Processing of Tuna Head By-Products into Antioxidant Peptide Ingredients for Aquaculture Feeds
Abstract
1. Introduction
2. Materials and Methods
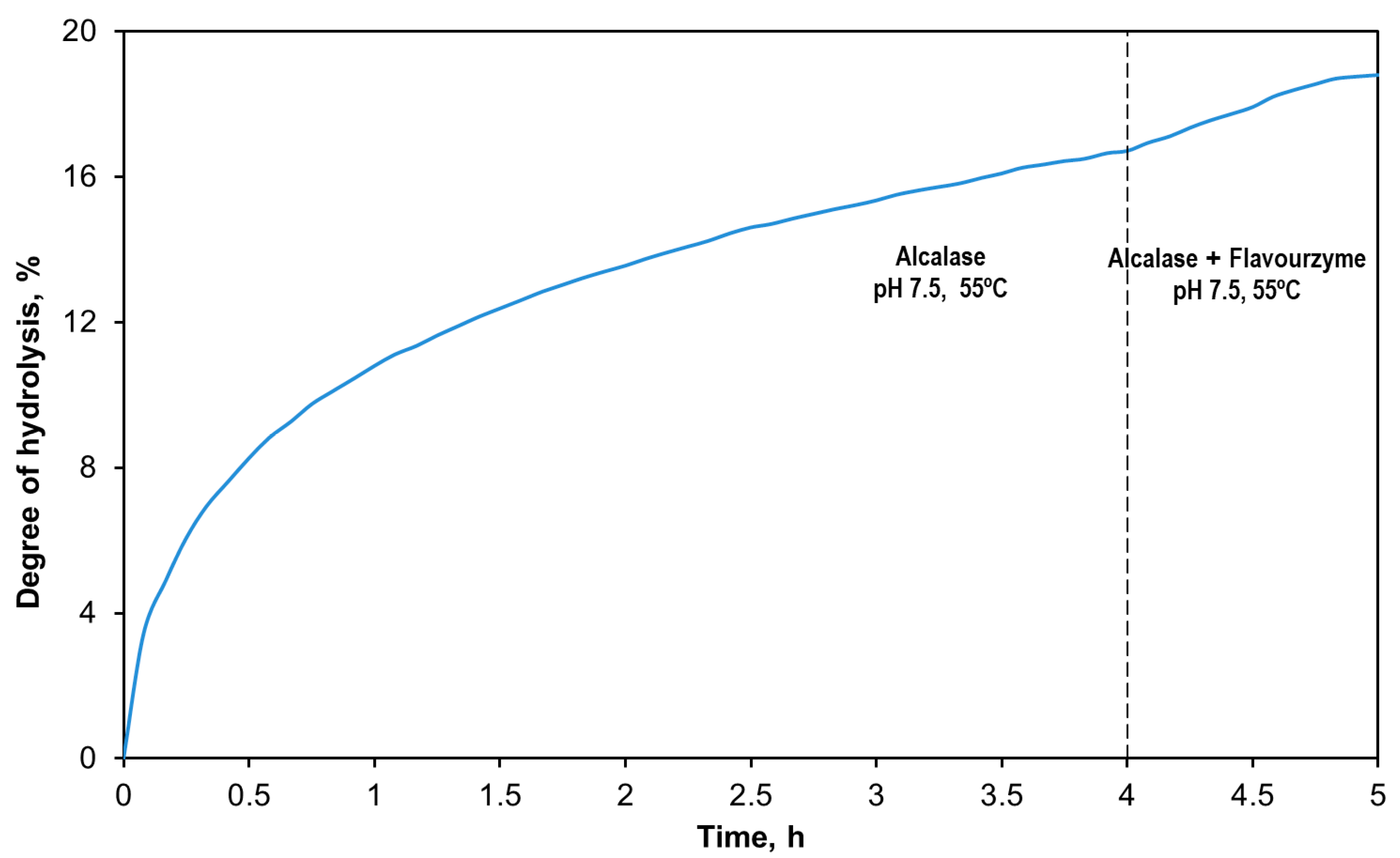
2.1. Production of Tuna Head Hydrolysates
2.2. Fractionation of the Tuna Head Hydrolysates with Ceramic Ultrafiltration Membranes
2.2.1. Operation of the Ceramic UF Membranes
2.2.2. Determination of Membrane Permeability
2.2.3. Modeling of the Permeate Flux
2.2.4. Cleaning Treatment of the Ceramic UF Membranes
2.3. Characterization of the Membrane Peptide Fractions
2.3.1. Determination of Soluble Protein of the Peptide Fractions
2.3.2. Amino Acid Composition of the Fractions F3F1 and F3R1
2.3.3. Molecular Weight Distribution of the Peptide Fractions
2.4. Determination of the Antioxidant Activities of the Peptide Fractions
2.5. Statistical Analysis
3. Results and Discussion
3.1. Production of the Tuna Head Hydrolysates
3.2. Fluid-Dynamic Study of the Membrane Filtration
3.2.1. Water Permeability of the Ceramic Membranes
3.2.2. Time Evolution of the Permeate Flux During Batch Concentration
3.2.3. Cleaning Efficiency of the Alkaline Treatment
3.3. Evaluation of the Peptide Fractions as Natural Antioxidants for Aquaculture Diets
3.3.1. Content of Soluble Protein and Amino Acid Composition of the Peptide Fractions
3.3.2. Molecular Weight Distribution of the Peptide Fractions
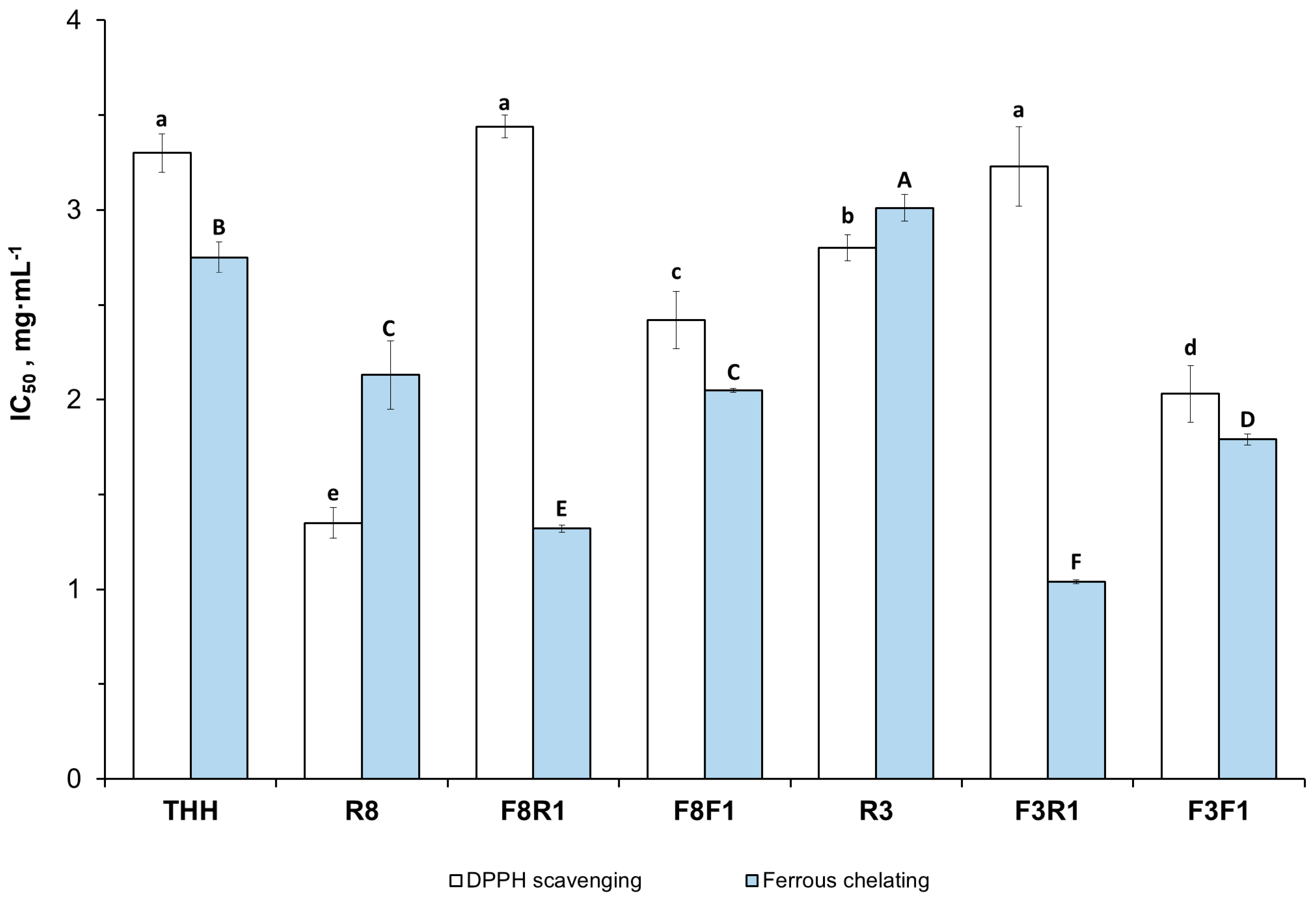
3.3.3. In Vitro Antioxidant Activities of the Peptide Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Ghalamara, S.; Brazinha, C.; Silva, S.; Pintado, M. Exploring Fish Processing By-Products as an Alternative Source of Bioactive Peptides: A Review on Extraction and Food Applications. Curr. Food Sci. Technol. Rep. 2024, 2, 377–391. [Google Scholar] [CrossRef]
- The Spanish Canned Seafood Industry in 2024: An Overview of the Sector|Gaictech®. Available online: https://www.gaictech.com/en/blog/the-spanish-canned-seafood-industry-in-2024-an-overview-of-the-sector/ (accessed on 10 June 2025).
- Ramakrishnan, S.R.; Jeong, C.-R.; Park, J.-W.; Cho, S.-S.; Kim, S.-J. A Review on the Processing of Functional Proteins or Peptides Derived from Fish By-Products and Their Industrial Applications. Heliyon 2023, 9, e14188. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Liu, S.; Wei, S.; Xia, Q.; Ji, H.; Deng, C.; Hao, J. Extraction of Fish Oil from Fish Heads Using Ultra-High Pressure Pre-Treatment Prior to Enzymatic Hydrolysis. Innov. Food Sci. Emerg. Technol. 2021, 70, 102670. [Google Scholar] [CrossRef]
- Ma, C.-C.; Wang, X.-C.; Tao, N.-P. Hydroxyapatite from the Skull of Tuna (Thunnus obesus) Head Combined with Chitosan to Restore Locomotive Function After Spinal Cord Injury. Front. Nutr. 2021, 8, 734498. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Souto-Montero, P.; Durán, S.; Pérez-Davila, S.; Vázquez, J.A.; González, P.; Serra, J. Valuable Ca/P Sources Obtained from Tuna Species’ By-Products Derived from Industrial Processing: Physicochemical and Features of Skeleton Fractions. Recycling 2024, 9, 109. [Google Scholar] [CrossRef]
- Wang, Y.M.; Li, X.Y.; Wang, J.; He, Y.; Chi, C.F.; Wang, B. Antioxidant Peptides from Protein Hydrolysate of Skipjack Tuna Milt: Purification, Identification, and Cytoprotection on H2O2 Damaged Human Umbilical Vein Endothelial Cells. Process Biochem. 2022, 113, 258–269. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhao, Y.-Q.; Wang, Y.-M.; Yang, X.-R.; Chi, C.-F.; Wang, B. Gelatins and Antioxidant Peptides from Skipjack Tuna (Katsuwonus pelamis) Skins: Purification, Characterization, and Cytoprotection on Ultraviolet-A Injured Human Skin Fibroblasts. Food Biosci. 2022, 50, 102138. [Google Scholar] [CrossRef]
- Sisa, A.; Martínez-Álvarez, O.; Gómez-Estaca, J.; Mosquera, M. Valorization of Yellowfin Tuna Tails: From Proteolytic Enzyme Production to Gelatin and Antioxidant Hydrolysate Extraction. Foods 2024, 13, 2034. [Google Scholar] [CrossRef]
- Wardani, D.W.; Ningrum, A.; Manikharda; Vanidia, N.; Munawaroh, H.S.H.; Susanto, E.; Show, P.L. In Silico and in Vitro Assessment of Yellowfin Tuna Skin (Thunnus albacares) Hydrolysate Antioxidation Effect. Food Hydrocoll. Health 2023, 3, 100126. [Google Scholar] [CrossRef]
- Qiao, Q.Q.; Luo, Q.B.; Suo, S.K.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Preparation, Characterization, and Cytoprotective Effects on HUVECs of Fourteen Novel Angiotensin-I-Converting Enzyme Inhibitory Peptides From Protein Hydrolysate of Tuna Processing By-Products. Front. Nutr. 2022, 9, 868681. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicrob. Proteins 2019, 11, 1015–1022. [Google Scholar] [CrossRef]
- Prasetyo, D.Y.B.; Agustini, T.W.; Anjani, G.; Riyadi, P.H. Evaluation of Immunomodulatory Properties of Fish-Protein Hydrolysate from Skipjack Tuna by-Products (Katsuwonus pelamis, Linnaeus 1758) in Streptozotocin-Nicotinamide-Induced Diabetic Rats. World’s Vet. J. 2025, 15, 117–125. [Google Scholar] [CrossRef]
- Aquaculture in Spain 2024. Available online: https://apromar.es/wp-content/uploads/2025/03/Informe2024_ENG_v2.pdf (accessed on 13 May 2025).
- Hamre, K.; Yúfera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.C.; Izquierdo, M. Fish Larval Nutrition and Feed Formulation: Knowledge Gaps and Bottlenecks for Advances in Larval Rearing. Rev. Aquac. 2013, 5, S26–S58. [Google Scholar] [CrossRef]
- Ahmed, N.; Thompson, S.; Glaser, M. Global Aquaculture Productivity, Environmental Sustainability, and Climate Change Adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, M.R.; Siddik, M.A.B.; Fotedar, R. Total Replacement of Fishmeal with Poultry Byproduct Meal Affected the Growth, Muscle Quality, Histological Structure, Antioxidant Capacity and Immune Response of Juvenile Barramundi, Lates Calcarifer. PLoS ONE 2020, 15, e0242079. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Howieson, J.; Siddik, M.A.B.; Foysal, M.J.; Fotedar, R. Supplementation of Tuna Hydrolysate and Insect Larvae Improves Fishmeal Replacement Efficacy of Poultry By-Product in Lates Calcarifer (Bloch, 1790) Juveniles. Sci. Rep. 2021, 11, 4997. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic Fish Protein Hydrolysates in Finfish Aquaculture: A Review. Rev. Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Rashidian, G.; Abedian Kenari, A.; Nikkhah, M. Dietary Effects of a Low-Molecular Weight Fraction (<10 KDa) from Shrimp Waste Hydrolysate on Growth Performance and Immunity of Rainbow Trout (Oncorhynchus Mykiss): Employing Nanodelivery Systems. Fish. Shellfish. Immunol. 2021, 118, 294–302. [Google Scholar] [CrossRef]
- Barroso, F.G.; Rodiles, A.; Vizcaino, A.J.; Martínez, T.F.; Alarcón, F.J. Evaluation of Feed Attractants in Juvenile Senegalese Sole, Solea Senegalensis. J. World Aquac. Soc. 2013, 44, 682–693. [Google Scholar] [CrossRef]
- Chotikachinda, R.; Tantikitti, C.; Benjakul, S.; Rustad, T.; Kumarnsit, E. Production of Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Viscera as Feeding Attractants for Asian Seabass (Lates calcarifer). Aquac. Nutr. 2013, 19, 773–784. [Google Scholar] [CrossRef]
- Valero, Y.; Saraiva-Fraga, M.; Costas, B.; Guardiola, F.A. Antimicrobial Peptides from Fish: Beyond the Fight against Pathogens. Rev. Aquac. 2020, 12, 224–253. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Kemp, D.C.; Kwon, J.Y. Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms. Molecules 2021, 26, 3225. [Google Scholar] [CrossRef] [PubMed]
- Aklakur, M. Natural Antioxidants from Sea: A Potential Industrial Perspective in Aquafeed Formulation. Rev. Aquac. 2018, 10, 385–399. [Google Scholar] [CrossRef]
- Chorhirankul, N.; Janssen, A.E.M.; Boom, R.M. UF Fractionation of Fish Protein Hydrolysate. Sep. Purif. Technol. 2024, 330, 125232. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Purification and Fractionation of Bioactive Peptides through Membrane Filtration: A Critical and Application Review. Trends Food Sci. Technol. 2023, 131, 118–128. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Saadaoui, H.; Espejo-Carpio, F.J.; Guadix, E.M.; Amar, R.B.; Pérez-Gálvez, R. Bi-Objective Optimization of Tuna Protein Hydrolysis to Produce Aquaculture Feed Ingredients. Food Bioprod. Process. 2019, 115, 26–35. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins, 1st ed.; Elsevier Applied Science Publishers LTD: London, UK, 1986. [Google Scholar]
- Steinhardt, H.; Beychok, S. Interaction of Proteins with Hydrogen Ions and Other Small Ions and Molecules. In The Proteins; Neurath, H., Ed.; Academic Press: New York, NY, USA, 1964; pp. 139–304. ISBN 9780123957245. [Google Scholar]
- Suki, A.; Fane, A.G.; Fell, C.J.D. Flux Decline in Protein Ultrafiltration. J. Memb. Sci. 1984, 21, 269–283. [Google Scholar] [CrossRef]
- Pérez-Gálvez, R.; Guadix, E.M.; Bergé, J.-P.; Guadix, A. Operation and Cleaning of Ceramic Membranes for the Filtration of Fish Press Liquor. J. Memb. Sci. 2011, 384, 142–148. [Google Scholar] [CrossRef]
- Pérez-Gálvez, R.; Guadix, E.M.; Bergé, J.-P.; Guadix, A. Processing Fish Press Waters Using Metallic and Ceramic Filtration. J. Chem. Technol. Biotechnol. 2013, 88, 1885–1890. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Picot, L.; Ravallec, R.; Martine, F.P.; Vandanjon, L.; Jaouen, P.; Chaplain-Derouiniot, M.; Guérard, F.; Chabeaud, A.; Legal, Y.; Alvarez, O.M.; et al. Impact of Ultrafiltration and Nanofiltration of an Industrial Fish Protein Hydrolysate on Its Bioactive Properties. J. Sci. Food Agric. 2010, 90, 1819–1826. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of Ferritin as a Lipid Oxidation Catalyst in Muscle Food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein Hydrolysates in Animal Nutrition: Industrial Production, Bioactive Peptides, and Functional Significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an Enzyme Preparation with Industrial Relevance: Automated Nine-Step Purification and Partial Characterization of Eight Enzymes. J. Agric. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef]
- Sierra-Lopera, L.M.; Zapata-Montoya, J.E. Optimization of Enzymatic Hydrolysis of Red Tilapia Scales (Oreochromis sp.) to Obtain Bioactive Peptides. Biotechnol. Rep. 2021, 30, e00611. [Google Scholar] [CrossRef] [PubMed]
- Remme, J.; Tveit, G.M.; Toldnes, B.; Slizyte, R.; Carvajal, A.K. Production of Protein Hydrolysates from Cod (Gadus Morhua) Heads: Lab and Pilot Scale Studies. J. Aquat. Food Prod. Technol. 2022, 31, 114–127. [Google Scholar] [CrossRef]
- Lin, M.-Z.; Chen, B.-H. An Improved Production Method of Bioactive Peptides from Sturgeon Fish Cartilage. Food Bioproc. Tech. 2024, 18, 2050–2069. [Google Scholar] [CrossRef]
- Corbatón-Báguena, M.-J.; Álvarez-Blanco, S.; Vincent-Vela, M.-C. Fouling Mechanisms of Ultrafiltration Membranes Fouled with Whey Model Solutions. Desalination 2015, 360, 87–96. [Google Scholar] [CrossRef]
- Dutournié, P.; Limousy, L.; Anquetil, J.; Déon, S. Modification of the Selectivity Properties of Tubular Ceramic Membranes after Alkaline Treatment. Membranes 2017, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, S.; Huang, T.; Dong, C.; Li, J.; Zhao, B.; Zhang, S. Chemical Cleaning of Ultrafiltration Membrane Fouled by Humic Substances: Comparison between Hydrogen Peroxide and Sodium Hypochlorite. Int. J. Environ. Res. Public Health 2019, 16, 2568. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Nasrullah, M.; Kamyab, H.; Suzana, N.; Ab Munaim, M.S.; Ab Wahid, Z.; Ali, I.H.; Salehi, R.; Chaiprapat, S. Fouling Characteristics and Cleaning Approach of Ultrafiltration Membrane during Xylose Reductase Separation. Bioprocess Biosyst. Eng. 2022, 45, 1125–1136. [Google Scholar] [CrossRef]
- Mourouzidis-Mourouzis, S.A.; Karabelas, A.J. Whey Protein Fouling of Large Pore-Size Ceramic Microfiltration Membranes at Small Cross-Flow Velocity. J. Memb. Sci. 2008, 323, 17–27. [Google Scholar] [CrossRef]
- Xing, S.; Liang, X.; Zhang, X.; Oliva-Teles, A.; Peres, H.; Li, M.; Wang, H.; Mai, K.; Kaushik, S.J.; Xue, M. Essential Amino Acid Requirements of Fish and Crustaceans, a Meta-Analysis. Rev. Aquac. 2024, 16, 1069–1086. [Google Scholar] [CrossRef]
- FAO. Nutritional Requirements. Available online: https://www.fao.org/fishery/affris/species-profiles/european-seabass/nutritional-requirements/en/ (accessed on 9 June 2025).
- Karami, Z.; Akbari-adergani, B. Bioactive Food Derived Peptides: A Review on Correlation between Structure of Bioactive Peptides and Their Functional Properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Comesaña, S.; Soengas, J.L.; Pérez, M.; Bermúdez, R.; Rotllant, J.; Valcarcel, J. Optimal and Sustainable Production of Tailored Fish Protein Hydrolysates from Tuna Canning Wastes and Discarded Blue Whiting: Effect of Protein Molecular Weight on Chemical and Bioactive Properties. Sci. Total Environ. 2024, 939, 173461. [Google Scholar] [CrossRef]
- Cai, Z.; Li, W.; Mai, K.; Xu, W.; Zhang, Y.; Ai, Q. Effects of Dietary Size-Fractionated Fish Hydrolysates on Growth, Activities of Digestive Enzymes and Aminotransferases and Expression of Some Protein Metabolism Related Genes in Large Yellow Croaker (Larimichthys crocea) Larvae. Aquaculture 2015, 440, 40–47. [Google Scholar] [CrossRef]
- Khosravi, S.; Bui, H.T.D.; Herault, M.; Fournier, V.; Kim, K.-D.; Lee, B.-J.; Kim, K.-W.; Lee, K.-J. Supplementation of Protein Hydrolysates to a Low-Fishmeal Diet Improves Growth and Health Status of Juvenile Olive Flounder, Paralichthys Olivaceus. J. World Aquac. Soc. 2018, 49, 897–911. [Google Scholar] [CrossRef]
- Ovissipour, M.; Kenari, A.A.; Nazari, R.; Motamedzadegan, A.; Rasco, B. Tuna Viscera Protein Hydrolysate: Nutritive and Disease Resistance Properties for Persian Sturgeon (Acipenser persicus L.) Larvae. Aquac. Res. 2014, 45, 591–601. [Google Scholar] [CrossRef]
- Printzi, A.; Jodet, S.; Fournier, V.; Collet, S.; Madec, L.; Simon, V.; Zambonino-Infante, J.-L.; Koumoundouros, G.; Mazurais, D. Effect of Early Peptide Diets on European Sea Bass (Dicentrarchus labrax) Skeletal Development. Aquaculture 2024, 584, 740657. [Google Scholar] [CrossRef]
- Kotzamanis, Y.P.; Gisbert, E.; Gatesoupe, F.J.; Infante, J.Z.; Cahu, C. Effects of Different Dietary Levels of Fish Protein Hydrolysates on Growth, Digestive Enzymes, Gut Microbiota, and Resistance to Vibrio Anguillarum in European Sea Bass (Dicentrarchus labrax) Larvae. Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2007, 147, 205–214. [Google Scholar] [CrossRef]
- Morais, S. The Physiology of Taste in Fish: Potential Implications for Feeding Stimulation and Gut Chemical Sensing. Rev. Fish. Sci. Aquac. 2017, 25, 133–149. [Google Scholar] [CrossRef]
- Li, X.; Fang, T.; Wang, J.; Wang, Z.; Guan, D.; Sun, H.; Yun, X.; Zhou, J. The Efficiency of Adding Amino Acid Mixtures to a Diet Free of Fishmeal and Soybean Meal as an Attractant in Yellow River Carp (Cyprinus carpio Var.). Aquac. Rep. 2022, 24, 101189. [Google Scholar] [CrossRef]
- Hu, X.; Ma, W.; Zhang, D.; Tian, Z.; Yang, Y.; Huang, Y.; Hong, Y. Application of Natural Antioxidants as Feed Additives in Aquaculture: A Review. Biology 2025, 14, 87. [Google Scholar] [CrossRef]
- Lundebyea, A.K.; Hovea, H.; Mågea, A.; Bohneb, V.J.B.; Hamrea, K. Levels of Synthetic Antioxidants (Ethoxyquin, Butylated Hydroxytoluene and Butylated Hydroxyanisole) in Fish Feed and Commercially Farmed Fish. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 1652–1657. [Google Scholar] [CrossRef]
- López-García, G.; Dublan-García, O.; Arizmendi-Cotero, D.; Gomez-Olivan, L.M. Antioxidant and Antimicrobial Peptides Derived from Food Proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Kizhakkethil, B.P.; George, J.C.; Abubacker, Z.A.; Ninan, G.; Chandragiri Nagarajarao, R. Antioxidant Peptides from Dark Meat of Yellowfin Tuna (Thunnus albacares): Process Optimization and Characterization. Waste Biomass Valorization 2021, 12, 1845–1860. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.M.; Li, L.Y.; Chi, C.F.; Wang, B. Twelve Antioxidant Peptides from Protein Hydrolysate of Skipjack Tuna (Katsuwonus pelamis) Roe Prepared by Flavourzyme: Purification, Sequence Identification, and Activity Evaluation. Front. Nutr. 2022, 8, 813780. [Google Scholar] [CrossRef]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, Z.-R.; Luo, H.-Y. Influence of Amino Acid Compositions and Peptide Profiles on Antioxidant Capacities of Two Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Dark Muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef]
- Krasae, K.; Worawattanamateekul, W.; Hinsui, J. Effects of Peptide Fractions and Amino Acids on Antioxidant Properties of Autolyzed Tuna Viscera Protein Hydrolysate. Food Res. 2023, 7, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-T.; Dong, C.-M. Research Progress of Metal Chelating Peptides. Food Health 2022, 4, 19. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Sun, X.; Sarteshnizi, R.A.; Boachie, R.T.; Okagu, O.D.; Abioye, R.O.; Neves, R.P.; Ohanenye, I.C.; Udenigwe, C.C. Peptide–Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency. Foods 2020, 9, 1402. [Google Scholar] [CrossRef]



| Membrane MWCO | Membrane Resistances | Resistance-in-Series Model, Equation (4) | % Cleaning Efficiency, Equation (5) | |||||
|---|---|---|---|---|---|---|---|---|
| Initial, RM Bar·m2·h·L−1 | Fouled, R1 Bar·m2·h·L−1 | Permeate Stream | J0 L·m−2·h−1 | J∞ L·m−2·h−1 | k h−1 | 1st Stage | 2nd Stage | |
| 8 kDa | 1.31 × 10−2 | 2.50 × 10−2 | F8 | 57.49 | 46.28 | 0.4794 | 66.87 | 23.13 |
| 3 kDa | 1.98 × 10−2 | 3.10 × 10−2 | F3 | 49.66 | 35.16 | 0.4933 | 99.79 | - |
| 1 kDa fed F8 | 2.12 × 10−2 | 3.75 × 10−2 | F8F1 | 35.04 | 24.75 | 0.7227 | 99.90 | - |
| 1 kDa fed F3 | 2.12 × 10−2 | 4.02 × 10−2 | F3F1 | 39.06 | 28.28 | 0.5892 | 99.24 | - |
| Content of Soluble Protein | Amino Acid Composition of F3R1 and F3F1 | |||
|---|---|---|---|---|
| Stream | Concentration, g·L−1 | Amino Acid | Mol Percentage | |
| F3R1 | F3F1 | |||
| THH | 9.35 ± 0.33 | ALA | 7.41 ± 0.15 | 6.73 ± 0.22 |
| R8 | 10.73 ± 0.26 | ARG | 18.16 ± 0.96 | 18.29 ± 0.67 |
| F8R1 | 6.36 ± 0.49 | ASN + ASP | 0.05 ± 0.00 | 0.05 ± 0.00 |
| F8F1 | 4.92 ± 0.29 | GLN + GLU | 1.50 ± 0.15 | 1.49 ± 0.21 |
| R3 | 10.29 ± 0.29 | GLY | 16.19 ± 0.67 | 17.46 ± 0.41 |
| F3R1 | 5.50 ± 0.56 | HIS | 3.86 ± 0.13 * | 4.62 ± 0.19 |
| F3F1 | 5.17 ± 0.14 | ILE | 4.93 ± 0.16 | 5.17 ± 0.13 |
| LEU | 8.22 ± 0.19 * | 9.06 ± 0.13 | ||
| LYS | 2.85 ± 0.12 | 2.86 ± 0.17 | ||
| MET | 4.71 ± 0.19 | 3.97 ± 0.21 | ||
| PHE | 8.65 ± 0.12 * | 8.04 ± 0.07 | ||
| PRO | 1.56 ± 0.07 * | 0.68 ± 0.12 | ||
| SER | 3.92 ± 0.25 | 3.39 ± 0.23 | ||
| THR | 4.51 ± 0.48 | 4.52 ± 0.78 | ||
| TYR | 5.64 ± 0.24 | 6.04 ± 0.14 | ||
| VAL | 7.85 ± 0.41 | 7.61 ± 0.25 | ||
| Total Amino Acids 1 | 100 ± 1.44 | 100 ± 1.27 | ||
| Total Essential Amino Acids 2 | 64.74 ± 1.21 | 64.14 ± 1.13 | ||
| Total Hydrophobic Amino Acids 3 | 63.16 ± 0.90 | 64.76 ± 0.63 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Gálvez, R.; Espejo-Carpio, F.J.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Processing of Tuna Head By-Products into Antioxidant Peptide Ingredients for Aquaculture Feeds. Antioxidants 2025, 14, 770. https://doi.org/10.3390/antiox14070770
Pérez-Gálvez R, Espejo-Carpio FJ, García-Moreno PJ, Guadix A, Guadix EM. Processing of Tuna Head By-Products into Antioxidant Peptide Ingredients for Aquaculture Feeds. Antioxidants. 2025; 14(7):770. https://doi.org/10.3390/antiox14070770
Chicago/Turabian StylePérez-Gálvez, Raúl, F. Javier Espejo-Carpio, Pedro J. García-Moreno, Antonio Guadix, and Emilia M. Guadix. 2025. "Processing of Tuna Head By-Products into Antioxidant Peptide Ingredients for Aquaculture Feeds" Antioxidants 14, no. 7: 770. https://doi.org/10.3390/antiox14070770
APA StylePérez-Gálvez, R., Espejo-Carpio, F. J., García-Moreno, P. J., Guadix, A., & Guadix, E. M. (2025). Processing of Tuna Head By-Products into Antioxidant Peptide Ingredients for Aquaculture Feeds. Antioxidants, 14(7), 770. https://doi.org/10.3390/antiox14070770








