expression. However, this pro-inflammatory signal may vary depending on digestion processes and the bioavailability of specific peptides. Despite this evidence, our findings indicate that digested chickpeas possess a peculiar ability to enhance epithelial integrity by upregulating tight junction protein expression, suggesting a dual role.
In addition, the antioxidant capacity observed in the digested products, particularly in the snack and chocolate matrices, may have contributed to the maintenance of epithelial barrier integrity. Oxidative stress is a well-known trigger of tight junction disruption and epithelial permeability, and the antioxidant compounds present in the chickpea- and dark chocolate-based snack could have mitigated oxidative damage at the intestinal level. Thus, the combination of antioxidant activity and anti-inflammatory modulation likely acted synergistically to preserve gut barrier function under inflammatory conditions.
Although the specific bioactive compounds responsible for the observed effects were not characterised in this study, the results highlight the functional potential of the chickpea- and dark chocolate-based snack.
Taken together, these results support the hypothesis that chickpeas and dark chocolate can contribute to gut health through distinct but complementary mechanisms. Their combination within a single food matrix may promote mechanistic synergy, enhancing the protective effect on the intestinal epithelium more effectively than either component alone.
This hypothesis aligns with the widely accepted concept of the “whole food effect,” which recognises that the health benefits of many plant-based foods arise not from a single compound, but from the synergistic interaction of multiple constituents within the food matrix, such as fibres, polyphenols, and other phytochemicals [
75]. This concept recognises the importance of studying foods as complex systems where interactions among nutrients and bioactive molecules may enhance biological activities. In this context, the protective effects on intestinal barrier integrity and modulation of inflammatory responses observed in this work could be attributed to the combined influence of the various bioactive constituents naturally present in chickpeas and dark chocolate.
However, it is important to emphasise that this study was able to comprehensively characterise the nature of the interaction between the two food components.
Although our results suggest that combining dark chocolate and chickpeas at half the individual doses in a single food matrix may result in effects comparable to those observed with full doses of the individual components, this observation alone is not sufficient to definitively define the interaction as additive or synergistic. The complexity of food matrices and the possibility of non-linear biological responses make it difficult to isolate the contribution of each component. Furthermore, the variability between different biomarkers further supports the idea that each component may act on distinct biological pathways.
Together, these findings suggest that the digested products not only act directly on intestinal epithelial cells but also modulate immune activity, thereby promoting intestinal homeostasis by modulating immune cell responses. This combined action highlights the potential of the chickpea-dark chocolate snack as a functional food, capable of supporting gut health and preventing chronic inflammation.
Beyond the scientific findings, it is important to consider the practical applications of the developed chickpea- and dark chocolate-based snack. While this study highlights its potential in promoting intestinal health and modulating inflammatory responses, such evidence may serve as a scientific foundation for further investigations focused on its commercial viability as a functional food.
Consumer acceptance is also a critical factor for the success of innovative food products and plays a pivotal role in the food industry. Understanding sensory preferences, willingness to pay (WTP), and purchasing motivations can help ensure successful market introduction [
76]. Although there is no direct consumer acceptance analysis for this product, studies have shown that clear communication of health benefits and product familiarity enhance the acceptance of functional and plant-based foods [
77].
Recent consumer research revealed a growing openness to trying novel plant-based products, especially when associated with attributes such as health, sensory pleasure, and sustainability [
78]. The gastronomic implications of these findings are significant: the perceptions gathered from diverse consumer profiles may help guide and accelerate the development and popularity of new plant-based snack options.
Moreover, a report on European consumer behaviour indicated a particular interest in legumes—such as chickpeas—with 43% of participants intending to replace animal products, 52% to increase consumption, and 53% to purchase them more regularly [
76]. This confirms the increasing potential of chickpea-based products in the European market.
In this scenario, ready-to-eat legume-based snacks are gaining popularity due to their nutritional benefits, palatability, and convenience.
These products fit well with current dietary trends, where traditional meals are being increasingly replaced by portable, nutrient-rich snacks [
79]. Furthermore, nutritional guidelines are increasingly recommending the redefinition of “snack foods” to prevent the overconsumption of highly processed, low-nutrient options [
80].
Therefore, the functional and nutritional benefits demonstrated in this work align with broader market demands for healthier snack alternatives. The development of a chickpea- and dark chocolate-based snack not only offers potential health benefits but also meets evolving consumer expectations for sustainable, nutritious, and enjoyable food options. These insights support its dual role as a scientifically validated and commercially promising functional food.